Recent Advances in Remote Pulmonary Artery Pressure Monitoring for Patients with Chronic Heart Failure: Current Evidence and Future Perspectives
Abstract
1. Introduction
2. Methods
3. Current Evidence
3.1. CardioMEMS HF System
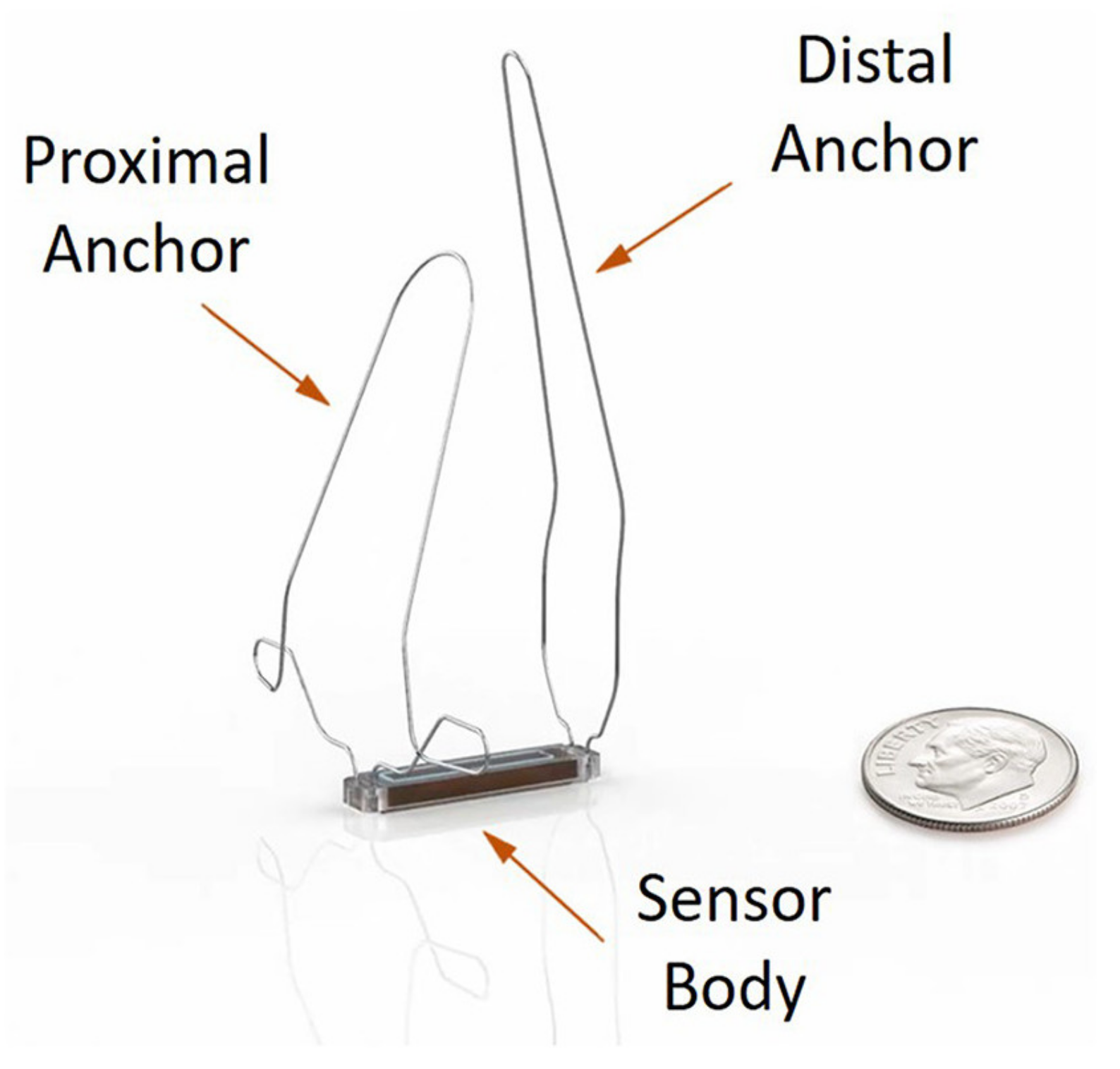
3.1.1. Device Information
3.1.2. Randomized Controlled Trials
3.1.3. Non-Randomized Trials
3.1.4. Cohort Studies
3.1.5. Clinically Relevant Subanalyses
3.2. Cordella HF System
3.2.1. Device Information
3.2.2. Non-Randomized Trials
3.2.3. Cohort Studies
3.3. Pulmonary Artery Pressure Monitoring to Assess Medical Therapy Effects
4. Ongoing Studies
4.1. CardioMEMS HF System
4.2. Cordella PAP Sensor
5. Differences between the Pulmonary Artery Pressure Monitoring Systems
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.P.; Sato, N.; Shah, A.N.; et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Jones, N.R.; Roalfe, A.K.; Adoki, I.; Hobbs, F.D.R.; Taylor, C.J. Survival of patients with chronic heart failure in the community: A systematic review and meta-analysis. Eur. J. Heart Fail. 2019, 21, 1306–1325. [Google Scholar] [CrossRef]
- Lindmark, K.; Boman, K.; Stalhammar, J.; Olofsson, M.; Lahoz, R.; Studer, R.; Proudfoot, C.; Corda, S.; Fonseca, A.F.; Costa-Scharplatz, M.; et al. Recurrent heart failure hospitalizations increase the risk of cardiovascular and all-cause mortality in patients with heart failure in Sweden: A real-world study. ESC Heart Fail. 2021, 8, 2144–2153. [Google Scholar] [CrossRef]
- Setoguchi, S.; Stevenson, L.W.; Schneeweiss, S. Repeated hospitalizations predict mortality in the community population with heart failure. Am. Heart J. 2007, 154, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Urbich, M.; Globe, G.; Pantiri, K.; Heisen, M.; Bennison, C.; Wirtz, H.S.; Di Tanna, G.L. A Systematic Review of Medical Costs Associated with Heart Failure in the USA (2014#x2013;2020). Pharmacoeconomics 2020, 38, 1219–1236. [Google Scholar] [CrossRef]
- Zito, A.; Princi, G.; Romiti, G.F.; Galli, M.; Basili, S.; Liuzzo, G.; Sanna, T.; Restivo, A.; Ciliberti, G.; Trani, C.; et al. Device-based remote monitoring strategies for congestion-guided management of patients with heart failure: A systematic review and meta-analysis. Eur. J. Heart Fail. 2022, 24, 2333–2341. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Abraham, W.T.; Albert, N.M.; Stough, W.G.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Pieper, K.; Sun, J.L.; Yancy, C.W.; et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: Findings from OPTIMIZE-HF. Arch. Intern. Med. 2008, 168, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Bennett, T.D.; St John Sutton, M.; Cho, Y.K.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M., Jr.; Abraham, W.T.; Smart, F.W.; Stevenson, L.W.; et al. Transition from chronic compensated to acute decompensated heart failure: Pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008, 118, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Perl, L. Implantable Hemodynamic Monitoring for Heart Failure Patients. J. Am. Coll. Cardiol. 2017, 70, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Verdejo, H.E.; Castro, P.F.; Concepcion, R.; Ferrada, M.A.; Alfaro, M.A.; Alcaino, M.E.; Deck, C.C.; Bourge, R.C. Comparison of a radiofrequency-based wireless pressure sensor to swan-ganz catheter and echocardiography for ambulatory assessment of pulmonary artery pressure in heart failure. J. Am. Coll. Cardiol. 2007, 50, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Brugts, J.J.; Radhoe, S.P.; Aydin, D.; Theuns, D.A.; Veenis, J.F. Clinical Update of the Latest Evidence for CardioMEMS Pulmonary Artery Pressure Monitoring in Patients with Chronic Heart Failure: A Promising System for Remote Heart Failure Care. Sensors 2021, 21, 2335. [Google Scholar] [CrossRef]
- Stevenson, L.W.; Zile, M.; Bennett, T.D.; Kueffer, F.J.; Jessup, M.L.; Adamson, P.; Abraham, W.T.; Manda, V.; Bourge, R.C. Chronic ambulatory intracardiac pressures and future heart failure events. Circ. Heart Fail. 2010, 3, 580–587. [Google Scholar] [CrossRef]
- Zile, M.R.; Adamson, P.B.; Cho, Y.K.; Bennett, T.D.; Bourge, R.C.; Aaron, M.F.; Aranda, J.M., Jr.; Abraham, W.T.; Stevenson, L.W.; Kueffer, F.J. Hemodynamic factors associated with acute decompensated heart failure: Part 1--insights into pathophysiology. J. Card. Fail. 2011, 17, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.B.; Abraham, W.T.; Bourge, R.C.; Costanzo, M.R.; Hasan, A.; Yadav, C.; Henderson, J.; Cowart, P.; Stevenson, L.W. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ. Heart Fail. 2014, 7, 935–944. [Google Scholar] [CrossRef]
- Givertz, M.M.; Stevenson, L.W.; Costanzo, M.R.; Bourge, R.C.; Bauman, J.G.; Ginn, G.; Abraham, W.T.; CHAMPION Trial Investigators. Pulmonary Artery Pressure-Guided Management of Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Bennett, T.D.; El Hajj, S.; Kueffer, F.J.; Baicu, C.F.; Abraham, W.T.; Bourge, R.C.; Warner Stevenson, L. Intracardiac Pressures Measured Using an Implantable Hemodynamic Monitor: Relationship to Mortality in Patients With Chronic Heart Failure. Circ. Heart Fail. 2017, 10, e003594. [Google Scholar] [CrossRef]
- Sharif, F.; Rosenkranz, S.; Bartunek, J.; Kempf, T.; Assmus, B.; Mahon, N.G.; Mullens, W. Safety and efficacy of a wireless pulmonary artery pressure sensor: Primary endpoint results of the SIRONA 2 clinical trial. ESC Heart Fail. 2022, 9, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Radhoe, S.P.; Brugts, J.J. CardioMEMS: A tool for remote hemodynamic monitoring of chronic heart failure patients. Future Cardiol. 2022, 18, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; de Groote, P.; McKenzie, S.; Brett, M.E.; Adamson, P.B.; CardioMEMS Post-Market Study Investigators. Rationale and design of the CardioMEMS Post-Market Multinational Clinical Study: COAST. ESC Heart Fail. 2020, 7, 865–872. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Krum, H. Telemonitoring of fluid status in heart failure: CHAMPION. Lancet 2011, 377, 616–618. [Google Scholar] [CrossRef]
- Abraham, W.T.; Stevenson, L.W.; Bourge, R.C.; Lindenfeld, J.A.; Bauman, J.G.; Adamson, P.B.; CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet 2016, 387, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Dagres, N.; Hindricks, G. Pulmonary pressure, telemedicine, and heart failure therapy. Lancet 2016, 387, 408–410. [Google Scholar] [CrossRef]
- Lindenfeld, J.; Zile, M.R.; Desai, A.S.; Bhatt, K.; Ducharme, A.; Horstmanshof, D.; Krim, S.R.; Maisel, A.; Mehra, M.R.; Paul, S.; et al. Haemodynamic-guided management of heart failure (GUIDE-HF): A randomised controlled trial. Lancet 2021, 398, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Lindenfeld, J.; Abraham, W.T.; Maisel, A.; Zile, M.; Smart, F.; Costanzo, M.R.; Mehra, M.R.; Ducharme, A.; Sears, S.F.; Desai, A.S.; et al. Hemodynamic-GUIDEd management of Heart Failure (GUIDE-HF). Am. Heart J. 2019, 214, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.A.; Mahaffey, K.W.; Mehran, R.; Nissen, S.E.; Wiviott, S.D.; Dunn, B.; Solomon, S.D.; Marler, J.R.; Teerlink, J.R.; Farb, A.; et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. Circulation 2018, 137, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Pellicori, P. To master heart failure, first master congestion. Lancet 2021, 398, 935–936. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Bodegard, J.; Norhammar, A.; Sartipy, P.; Thuresson, M.; Cowie, M.R.; Fonarow, G.C.; Vaduganathan, M.; Coats, A.J.S. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: A multinational observational study (US, UK and Sweden). Eur. J. Heart Fail. 2021, 23, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Angermann, C.E.; Assmus, B.; Anker, S.D.; Asselbergs, F.W.; Brachmann, J.; Brett, M.E.; Brugts, J.J.; Ertl, G.; Ginn, G.; Hilker, L.; et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: The CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF). Eur. J. Heart Fail. 2020, 22, 1891–1901. [Google Scholar] [CrossRef]
- Shavelle, D.M.; Desai, A.S.; Abraham, W.T.; Bourge, R.C.; Raval, N.; Rathman, L.D.; Heywood, J.T.; Jermyn, R.A.; Pelzel, J.; Jonsson, O.T.; et al. Lower Rates of Heart Failure and All-Cause Hospitalizations During Pulmonary Artery Pressure-Guided Therapy for Ambulatory Heart Failure: One-Year Outcomes From the CardioMEMS Post-Approval Study. Circ. Heart Fail. 2020, 13, e006863. [Google Scholar] [CrossRef]
- Heywood, J.T.; Zalawadiya, S.; Bourge, R.C.; Costanzo, M.R.; Desai, A.S.; Rathman, L.D.; Raval, N.; Shavelle, D.M.; Henderson, J.D.; Brett, M.E.; et al. Sustained Reduction in Pulmonary Artery Pressures and Hospitalizations During 2 Years of Ambulatory Monitoring. J. Card. Fail. 2022, 29, 56–66. [Google Scholar] [CrossRef]
- Cowie, M.R.; Flett, A.; Cowburn, P.; Foley, P.; Chandrasekaran, B.; Loke, I.; Critoph, C.; Gardner, R.S.; Guha, K.; Betts, T.R.; et al. Real-world evidence in a national health service: Results of the UK CardioMEMS HF System Post-Market Study. ESC Heart Fail. 2022, 9, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Codina, P.; Altisent, O.A.; Santiago-Vacas, E.; Domingo, M.; Lupon, J.; Bayes-Genis, A. A new option for monitoring heart failure. First experience in Spain with CardioMEMS. Med. Clin. 2021, 156, 26–28. [Google Scholar] [CrossRef]
- Vaz Ferreira, V.; Pereira-da-Silva, T.; Cacela, D.; Cruz Ferreira, R. Remote invasive monitoring of pulmonary artery pressures in heart failure patients: Initial experience in Portugal in the context of the Covid-19 pandemic. Rev. Port. Cardiol. 2022, 41, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Brugts, J.J.; Veenis, J.F.; Radhoe, S.P.; Linssen, G.C.M.; van Gent, M.; Borleffs, C.J.W.; van Ramshorst, J.; van Pol, P.; Tukkie, R.; Spee, R.F.; et al. A randomised comparison of the effect of haemodynamic monitoring with CardioMEMS in addition to standard care on quality of life and hospitalisations in patients with chronic heart failure: Design and rationale of the MONITOR HF multicentre randomised clinical trial. Neth. Heart J. 2020, 28, 16–26. [Google Scholar]
- Desai, A.S.; Bhimaraj, A.; Bharmi, R.; Jermyn, R.; Bhatt, K.; Shavelle, D.; Redfield, M.M.; Hull, R.; Pelzel, J.; Davis, K.; et al. Ambulatory Hemodynamic Monitoring Reduces Heart Failure Hospitalizations in “Real-World” Clinical Practice. J. Am. Coll. Cardiol. 2017, 69, 2357–2365. [Google Scholar] [CrossRef]
- Abraham, J.; Bharmi, R.; Jonsson, O.; Oliveira, G.H.; Artis, A.; Valika, A.; Capodilupo, R.; Adamson, P.B.; Roberts, G.; Dalal, N.; et al. Association of Ambulatory Hemodynamic Monitoring of Heart Failure With Clinical Outcomes in a Concurrent Matched Cohort Analysis. JAMA Cardiol. 2019, 4, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Kishino, Y.; Kuno, T.; Malik, A.H.; Lanier, G.M.; Sims, D.B.; Ruiz Duque, E.; Briasoulis, A. Effect of pulmonary artery pressure-guided therapy on heart failure readmission in a nationally representative cohort. ESC Heart Fail. 2022, 9, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G.P.; Minniefield, N.; Raju, B.; Patel, N.; Michelis, K.; Van Zyl, J.; Cheeran, D.; Alam, A. Effectiveness and Safety Profile of Remote Pulmonary Artery Hemodynamic Monitoring in a “Real-World” Veterans Affairs Healthcare System. Am. J. Cardiol. 2022, 184, 56–62. [Google Scholar] [CrossRef]
- Feldman, D.S.; Moazami, N.; Adamson, P.B.; Vierecke, J.; Raval, N.; Shreenivas, S.; Cabuay, B.M.; Jimenez, J.; Abraham, W.T.; O’Connell, J.B.; et al. The Utility of a Wireless Implantable Hemodynamic Monitoring System in Patients Requiring Mechanical Circulatory Support. ASAIO J. 2018, 64, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Varma, N.; Bourge, R.C.; Stevenson, L.W.; Costanzo, M.R.; Shavelle, D.; Adamson, P.B.; Ginn, G.; Henderson, J.; Abraham, W.T.; CHAMPION Investigator Group. Remote Hemodynamic-Guided Therapy of Patients With Recurrent Heart Failure Following Cardiac Resynchronization Therapy. J. Am. Heart Assoc. 2021, 10, e017619. [Google Scholar] [CrossRef]
- Adamson, P.B.; Abraham, W.T.; Stevenson, L.W.; Desai, A.S.; Lindenfeld, J.; Bourge, R.C.; Bauman, J. Pulmonary Artery Pressure-Guided Heart Failure Management Reduces 30-Day Readmissions. Circ. Heart Fail. 2016, 9, e002600. [Google Scholar] [CrossRef]
- Bohm, M.; Assmus, B.; Anker, S.D.; Asselbergs, F.W.; Brachmann, J.; Brett, M.E.; Brugts, J.J.; Ertl, G.; Wang, A.; Hilker, L.; et al. Less loop diuretic use in patients on sacubitril/valsartan undergoing remote pulmonary artery pressure monitoring. ESC Heart Fail. 2022, 9, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Angermann, C.E.; Alkhlout, B.; Asselbergs, F.W.; Schnupp, S.; Brugts, J.J.; Nordbeck, P.; Zhou, Q.; Brett, M.E.; Ginn, G.; et al. Effects of remote haemodynamic-guided heart failure management in patients with different subtypes of pulmonary hypertension: Insights from the MEMS-HF study. Eur. J. Heart Fail. 2022, 24, 2320–2330. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Henderson, J.; Axsom, K.M.; Costanzo, M.R.; Adamson, P.B.; Miller, A.B.; Brett, M.E.; Givertz, M.M. Remote Hemodynamic Monitoring Equally Reduces Heart Failure Hospitalizations in Women and Men in Clinical Practice: A Sex-Specific Analysis of the CardioMEMS Post-Approval Study. Circ. Heart Fail. 2021, 14, e007892. [Google Scholar] [CrossRef]
- Brinkley, D.M.; Guglin, M.E.; Bennett, M.K.; Redfield, M.M.; Abraham, W.T.; Brett, M.E.; Dirckx, N.; Adamson, P.B.; Stevenson, L.W. Pulmonary Artery Pressure Monitoring Effectively Guides Management to Reduce Heart Failure Hospitalizations in Obesity. JACC Heart Fail. 2021, 9, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Veenis, J.F.; Rocca, H.B.; Linssen, G.C.M.; Erol-Yilmaz, A.; Pronk, A.C.B.; Engelen, D.J.M.; van Tooren, R.M.; Koornstra-Wortel, H.J.J.; de Boer, R.A.; van der Meer, P.; et al. Impact of sex-specific target dose in chronic heart failure patients with reduced ejection fraction. Eur. J. Prev. Cardiol. 2021, 28, 957–965. [Google Scholar] [CrossRef]
- Mullens, W.; Sharif, F.; Dupont, M.; Rothman, A.M.K.; Wijns, W. Digital health care solution for proactive heart failure management with the Cordella Heart Failure System: Results of the SIRONA first-in-human study. Eur. J. Heart Fail. 2020, 22, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Dauw, J.; Sokolski, M.; Middleton, J.T.; Nijst, P.; Dupont, M.; Forouzan, O.; Rothman, A.M.K.; Ruschitzka, F.; Flammer, A.J.; Mullens, W. Ambulatory haemodynamic-guided management reduces heart failure hospitalizations in a multicentre European heart failure cohort. ESC Heart Fail. 2022, 9, 3858–3867. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.S.; Havakuk, O.; McLeod, J.M.; Hwang, J.; Kwong, H.Y.; Shavelle, D.; Zile, M.R.; Elkayam, U.; Fong, M.W.; Grazette, L.P. Acute pulmonary pressure change after transition to sacubitril/valsartan in patients with heart failure reduced ejection fraction. ESC Heart Fail. 2021, 8, 1706–1710. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Gholkar, G.; Tolia, S.; Kado, H.; Zughaib, M. Effect of sacubitril/valsartan on cardiac filling pressures in patients with left ventricular systolic dysfunction. Int. J. Cardiol. 2018, 271, 169–173. [Google Scholar] [CrossRef]
- Codina, P.; Domingo, M.; Barcelo, E.; Gastelurrutia, P.; Casquete, D.; Vila, J.; Abdul-Jawad Altisent, O.; Spitaleri, G.; Cediel, G.; Santiago-Vacas, E.; et al. Sacubitril/valsartan affects pulmonary arterial pressure in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail. 2022, 9, 2170–2180. [Google Scholar] [CrossRef]
- Mullens, W.; Martens, P.; Forouzan, O.; Dauw, J.; Vercammen, J.; Luwel, E.; Ceyssens, W.; Kockaerts, V.; Ameloot, K.; Dupont, M. Effects of dapagliflozin on congestion assessed by remote pulmonary artery pressure monitoring. ESC Heart Fail. 2020, 7, 2071–2073. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. SGLT-2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Brunner-La Rocca, H.P.; Linssen, G.C.; Smeele, F.J.; van Drimmelen, A.A.; Schaafsma, H.J.; Westendorp, P.H.; Rademaker, P.C.; van de Kamp, H.J.; Hoes, A.W.; Brugts, J.J.; et al. Contemporary Drug Treatment of Chronic Heart Failure With Reduced Ejection Fraction: The CHECK-HF Registry. JACC Heart Fail. 2019, 7, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef]
- Stork, S.; Bernhardt, A.; Bohm, M.; Brachmann, J.; Dagres, N.; Frantz, S.; Hindricks, G.; Kohler, F.; Zeymer, U.; Rosenkranz, S.; et al. Pulmonary artery sensor system pressure monitoring to improve heart failure outcomes (PASSPORT-HF): Rationale and design of the PASSPORT-HF multicenter randomized clinical trial. Clin. Res. Cardiol. 2022, 111, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Adamson, P.B.; Costanzo, M.R.; Eigler, N.; Gold, M.; Klapholz, M.; Maurer, M.; Saxon, L.; Singh, J.; Troughton, R. Hemodynamic Monitoring in Advanced Heart Failure: Results from the LAPTOP-HF Trial. J. Card. Fail. 2016, 22, 940. [Google Scholar] [CrossRef]
- Radhoe, S.P.; Veenis, J.F.; Brugts, J.J. Invasive Devices and Sensors for Remote Care of Heart Failure Patients. Sensors 2021, 21, 2014. [Google Scholar] [CrossRef]




| First Author | Year | PAP Monitoring Device | Study Design | Study Name | Study Population | Sample Size | Comparator | HFH Outcomes |
|---|---|---|---|---|---|---|---|---|
| Abraham | 2011 | CardioMEMS | RCT | CHAMPION | NYHA III HF and HFH < 12 months | 550 | Control group | HR 0.72; 95% CI 0.60–0.85; p = 0.0002 (6 months follow-up) HR 0.63; 95% CI 0.52–0.77; p < 0.0001) (15 months mean follow-up) |
| Abraham | 2016 | CardioMEMS | RCT | CHAMPION (extended follow-up) | NYHA III HF and HFH < 12 months | 347 | Control group and patient own historic control | HR 0.67; 95% CI 0.55–0.80; p < 0.0001 (18 months mean follow-up, compared to control group) HR 0.52; 95% CI 0.40–0.69; p < 0.0001 (13 months mean follow-up, compared to historic control) |
| Lindenfeld | 2021 | CardioMEMS | RCT | GUIDE-HF | NYHA II–IV HF and HFH < 12 months or elevated NT-proBNP/BNP | 1000 | Control group | HR 0.88; 95% CI 0.74–1.05; p = 0.16 (main analysis) HR 0.81; 95% CI 0.66–1.00; p = 0.049 (pre-COVID analysis) |
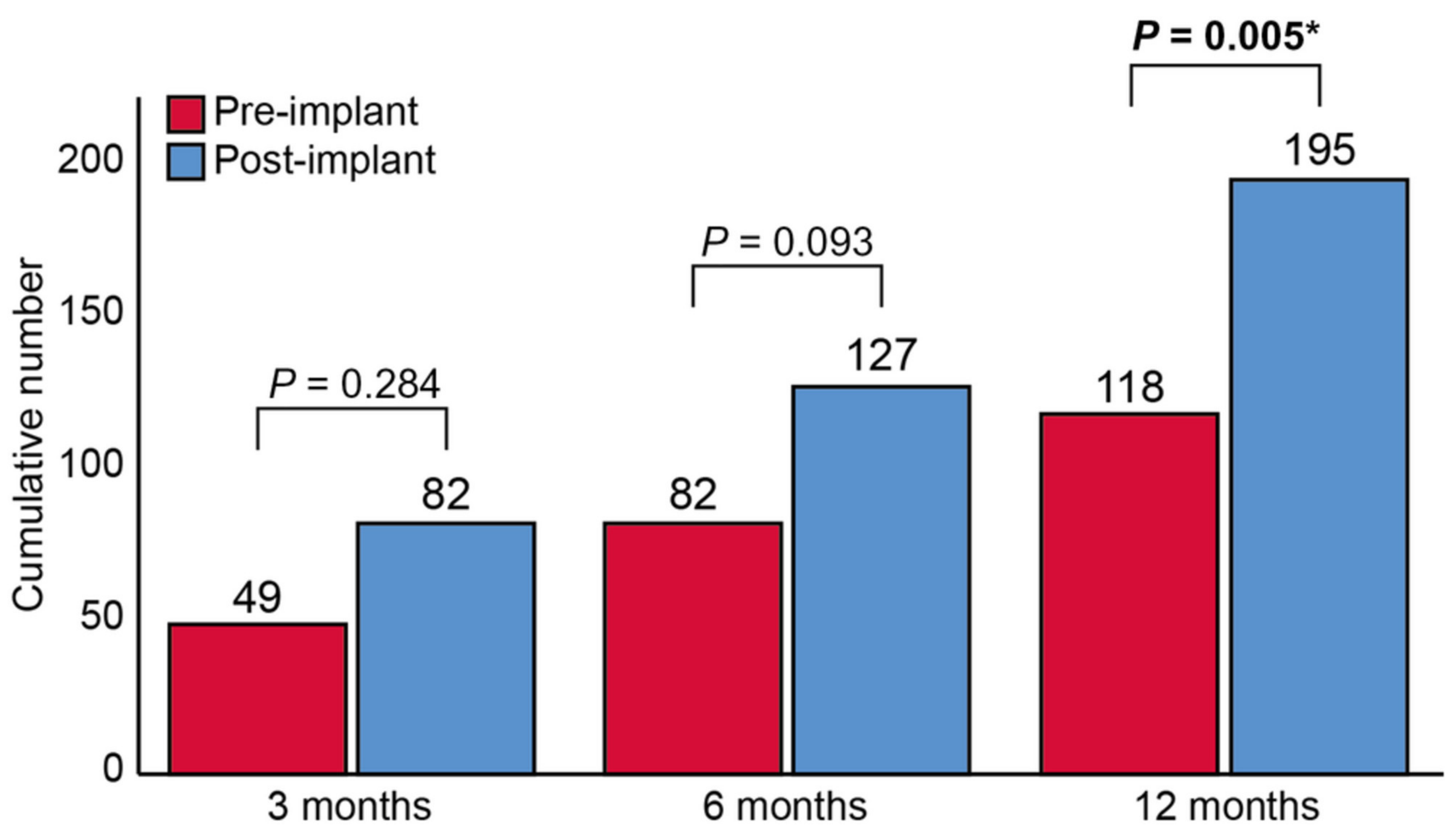
| Angermann | 2020 | CardioMEMS | Non-randomized trial and Post-marketing | MEMS-HF | NYHA III HF and HFH < 12 months | 236 | Patient own historic control | HR 0.38; 95% CI 0.31–0.48; p < 0.0001 (12 months post-implant versus 12 months pre-implant) |
| Shavelle | 2020 | CardioMEMS | Non-randomized trial and Post-marketing | CardioMEMS post-approval study | NYHA III HF and HFH < 12 months | 1200 | Patient own historic control | HR 0.43; 95% CI 0.39–0.47; p < 0.0001 (12 months post-implant versus 12 months pre-implant) |
| Heywood | 2022 | CardioMEMS | Non-randomized trial and Post-marketing | CardioMEMS post-approval study (extended follow-up) | NYHA III HF and HFH < 12 months | 710 | Patient own historic control | HR 0.30; 95% CI 0.25–0.35; p < 0.0001 (24 months post-implant versus 12 months pre-implant HR 0.62; 95% CI 0.56–0.69; p < 0.0001 (24 months post-implant versus 12 months post-implant) |
| Cowie | 2022 | CardioMEMS | Non-randomized trial | COAST (UK results) | NYHA III HF and HFH < 12 months | 100 | Patient own historic control | HR 0.18; 95% CI 0.12–0.28; p < 0.0001 (12 months post-implant versus 12 months pre-implant) |
| Desai | 2017 | CardioMEMS | Cohort study | … | HF patients with an implanted CardioMEMS and ≥6 months follow-up | 1114 | Patient own historic control | HR 0.55; 95% CI 0.49–0.61; p < 0.001 (6 months post-implant versus 6 months pre-implant) HR 0.66; 95% CI 0.57–0.76; p < 0.001 (12 months post-implant versus 12 months pre-implant) |
| Abraham | 2019 | CardioMEMS | Cohort study | … | HF patients with an implanted CardioMEMS and >12 months follow-up | 2174 | Matched control group | HR 0.76; 95% CI 0.65–0.89; p < 0.01 (12 months follow-up) |
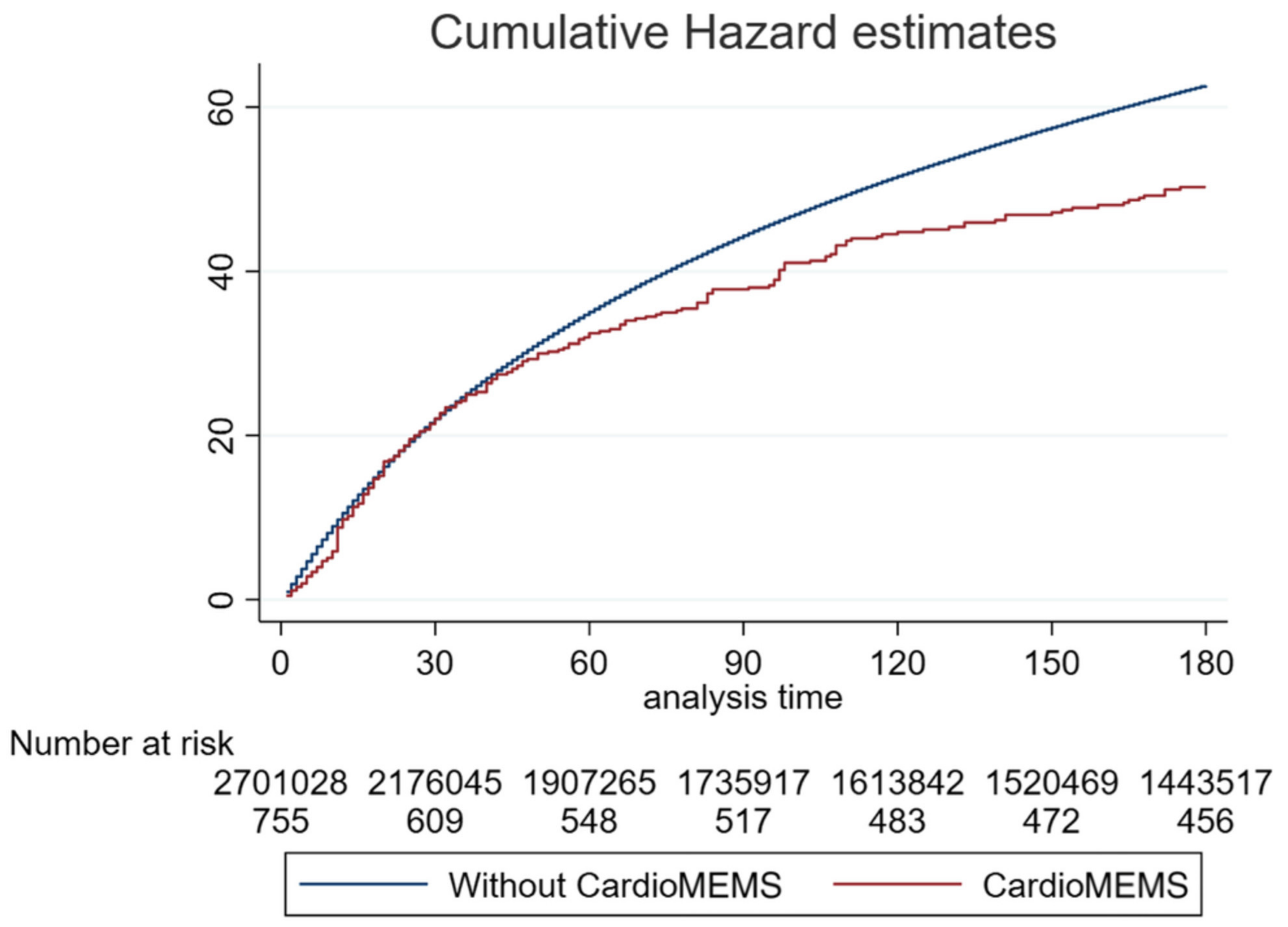
| Kishino | 2022 | CardioMEMS | Cohort study | … | HF patients with an implanted CardioMEMS AND an HFH | 3763 | Matched control group | HR 0.75; 95% CI 0.63–0.89; p = 0.001 (30 days follow-up) HR 0.73; 95% CI 0.63–0.86 p < 0.001 (90 days follow-up) HR 0.80; 95% CI 0.71–0.91; p = 0.001 (180 days follow-up) |
| Milligan | 2022 | CardioMEMS | Cohort study | … | NYHA III HF and an implanted CardioMEMS | 53 | Patient own historic control | IRR 0.48; 95% CI 0.32–0.70; p < 0.001 (6 months post-implant versus 6 months pre-implant) IRR 0.56; 95% CI 0.41–0.76; p < 0.001 (12 months post-implant versus 12 months pre-implant) |
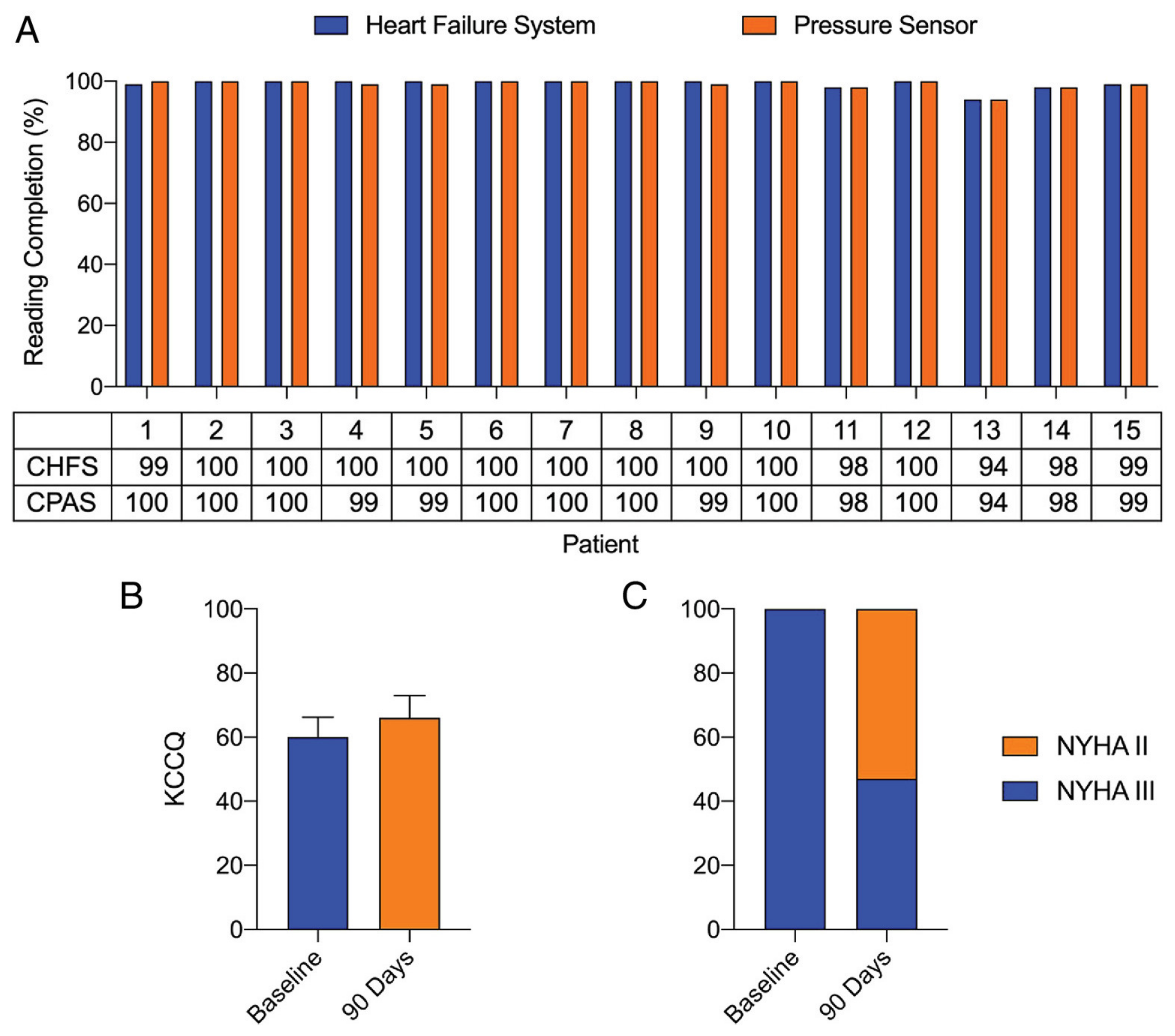
| Mullens | 2020 | Cordella | Non-randomized trial | SIRONA | NYHA III HF and HFH < 12 months | 15 | … | … |
| Sharif | 2022 | Cordella | Non-randomized trial | SIRONA 2 | NYHA III HF and HFH < 12 months or elevated NT-proBNP/BNP | 75 | … | 8 HFH (90 days follow-up) 11 HFH (6 months follow-up) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clephas, P.R.D.; Aydin, D.; Radhoe, S.P.; Brugts, J.J. Recent Advances in Remote Pulmonary Artery Pressure Monitoring for Patients with Chronic Heart Failure: Current Evidence and Future Perspectives. Sensors 2023, 23, 1364. https://doi.org/10.3390/s23031364
Clephas PRD, Aydin D, Radhoe SP, Brugts JJ. Recent Advances in Remote Pulmonary Artery Pressure Monitoring for Patients with Chronic Heart Failure: Current Evidence and Future Perspectives. Sensors. 2023; 23(3):1364. https://doi.org/10.3390/s23031364
Chicago/Turabian StyleClephas, Pascal R. D., Dilan Aydin, Sumant P. Radhoe, and Jasper J. Brugts. 2023. "Recent Advances in Remote Pulmonary Artery Pressure Monitoring for Patients with Chronic Heart Failure: Current Evidence and Future Perspectives" Sensors 23, no. 3: 1364. https://doi.org/10.3390/s23031364
APA StyleClephas, P. R. D., Aydin, D., Radhoe, S. P., & Brugts, J. J. (2023). Recent Advances in Remote Pulmonary Artery Pressure Monitoring for Patients with Chronic Heart Failure: Current Evidence and Future Perspectives. Sensors, 23(3), 1364. https://doi.org/10.3390/s23031364






