Overestimation of Oxygen Saturation Measured by Pulse Oximetry in Hypoxemia. Part 1: Effect of Optical Pathlengths-Ratio Increase
Abstract
1. Introduction
2. Background
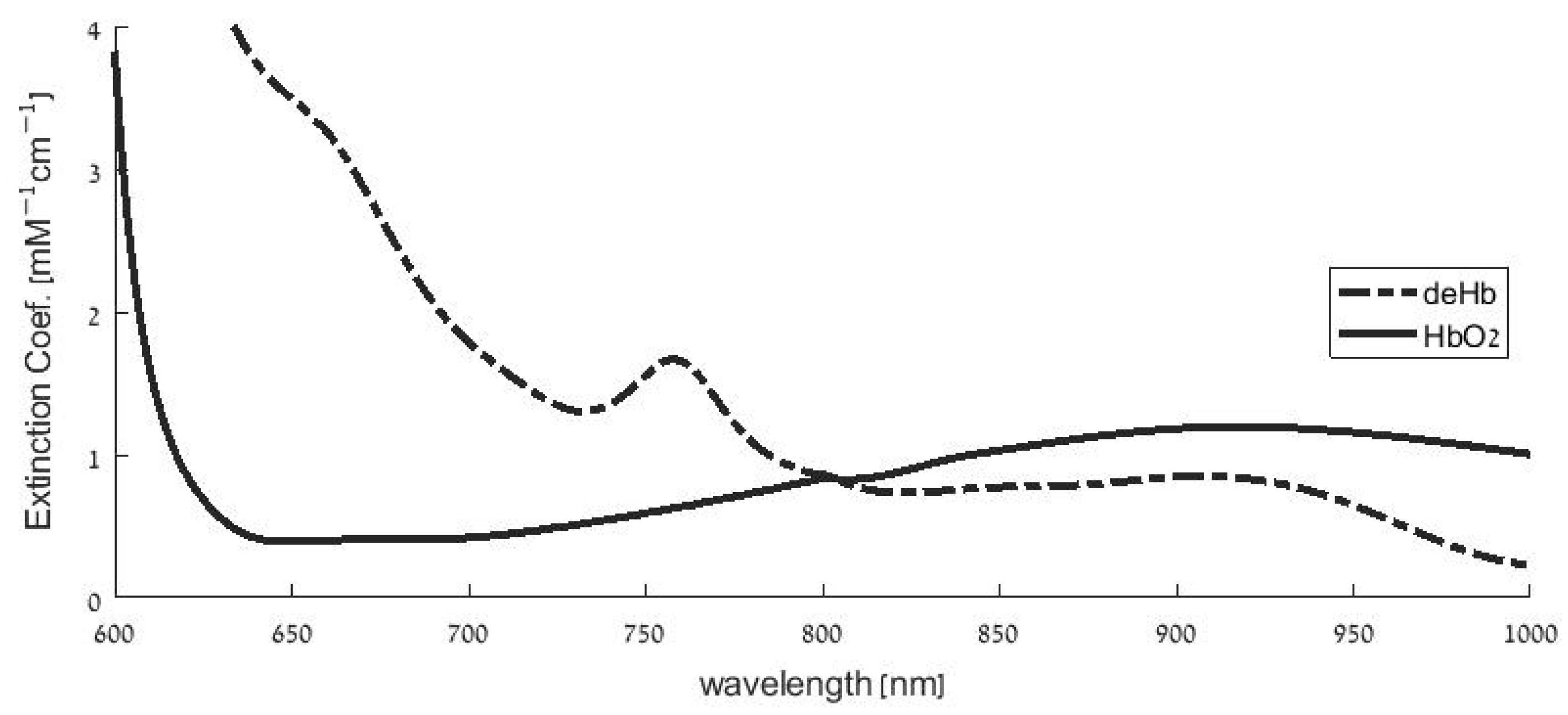
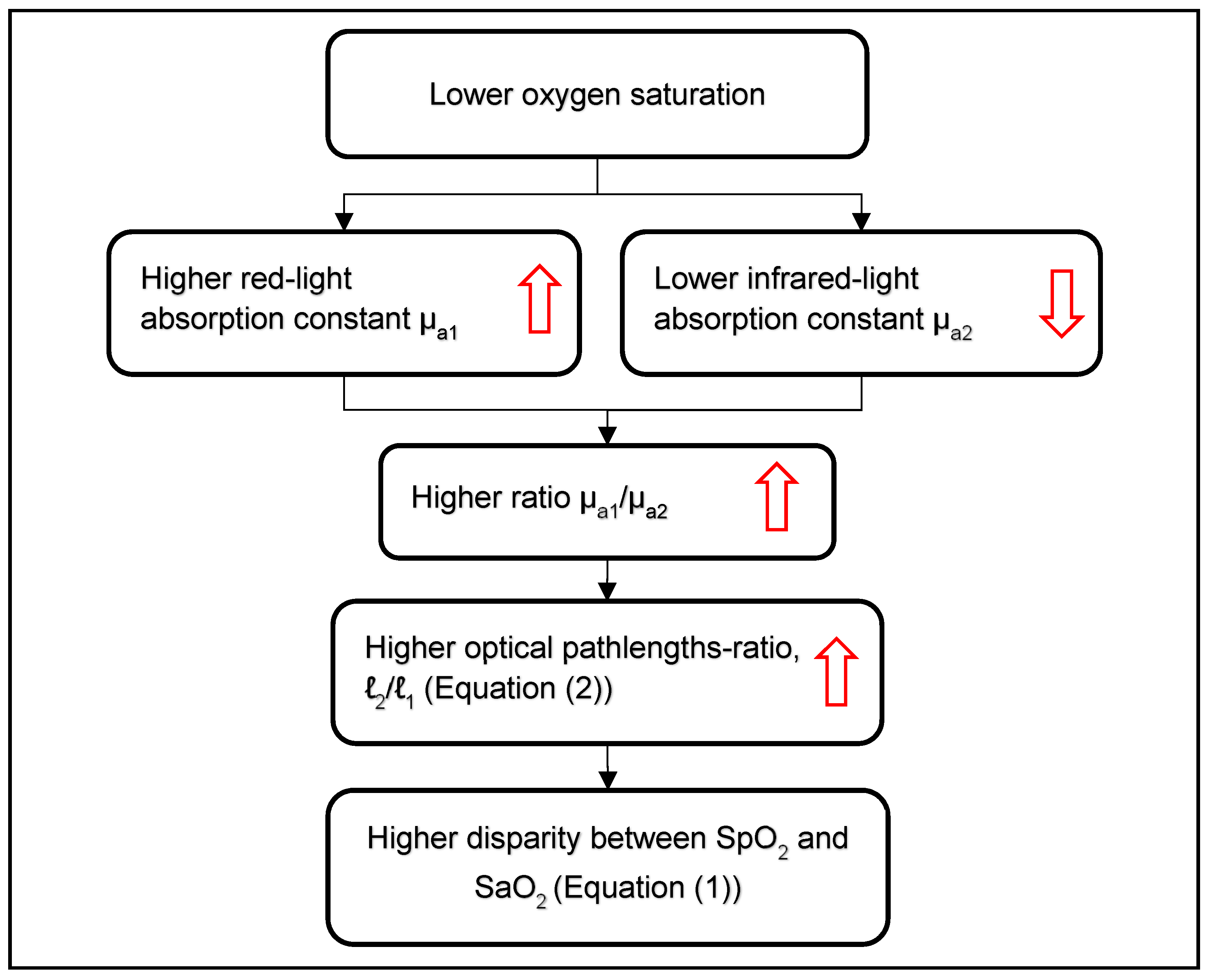
2.1. Overestimation of SpO2 in Hypoxemia
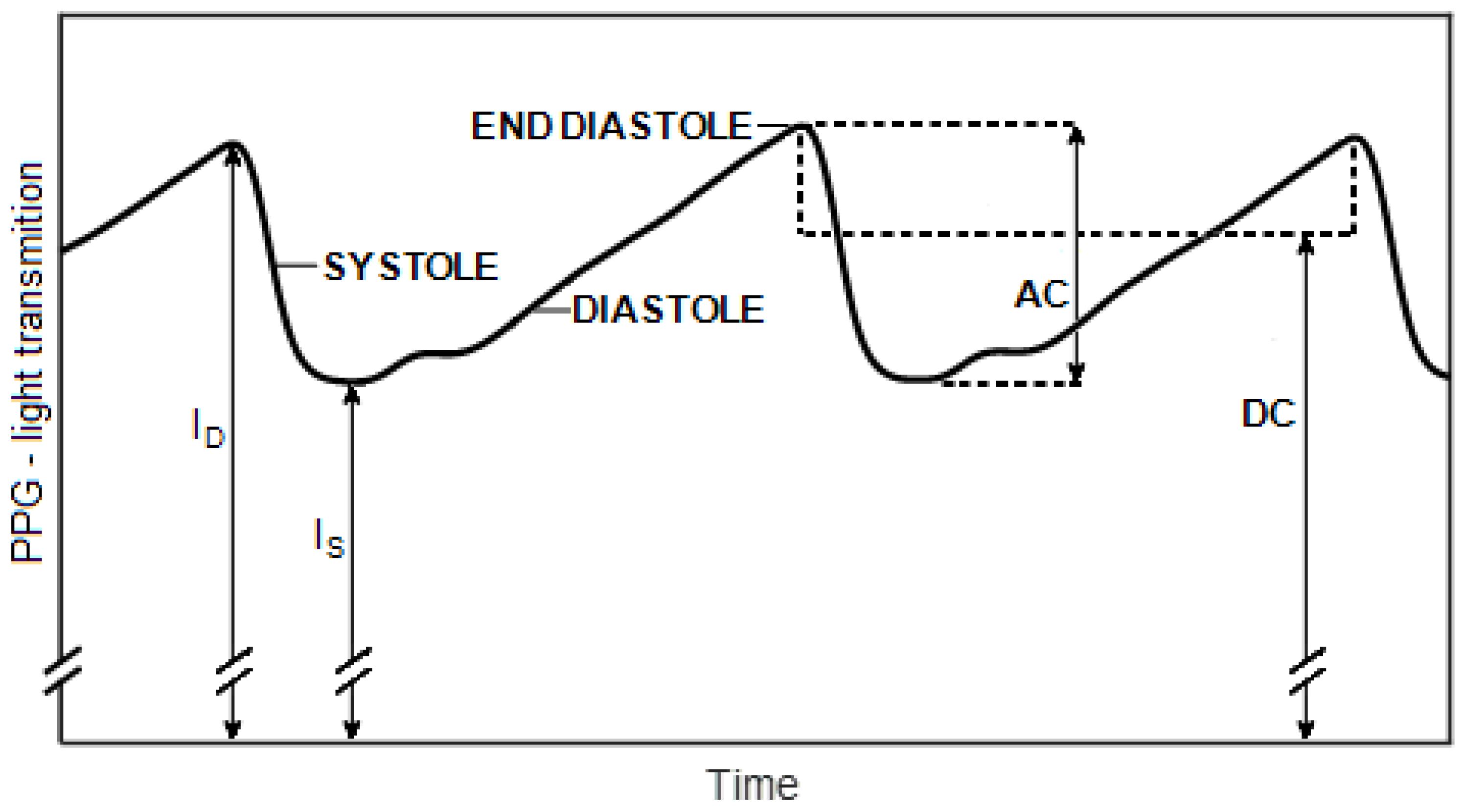
2.2. Pulse Oximetry
3. Materials and Methods
3.1. The Patients and the Clinical Examinations
3.2. The Two-Infrared Pulse Oximeter and Determination of the SpO2
3.3. Statistical Methods
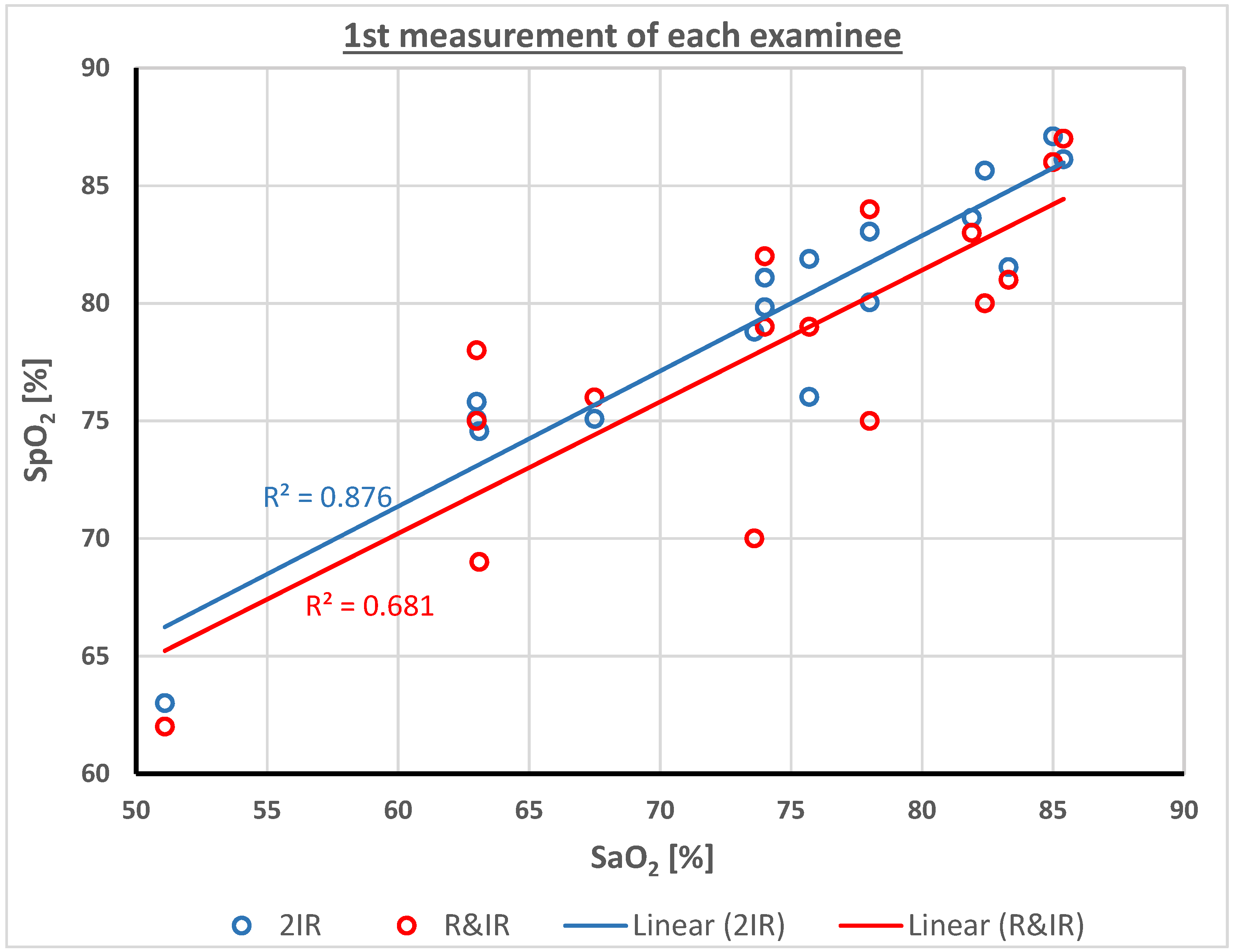
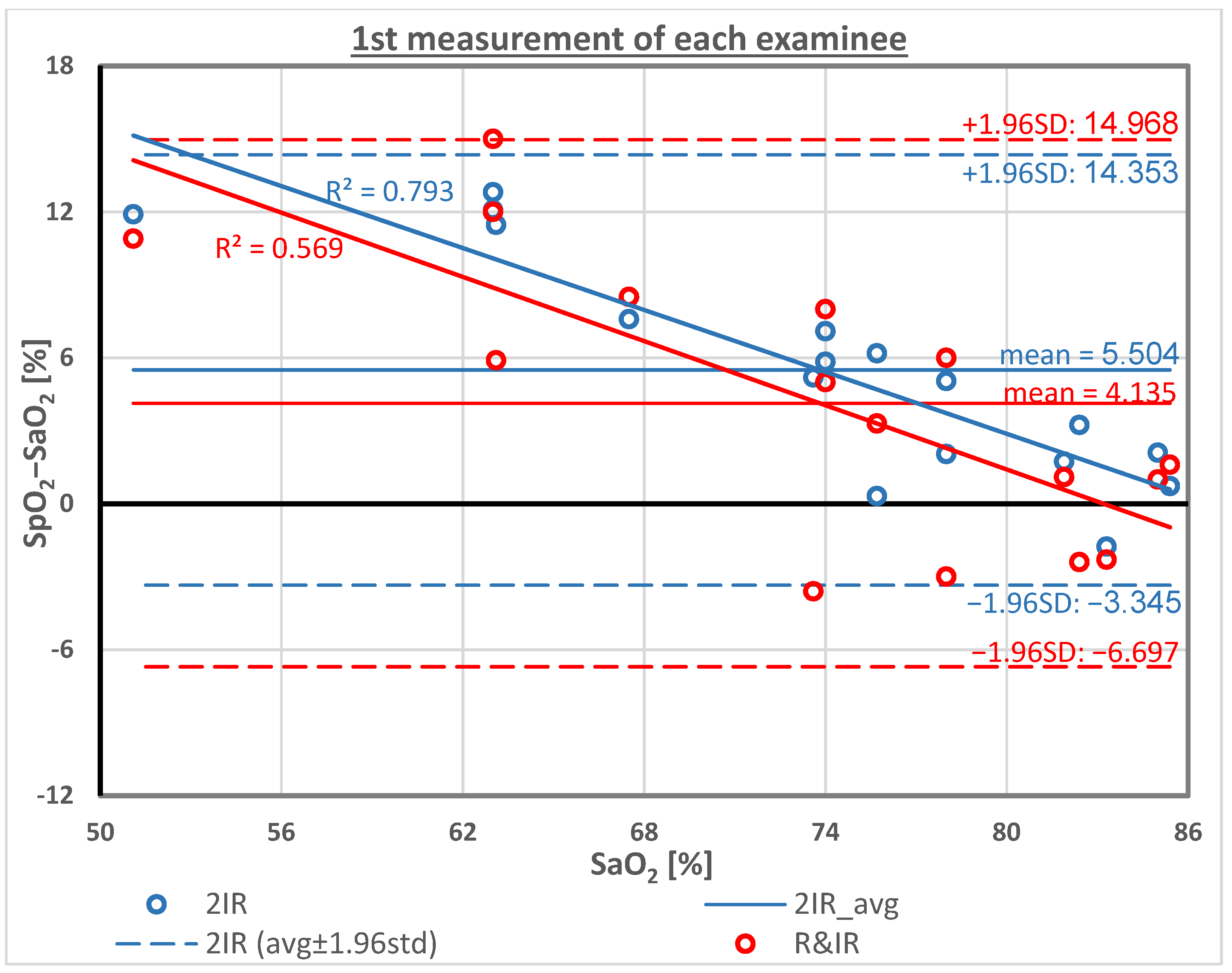
4. Results
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med. Devices 2014, 7, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Pulse Oximeter Accuracy and Limitations: FDA Safety Communication. Issued: February 19, 2021. Available online: https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication (accessed on 26 July 2022).
- Wilson, B.J.; Cowan, H.J.; Lord, J.A.; Zuege, D.J.; Zygun, D.A. The accuracy of pulse oximetry in emergency department patients with severe sepsis and septic shock: A retrospective cohort study. BMC Emerg. Med. 2010, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Lee, J.H.; Song, I.K.; Kim, H.S.; Jang, Y.E.; Yoo, S.; Kim, J.T. Accuracy of pulse oximeters at low oxygen saturations in children with congenital cyanotic heart disease: An observational study. Paediatr. Anaesth. 2019, 29, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.U.; Char, D.S.; Feinstein, J.A.; Verma, A.; Shiboski, S.C.; Ramamoorthy, C. Accuracy of Pulse Oximeters Intended for Hypoxemic Pediatric Patients. Pediatr. Crit. Care Med. 2016, 17, 315–320. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Helias, M.; Raia, L.; Nicolas, E.; Jaubert, P.; Benghanem, S.; Ait Hamou, Z.; Dupland, P.; Charpentier, J.; Pène, F.; et al. Impact of COVID-19 on the association between pulse oximetry and arterial oxygenation in patients with acute respiratory distress syndrome. Sci. Rep. 2022, 12, 1462. [Google Scholar] [CrossRef]
- Okunlola, O.E.; Lipnick, M.S.; Batchelder, P.B.; Bernstein, M.; Feiner, J.R.; Bickler, P.E. Pulse Oximeter Performance, Racial Inequity, and the Work Ahead. Respir. Care 2022, 67, 252–257. [Google Scholar] [CrossRef]
- Bickler, P.E.; Feiner, J.R.; Severinghaus, J.W. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology 2005, 102, 715–719. [Google Scholar] [CrossRef]
- Feiner, J.R.; Severinghaus, J.W.; Bickler, P.E. Dark skin decreases the accuracy of pulse oximeters at low oxygen saturation: The effects of oximeter probe type and gender. Anesth. Analg. 2007, 105, S18–S23. [Google Scholar] [CrossRef]
- Ross, P.A.; Newth, C.J.; Khemani, R.G. Accuracy of pulse oximetry in children. Pediatrics 2014, 133, 22–29. [Google Scholar] [CrossRef]
- Ascha, M.; Bhattacharyya, A.; Ramos, J.A.; Tonelli, A.R. Pulse Oximetry and Arterial Oxygen Saturation during Cardiopulmonary Exercise Testing. Med. Sci. Sports Exerc. 2018, 50, 1992–1997. [Google Scholar] [CrossRef]
- Wong, A.I.; Charpignon, M.; Kim, H.; Josef, C.; de Hond, A.A.H.; Fojas, J.J.; Tabaie, A.; Liu, X.; Mireles-Cabodevila, E.; Carvalho, L.; et al. Analysis of Discrepancies Between Pulse Oximetry and Arterial Oxygen Saturation Measurements by Race and Ethnicity and Association With Organ Dysfunction and Mortality. JAMA Netw. Open 2021, 4, e2131674. [Google Scholar] [CrossRef] [PubMed]
- Crooks, C.J.; West, J.; Morling, J.R.; Simmonds, M.; Juurlink, I.; Briggs, S.; Cruickshank, S.; Hammond-Pears, S.; Shaw, D.; Card, T.R.; et al. Pulse oximeter measurement error of oxygen saturation in patients with SARS-CoV-2 infection stratified by smoking status. Eur. Respir. J. 2022, 60, 2201190. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; McAuley, D.F.; Giles, S.; Routledge, H.; Gao, F. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit Care 2003, 7, R67–R71. [Google Scholar] [CrossRef]
- Seguin, P.; Le Rouzo, A.; Tanguy, M.; Guillou, Y.M.; Feuillu, A.; Mallédant, Y. Evidence for the need of bedside accuracy of pulse oximetry in an intensive care unit. Crit. Care Med. 2000, 28, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.J.; Schuetz, W.H.; Proeschel, P.A.; Jaklin, C. Accuracy of pulse oximetry in children with cyanotic congenital heart disease. J. Cardiothorac. Vasc. Anesth. 1993, 7, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat-Yazdi, F.; Torres, A., Jr.; Fortuna, R.; Geiss, D.M. Pulse oximeter accuracy and precision affected by sensor location in cyanotic children. Pediatr. Crit. Care Med. 2008, 9, 393–397. [Google Scholar] [CrossRef]
- Murphy, D.; Pak, Y.; Cleary, J.P. Pulse Oximetry Overestimates Oxyhemoglobin in Neonates with Critical Congenital Heart Disease. Neonatology 2016, 109, 213–218. [Google Scholar] [CrossRef]
- Griksaitis, M.J.; Scrimgeour, G.E.; Pappachan, J.V.; Baldock, A.J. Accuracy of the Masimo SET® LNCS neo peripheral pulse oximeter in cyanotic congenital heart disease. Cardiol. Young. 2016, 26, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Scrimgeour, G.E.; Griksaitis, M.J.; Pappachan, J.V.; Baldock, A.J. The Accuracy of Noninvasive Peripheral Pulse Oximetry After Palliative Cardiac Surgery in Patients With Cyanotic Congenital Heart Disease. World J. Pediatr. Congenit. Heart Surg. 2017, 8, 32–38. [Google Scholar] [CrossRef]
- Foglia, E.E.; Whyte, R.K.; Chaudhary, A.; Mott, A.; Chen, J.; Propert, K.J.; Schmidt, B. The Effect of Skin Pigmentation on the Accuracy of Pulse Oximetry in Infants with Hypoxemia. J. Pediatr. 2017, 182, 375–377.e2. [Google Scholar] [CrossRef]
- Wackernagel, D.; Blennow, M.; Hellström, A. Accuracy of pulse oximetry in preterm and term infants is insufficient to determine arterial oxygen saturation and tension. Acta Paediatr. 2020, 109, 2251–2257. [Google Scholar] [CrossRef]
- Vesoulis, Z.; Tims, A.; Lodhi, H.; Lalos, N.; Whitehead, H. Racial discrepancy in pulse oximeter accuracy in preterm infants. J. Perinatol. 2022, 42, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.J.; Shen, Y.; Lu, L.; Zhang, R.Y.; Zhang, Q.; Shen, W.F. Increased blood glycohemoglobin A1c levels lead to overestimation of arterial oxygen saturation by pulse oximetry in patients with type 2 diabetes. Cardiovasc. Diabetol. 2012, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Gürün Kaya, A.; Öz, M.; Akdemir Kalkan, İ.; Gülten, E.; Çınar, G.; Azap, A.; Kaya, A. Is pulse oximeter a reliable tool for non-critically ill patients with COVID-19? Int. J. Clin. Pract. 2021, 75, e14983. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Baig, N.; McDonnell, T.; Bentley, A. Discrepancy between SpO2 and SaO2 in patients with COVID-19. Anaesthesia 2021, 76, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, M.; Nitzan, I.; Arieli, Y. The Various Oximetric Techniques Used for the Evaluation of Blood Oxygenation. Sensors 2020, 20, 4844. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse oximetry: Understanding its basic principles facilitates appreciation of its limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, P.A.; Chatterjee, S. The Origin of Photoplethysmography, Ch. 2. In Photoplethysmography; Kyriacou, P.A., Allen, J., Eds.; Elsevier, Academic Press: London, UK, 2021. [Google Scholar]
- Kim, J.G.; Liu, H. Variation of haemoglobin extinction coefficients can cause errors in the determination of haemoglobin concentration measured by near-infrared spectroscopy. Phys. Med. Biol. 2007, 52, 6295. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G. (Gwangju Institute of Science and Technology, Gwangju, Republic of Korea); Liu, H. (Joint Graduate Program in Biomedical Engineering, University of Texas at Arlington/University of Texas Southwestern Medical Center, Dallas, TX, USA). Personal Communication, 2007.
- Yossef Hay, O.; Cohen, M.; Nitzan, I.; Kasirer, Y.; Shahroor-Karni, S.; Yitzhaky, Y.; Engelberg, S.; Nitzan, M. Pulse Oximetry with Two Infrared Wavelengths without Calibration in Extracted Arterial Blood. Sensors 2018, 18, 3457. [Google Scholar] [CrossRef] [PubMed]
- Zourabian, A.; Siegel, A.; Chance, B.; Ramanujan, N.; Rode, M.; Boas, D.A. Trans-abdominal monitoring of fetal arterial blood oxygenation using pulse oximetry. J. Biomed. Opt. 2000, 5, 391–405. [Google Scholar] [CrossRef]
- Nitzan, M.; Babchenko, A.; Khanokh, B.; Taitelbaum, H. Measurement of oxygen saturation in venous blood by dynamic near infrared spectroscopy. J. Biomed. Opt. 2000, 5, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Wolf, M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J. Biomed. Opt. 2013, 18, 105004. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Perpetuini, D.; Filippini, C.; Cardone, D.; Merla, A. Differential pathlength factor in continuous wave functional near-infrared spectroscopy: Reducing hemoglobin’s cross talk in high-density recordings. Neurophotonics 2019, 6, 035005. [Google Scholar] [CrossRef]
- Mendelson, Y. Pulse oximetry: Theory and applications for noninvasive monitoring. Clin. Chem. 1992, 38, 1601–1607. [Google Scholar] [CrossRef]
- Mannheimer, P.D. The Light-Tissue Interaction of Pulse Oximetry. Anesth. Analg. 2007, 105, S10–S17. [Google Scholar] [CrossRef]
- Hornberger, C.; Wabnitz, H. Approaches for calibration and validation of near-infrared optical methods for oxygenation monitoring. Biomed. Tech. 2018, 63, 537–546. [Google Scholar] [CrossRef]
- Friebel, M.; Helfmann, J.; Netz, U.; Meinke, M. Influence of oxygen saturation on the optical scattering properties of human red blood cells in the spectral range 250 to 2000 nm. J. Biomed. Opt. 2009, 14, 034001. [Google Scholar] [CrossRef] [PubMed]
- Jacques 2013, Jacques SL Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61.
- Jonasson, H.; Fredriksson, I.; Bergstrand, S.; Östgren, C.J.; Larsson, M.; Strömberg, T. In vivo characterization of light scattering properties of human skin in the 475- to 850-nm wavelength range in a Swedish cohort. J. Biomed. Opt. 2018, 23, 1–6. [Google Scholar] [CrossRef]
- Nitzan, M.; Noach, S.; Tobal, E.; Adar, Y.; Miller, Y.; Shalom, E.; Engelberg, S. Calibration-free pulse oximetry based on two wavelengths in the infrared—A preliminary study. Sensors 2014, 14, 7420–7434. [Google Scholar] [CrossRef]
- ISO 80601-2-61 Standard. Available online: https://www.iso.org/standard/67963.html (accessed on 21 December 2022).
- Batchelder, P.B.; Raley, D.M. Maximizing the laboratory setting for testing devices and understanding statistical output in pulse oximetry. Anesth. Analg. 2007, 105, S85–S94. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Pretty, C.G.; Amies, A.C.; Balmer, J.; Banna, H.E.; Shaw, G.M.; Geoffrey Chase, J. Proof of concept non-invasive estimation of peripheral venous oxygen saturation. Biomed. Eng. Online 2017, 16, 60. [Google Scholar] [CrossRef] [PubMed]

| Pat. # | SaO2 [%] | SpO2 [%] | SpO2−SaO2 [%] | SpO2 Corrct. [%] | SpO2−SaO2 Corrct. [%] | ||||
|---|---|---|---|---|---|---|---|---|---|
| R&IR | 2IR | R&IR | 2IR | Nellcor | 2IR | Nellcor | 2IR | ||
| 1 | 83.3 | 81 | 81.5 | −2.3 | −1.8 | 79.3 | 77.7 | −4.0 | −5.6 |
| 2 | 63.1 | 69 | 74.6 | 5.9 | 11.5 | 57.8 | 65.6 | −5.3 | 2.5 |
| 3 | 63.0 | 78 | 75.1 | 15.0 | 12.1 | 73.9 | 66.4 | 10.9 | 3.4 |
| 4 | 82.4 | 80 | 85.6 | −2.4 | 3.2 | 77.5 | 84.8 | −4.9 | 2.4 |
| 5 | 63.0 | 75 | 75.8 | 12.0 | 12.8 | 68.6 | 67.7 | 5.6 | 4.7 |
| 6 | 78.0 | 75 | 80.0 | −3.0 | 2.0 | 68.6 | 75.1 | −9.4 | −2.9 |
| 7 | 67.5 | 76 | 75.1 | 8.5 | 7.6 | 70.3 | 66.5 | 2.8 | −1.0 |
| 8 | 73.6 | 70 | 78.8 | −3.6 | 5.2 | 59.6 | 72.9 | −14.0 | −0.7 |
| 9 | 85.0 | 86 | 87.1 | 1.0 | 2.1 | 88.2 | 87.3 | 3.2 | 2.3 |
| 10 | 51.1 | 62 | 63.0 | 10.9 | 11.9 | 45.3 | 45.5 | −5.8 | −5.6 |
| 11 | 74.0 | 82 | 81.1 | 8.0 | 7.1 | 81.1 | 76.9 | 7.1 | 2.9 |
| 12 | 75.7 | 79 | 76.0 | 3.3 | 0.3 | 75.7 | 68.1 | 0.0 | −7.6 |
| 13 | 85.4 | 87 | 86.1 | 1.6 | 0.7 | 90.0 | 85.6 | 4.6 | 0.2 |
| 14 | 74.0 | 79 | 79.8 | 5 | 5.8 | 75.7 | 74.7 | 1.7 | 0.7 |
| 15 | 78.0 | 84 | 83.0 | 6 | 5.0 | 84.6 | 80.3 | 6.6 | 2.3 |
| 16 | 81.9 | 83 | 83.6 | 1.1 | 1.7 | 82.8 | 81.3 | 0.9 | −0.6 |
| 17 | 75.7 | 79 | 81.9 | 3.3 | 6.2 | 75.7 | 78.3 | 0.0 | 2.6 |
| Mean | 73.8 | 77.9 | 79.3 | 4.1 | 5.5 | 73.8 | 73.8 | 0.0 | 0.0 |
| SD | 9.5 | 6.4 | 5.8 | 5.5 | 4.5 | 11.5 | 10.1 | 6.5 | 3.6 |
| SpO2_R-IR (%) | SpO2_2IR (%) | |
|---|---|---|
| Standard deviation of SpO2−SaO2 after calibration * | 6.5 | 3.6 |
| Arms, the rms of SpO2−SaO2 after calibration | 6.3 | 3.5 |
| Correlation coefficient of SpO2 with SaO2 ** | 0.83 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elron, E.; Bromiker, R.; Gleisner, O.; Yosef-Hai, O.; Goldberg, O.; Nitzan, I.; Nitzan, M. Overestimation of Oxygen Saturation Measured by Pulse Oximetry in Hypoxemia. Part 1: Effect of Optical Pathlengths-Ratio Increase. Sensors 2023, 23, 1434. https://doi.org/10.3390/s23031434
Elron E, Bromiker R, Gleisner O, Yosef-Hai O, Goldberg O, Nitzan I, Nitzan M. Overestimation of Oxygen Saturation Measured by Pulse Oximetry in Hypoxemia. Part 1: Effect of Optical Pathlengths-Ratio Increase. Sensors. 2023; 23(3):1434. https://doi.org/10.3390/s23031434
Chicago/Turabian StyleElron, Eyal, Ruben Bromiker, Ori Gleisner, Ohad Yosef-Hai, Ori Goldberg, Itamar Nitzan, and Meir Nitzan. 2023. "Overestimation of Oxygen Saturation Measured by Pulse Oximetry in Hypoxemia. Part 1: Effect of Optical Pathlengths-Ratio Increase" Sensors 23, no. 3: 1434. https://doi.org/10.3390/s23031434
APA StyleElron, E., Bromiker, R., Gleisner, O., Yosef-Hai, O., Goldberg, O., Nitzan, I., & Nitzan, M. (2023). Overestimation of Oxygen Saturation Measured by Pulse Oximetry in Hypoxemia. Part 1: Effect of Optical Pathlengths-Ratio Increase. Sensors, 23(3), 1434. https://doi.org/10.3390/s23031434









