Simulation and Analysis of Anodized Aluminum Oxide Membrane Degradation
Abstract
:1. Introduction
2. Materials and Methods
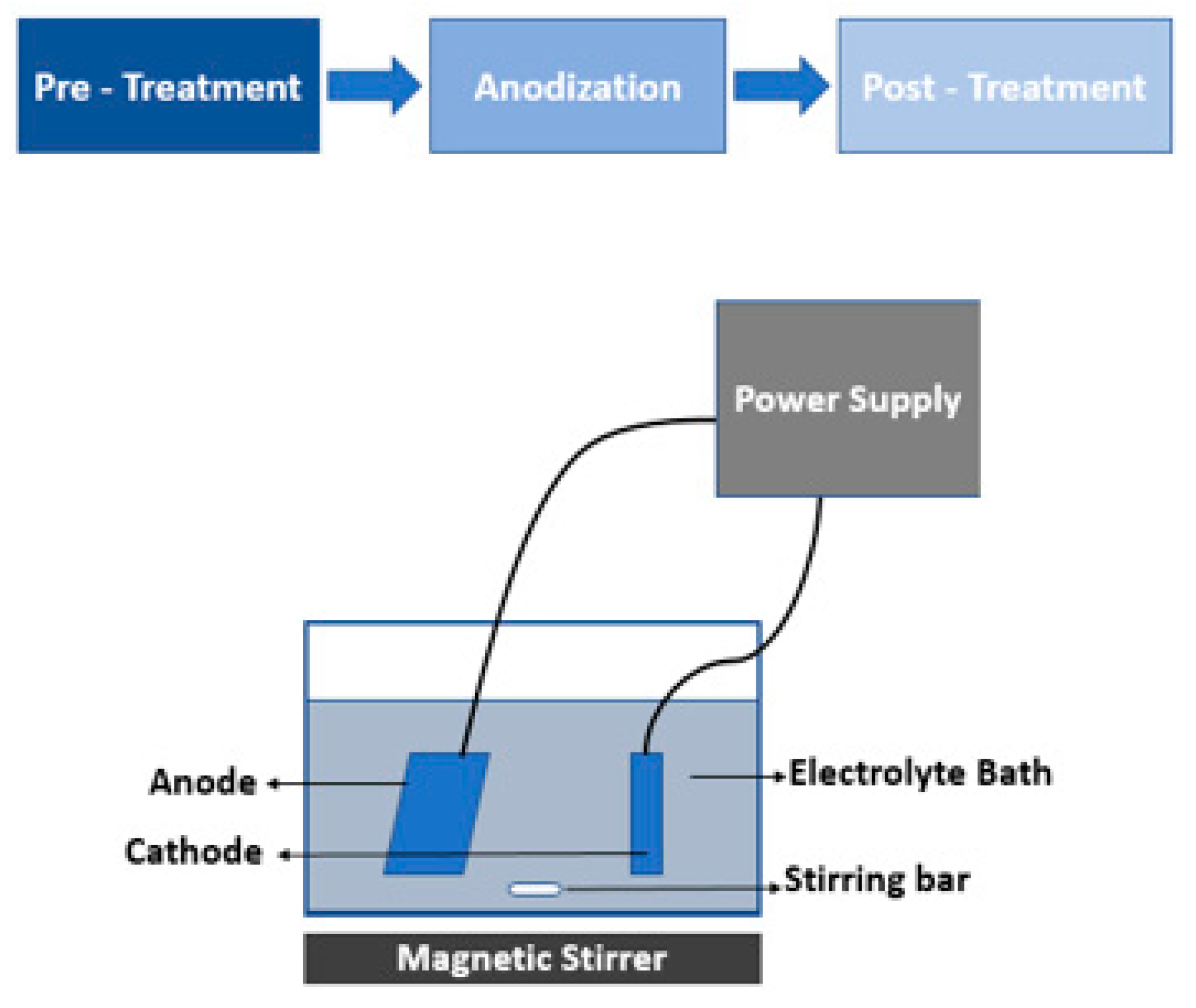
3. Fabrication
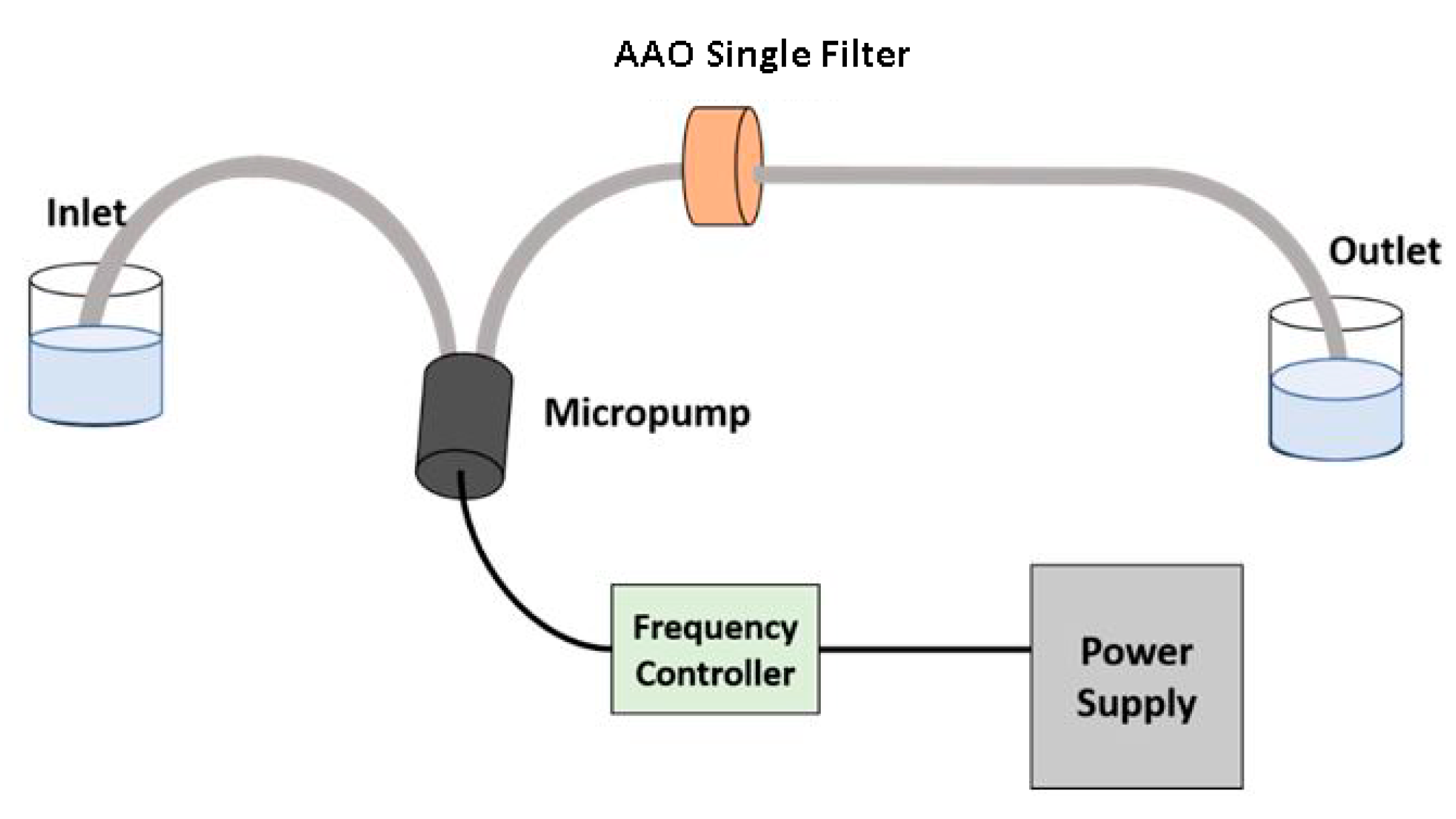
4. Experimental Design for Filtration
5. Results and Discussion
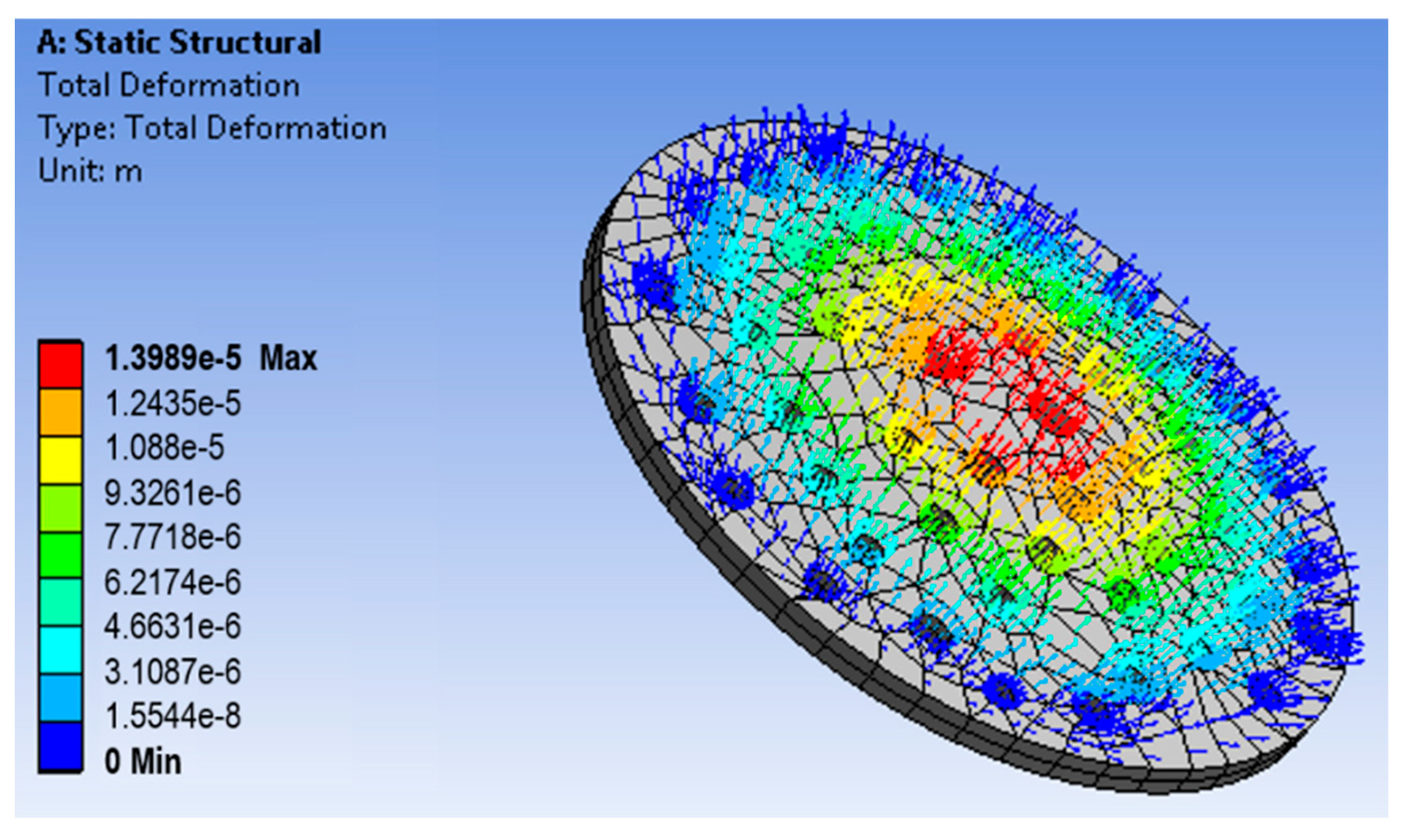
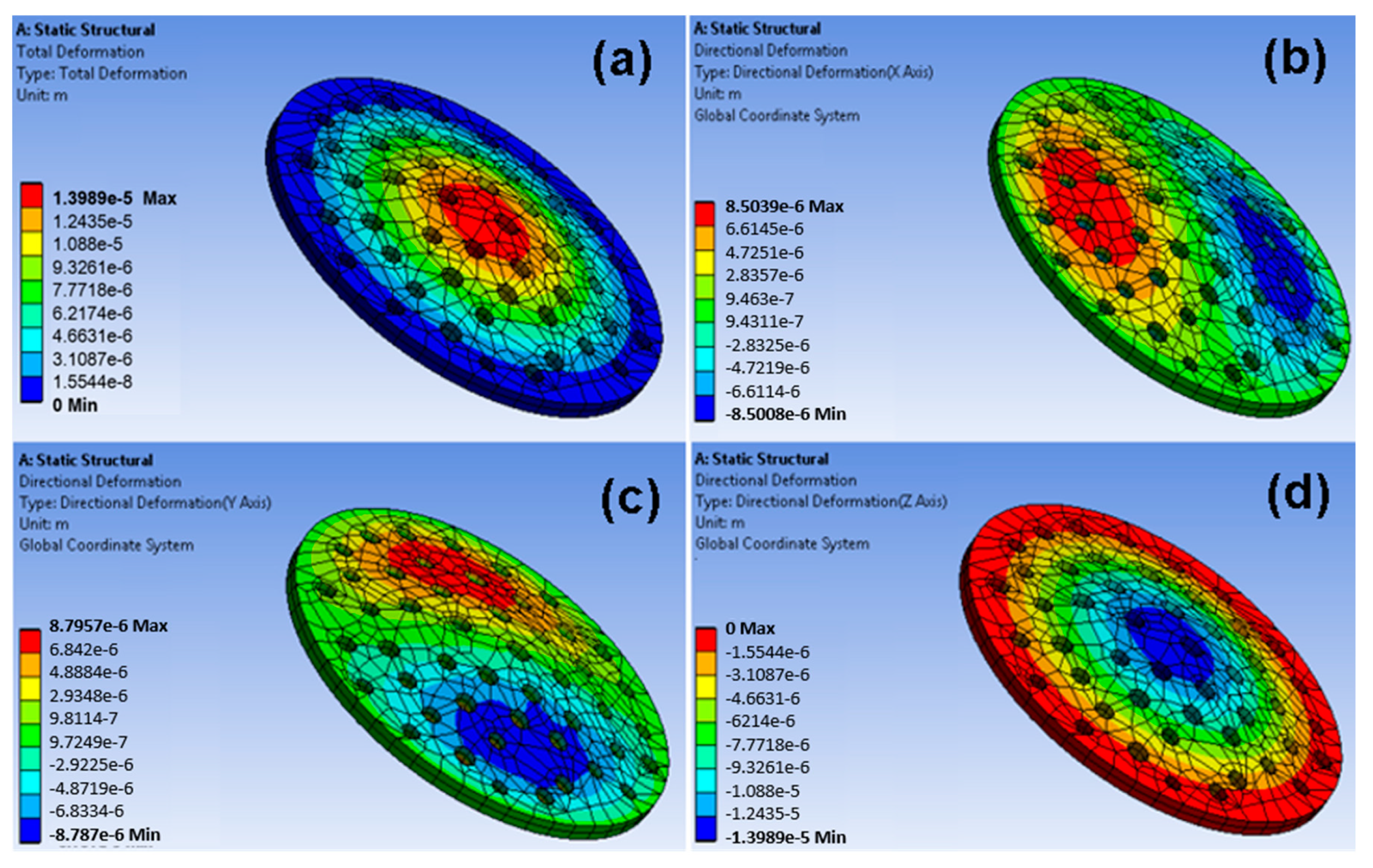
5.1. Computational Analysis
5.2. Numerical Analysis
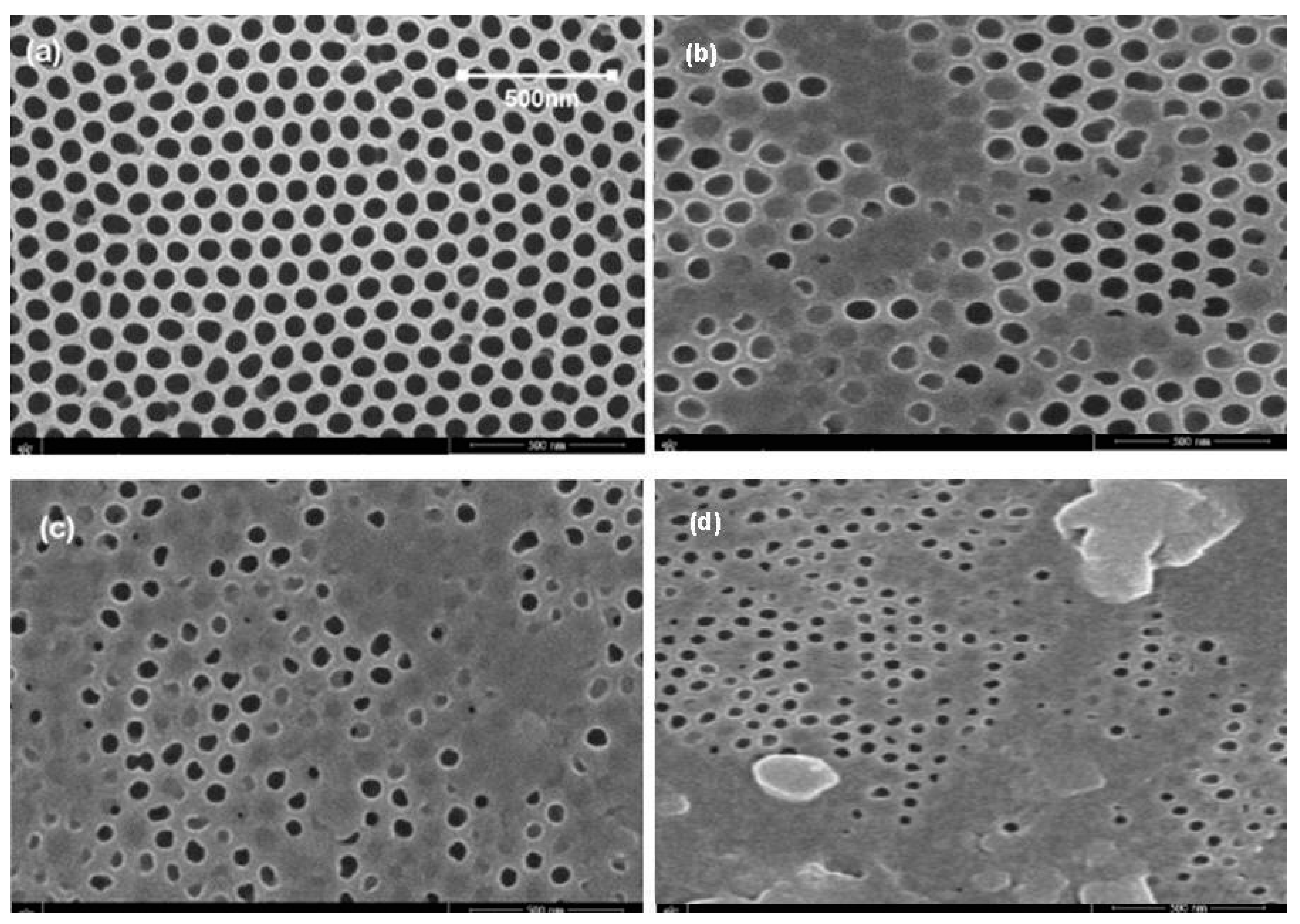
5.3. Membrane Morphology
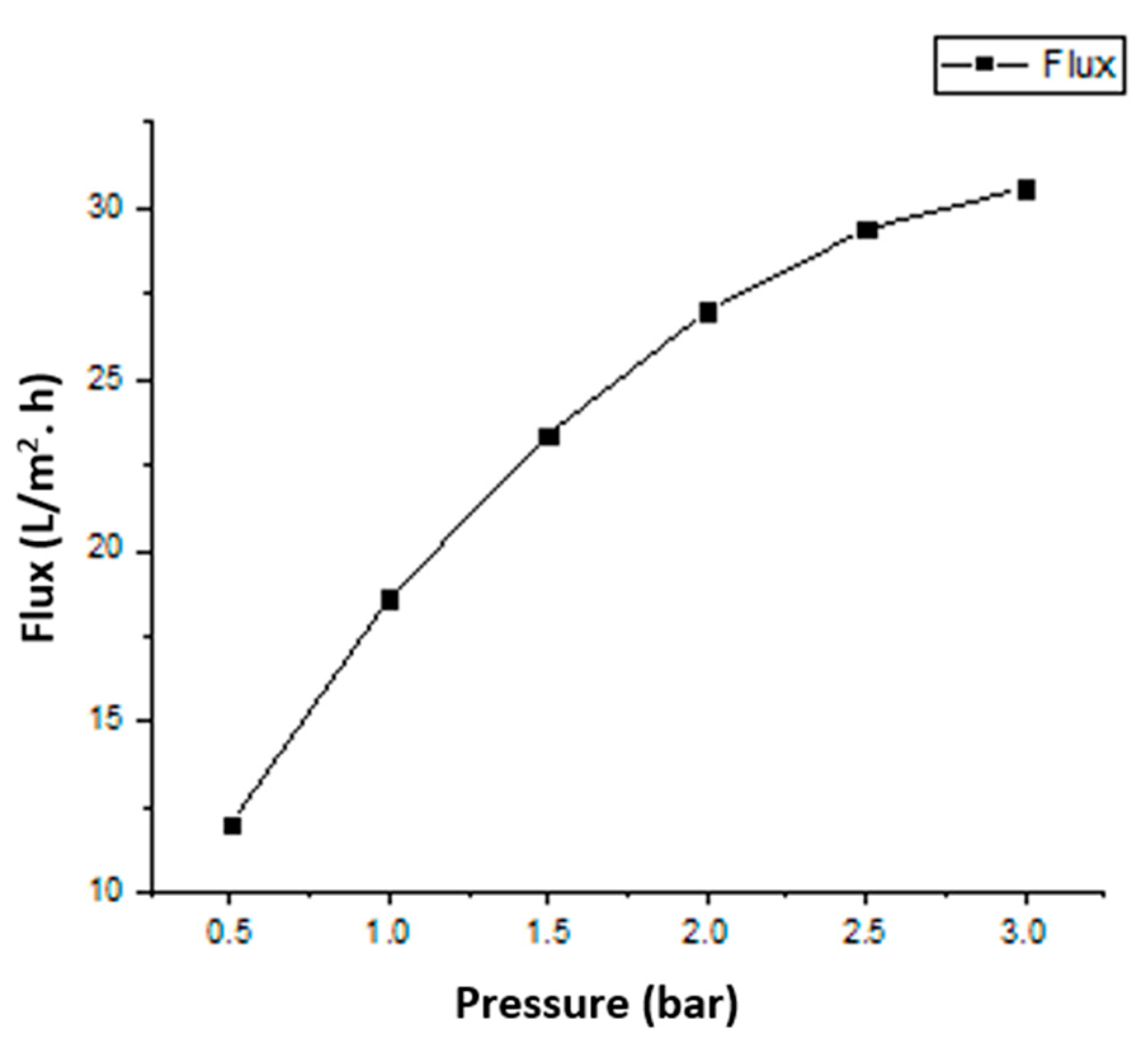
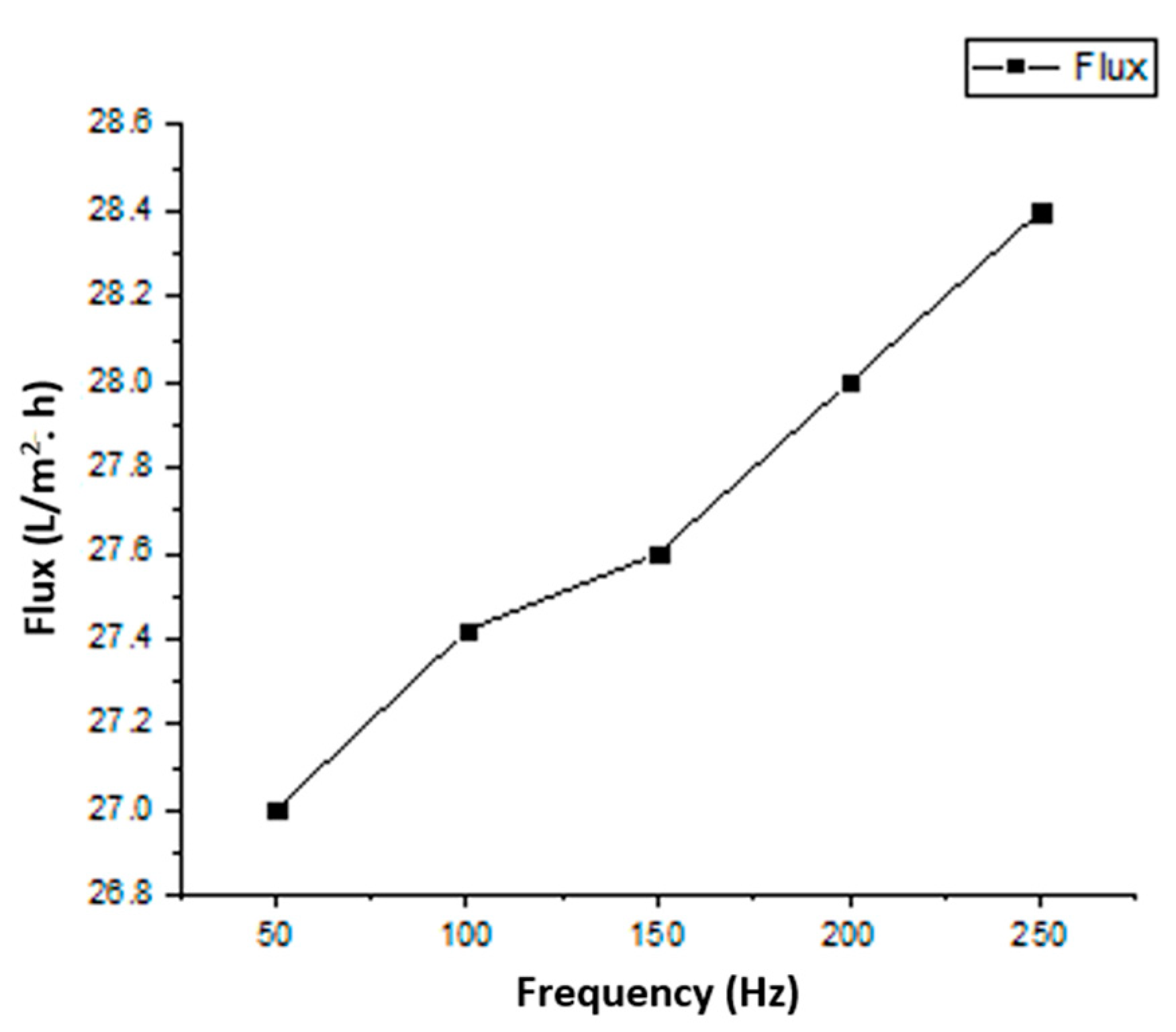
5.4. Permeation Analysis
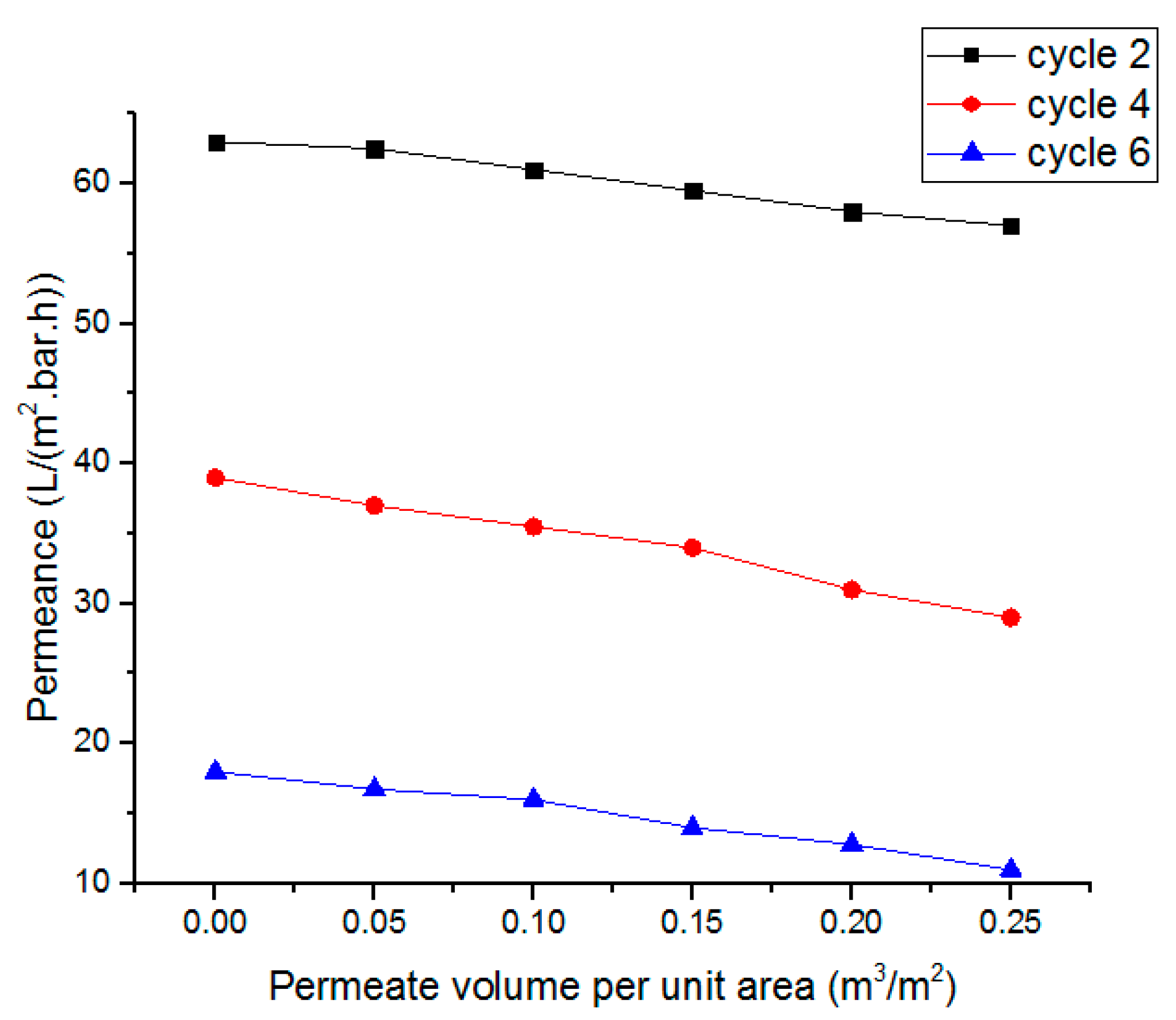
5.5. Membrane Degradation Analysis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caton, P.F.; White, R.M. MEMS Microfilter with Acoustic Cleaning. In Technical Digest. MEMS 2001. Proceedings of the 14th IEEE International Conference on Micro Electro Mechanical Systems (Cat. No.01CH37090), Interlaken, Switzerland, 25 January 2001; IEEE: Piscataway, NJ, USA, 2001; pp. 479–482. [Google Scholar] [CrossRef]
- Rosen, Y.; Gurman, P. MEMS and Microfluidics for Diagnostics Devices. Curr. Pharm. Biotechnol. 2010, 11, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.-Y.; Chen, P.-S.; Liao, T.-W.; Juang, Y.-J. Microwell-Assisted Filtration with Anodic Aluminum Oxide Membrane for Raman Analysis of Algal Cells. Algal Res. 2018, 33, 412–418. [Google Scholar] [CrossRef]
- Song, J.; Oh, H.; Kong, H.; Jang, J. Polyrhodanine Modified Anodic Aluminum Oxide Membrane for Heavy MetalIons Removal. J. Hazard. Mater. 2011, 187, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M. Manganese Ferrite Nanoparticle: Synthesis, Characterization, and Photocatalytic Dye Degradation Ability. Desalination Water Treat. 2015, 53, 84–90. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, A.; Taghizadeh, M.; Abdi, J. InSitu Deposition of Ag/Ag Clon the Surface of Magnetic Metal-Organic Framework Nanocomposite and Its Application for the Visible-Light Photocatalytic Degradation of Rhodamine Dye. J. Hazard. Mater. 2019, 378, 120741. [Google Scholar] [CrossRef]
- Mahmoodi, N.M. Synthesis of Core–Shell Magnetic Adsorbent Nanoparticle and Selectivity Analysis for Binary System DyeRemoval. J. Ind. Eng. Chem. 2014, 20, 2050–2058. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Hayati, B.; Arami, M. Textile Dye Removal from Single and Ternary Systems Using Date Stones: Kinetic, Isotherm, and Thermodynamic Studies. J. Chem. Eng. Data 2010, 55, 4638–4649. [Google Scholar] [CrossRef]
- Oveisi, M.; Mahmoodi, N.M.; Asli, M.A. Facile and Green Synthesis of Metal Organic Framework/ Inorganic Nanofiber Using Electrospinning for Recyclable Visible Light Photocatalysis. J. Clean. Prod. 2019, 222, 669–684. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Z.; Gao, J. Solar steam-driven membrane filtration for high flux water purification. Nat. Water 2023, 1, 391–398. [Google Scholar] [CrossRef]
- Peter-Varbanets, M.; Zurbrügg, C.; Swartz, C.; Pronk, W. Decentralized Systems for Potable Water and the Potential of Membrane Technology. Water Res. 2009, 43, 245–265. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Rahul, A.K.; Shekhar, S.; Kumar, S. Removal of heavy metal from wastewater using ion exchange with membrane filtration from Swarnamukhi river in Tirupati. Mater. Today Proc. 2023, 78, 1–6. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Madaeni, S.S. The Application of Membrane Technology for Water Disinfection. Water Res. 1999, 33, 301–308. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L. (Eds.) Comprehensive Membrane Science and Engineering; Newnes: Oxford, UK; Elsevier: Amsterdam, Netherlands, 2010; Volume 1. [Google Scholar]
- Asefi, D.; Mahmoodi, N.M.; Arami, M. Effect of Nonionic Co-Surfactants on Corrosion Inhibition Effect of Cationic Gemini Surfactant. Colloids Surf. A Physicochem Eng. Asp. 2010, 355, 183–186. [Google Scholar] [CrossRef]
- Wendorff, J.H.; Agarwal, S.; Greiner, A. Electrospinning: Materials, Processing, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Belwalkar, A.; Grasing, E.; Vangeertruyden, W.; Huang, Z.; Misiolek, W. Effect of Processing Parameters on Pore Structure and Thickness of Anodic Aluminum Oxide (AAO) Tubular Membranes. J. Membr. Sci. 2008, 319, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Sulka, G.D.; Parkoła, K.G. Anodising Potential Influence on Well-Ordered Nanostructures Formed by Anodisation of Aluminium in Sulphuric Acid. Thin Solid Films 2006, 515, 338–345. [Google Scholar] [CrossRef]
- Wu, Y.C.; Cui, J.W.; Wang, Y.; Xu, G.Q.; Zhang, X.Y. Effects of Pretreatment and the First Anodization Time on Morphology of Anodic Aluminium Oxide Templates. Adv. Mater. Res. 2011, 181–182, 707–711. [Google Scholar] [CrossRef]
- McQuaig, M.K.; Toro, A.; Van Geertruyden, W.; Misiolek, W.Z. The Effect of High Temperature Heat Treatment on the Structure and Properties of Anodic Aluminum Oxide. J. Mater. Sci. 2011, 46, 243–253. [Google Scholar] [CrossRef]
- Adiga, S.P.; Jin, C.; Curtiss, L.A.; Monteiro-Riviere, N.A.; Narayan, R.J. Nanoporous Membranes for Medical and Biological Applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 568–581. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in Nano-Engineered Anodic Aluminum Oxide Membrane Development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef]
- Macias, G.; Hernández-Eguía, L.P.; Ferré-Borrull, J.; Pallares, J.; Marsal, L.F. Gold-Coated Ordered Nanoporous Anodic Alumina Bilayers for Future Label-Free Interferometric Biosensors. ACS Appl. Mater. Interfaces 2013, 5, 8093–8098. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.M.; Rao, S.; Seo, Y.-S.; Schadt, K.; Hao, Y.; Chiao, J.-C. Micro PH Sensors Based on Iridium Oxide Nanotubes. IEEE Trans. Nanotechnol. 2014, 13, 945–953. [Google Scholar] [CrossRef]
- Shimizu, K.; Habazaki, H.; Skeldon, P.; Thompson, G.E.; Wood, G.C. Comparison of Depth Profiling Analysis of a Thick, Electrolytically-Coloured Porous Alumina Film by EPMA and GDOES. Surf. Interface Anal. 1999, 27, 1046–1049. [Google Scholar] [CrossRef]
- Patel, Y.; Janusas, G.; Palevicius, A.; Vilkauskas, A. Development of Nanoporous AAO Membrane for Nano Filtration Using the Acoustophoresis Method. Sensors 2020, 20, 3833. [Google Scholar] [CrossRef] [PubMed]
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural Features of Oxide Coatings on Aluminum. J. Electrochem. Soc. 1953, 100, 411–419. [Google Scholar] [CrossRef]
- Martin, C.R. Membrane-Based Synthesis of Nanomaterials. Chem. Mater. 1996, 8, 1739–1746. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhou, H.; Xie, C.; Huang, Z.; Fu, C.; Kuang, Y. Preparation of Self-Ordered Nanoporous Anodic Aluminum Oxide Membranes by Combination of Hard Anodization and Mild Anodization. Thin Solid Films 2014, 552, 75–81. [Google Scholar] [CrossRef]
- Heo, J.; Kwon, H.J.; Jeon, H.; Kim, B.; Kim, S.J.; Lim, G. Ultra-High-Aspect-Orthogonal and Tunable Three Dimensional Polymeric Nanochannel Stack Array for BioMEMS Applications. Nanoscale 2014, 6, 9681–9688. [Google Scholar] [CrossRef]
- Jivani, R.R.; Lakhtaria, G.J.; Patadiya, D.D.; Patel, L.D.; Jivani, N.P.; Jhala, B.P. RETRACTED: Biomedical Microelectromechanical Systems (BioMEMS): Revolution in Drug Delivery and Analytical Techniques. Saudi Pharm. J. 2016, 24, 1–20. [Google Scholar] [CrossRef]
- Ashraf, M.W.; Manzoor, S.; ShahzadSarfraz, M.; Wasim, M.F.; Ali, B.; Akhlaq, M.; Rujita, C.; Popa, A. Fabrication and Fuzzy Analysis of AAO Membrane with Manipulated Pore Diameter for Applications in Biotechnology. J. Intell. Fuzzy Syst. 2020, 38, 5857–5864. [Google Scholar] [CrossRef]
- Ashraf, M.W.; Qureshi, M.Z.; Ghaffar, F.; Tayyaba, S.; Afzulpurkar, N. Structural Study of AAO Membrane during the Dialysis Process. In Proceedings of the 2016 International Conference on Intelligent Systems Engineering (ICISE), Islamabad, Pakistan, 15–17 January 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 226–231. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Berry, C.; Singh, P.; Berchmans, S.; Fawcett, D. Biocompatibility of Synthesised Nano-Porous Anodic Aluminium Oxide Membranes for Use as a Cell Culture Substrate for Madin-Darby Canine Kidneys Cells: A Preliminary Study. J. Tissue Sci. Eng. 2012, 3. [Google Scholar] [CrossRef]
- Phuong, N.; Andisetiawan, A.; Van Lam, D.; Kim, J.H.; Choi, D.-S.; Whang, K.-H.; Nham, J.; Lee, Y.J.; Yoo, Y.-E.; Yoon, J.S. Nano Sand Filter with Functionalized Nanoparticles Embedded in Anodic Aluminum Oxide Templates. Sci. Rep. 2016, 6, 37673. [Google Scholar] [CrossRef] [PubMed]
- Khan Kasi, A.; Khan Kasi, J.; Afzulpurkar, N.; Bohez, E.; Tuantranont, A.; Mahaisavariya, B. Novel Anodic Aluminum Oxide (AAO) Nanoporous Membrane for Wearable Hemodialysis Device. In Proceedings of the International Conference on Communications and Electronics 2010, Trang, Vietnam, 11–13 August 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 98–101. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Yang, W.-T.; Wu, J.-C. Isolation and Detection of Exosomes via AAO Membrane and QCM Measurement. Microelectron. Eng. 2019, 216, 111094. [Google Scholar] [CrossRef]
- Liu, Y.; Lou, J.; Ni, M.; Song, C.; Wu, J.; Dasgupta, N.P.; Tao, P.; Shang, W.; Deng, T. Bioinspired Bifunctional Membrane for Efficient Clean Water Generation. ACS Appl. Mater. Interfaces 2016, 8, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Nikam, S.B.; SK, A. Enantioselective Separation Using Chiral Amino Acid Functionalized Polyfluorene Coated on Mesoporous Anodic Aluminum Oxide Membranes. Anal. Chem. 2020, 92, 6850–6857. [Google Scholar] [CrossRef]
- Jafari, A.; Mahvi, A.H.; Nasseri, S.; Rashidi, A.; Nabizadeh, R.; Rezaee, R. Ultrafiltration of Natural Organic Matter from Water by Vertically Aligned Carbon Nanotube Membrane. J. Environ. Health Sci. Eng. 2015, 13, 51. [Google Scholar] [CrossRef]
- Manzoor, S. Coupled Field Simulation, Fabrication, and Analysis AAO Membranefor Filtation. Ph.D. Dissertation, GC University Lahore, Lahore, Pakistan, 23 September 2022. [Google Scholar]
- Qasim, F.; Ashraf, M.W.; Tayyaba, S.; Tariq, M.I.; Herrera-May, A.L. Simulation, Fabrication and Microfiltration Using Dual Anodic Aluminum Oxide Membrane. Membranes 2023, 13, 825. [Google Scholar] [CrossRef]


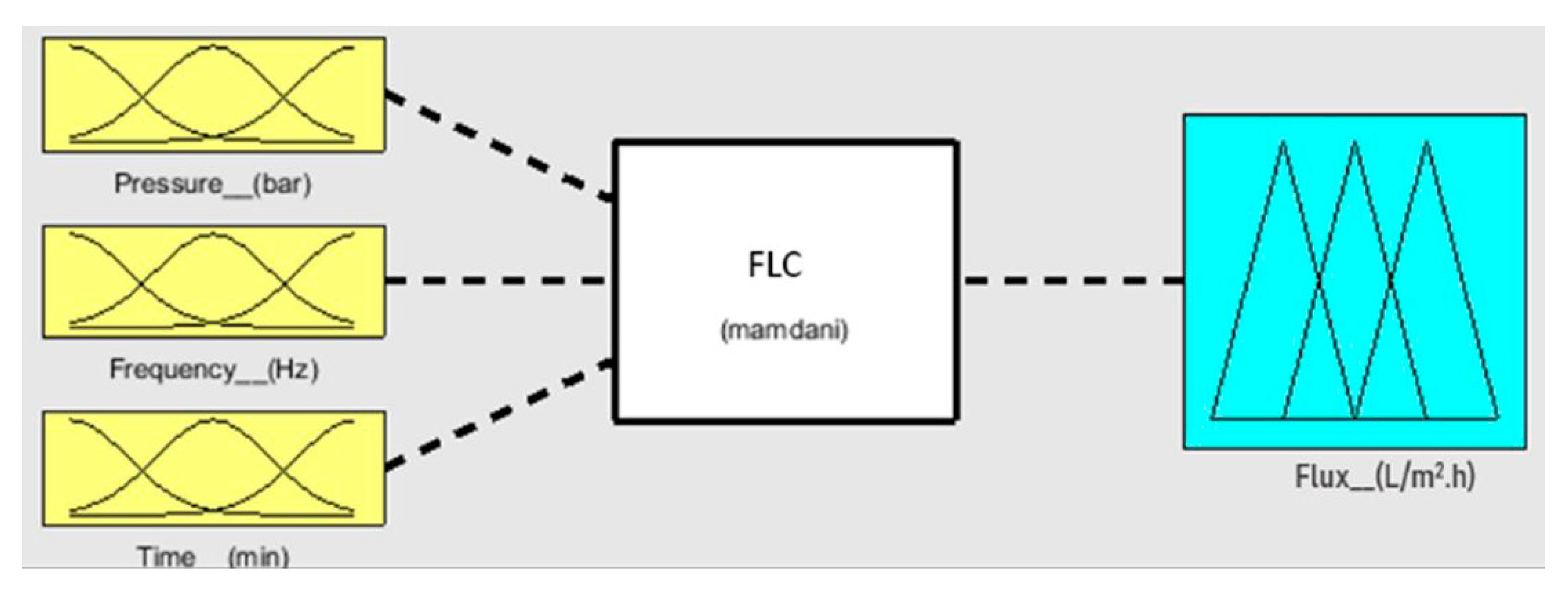
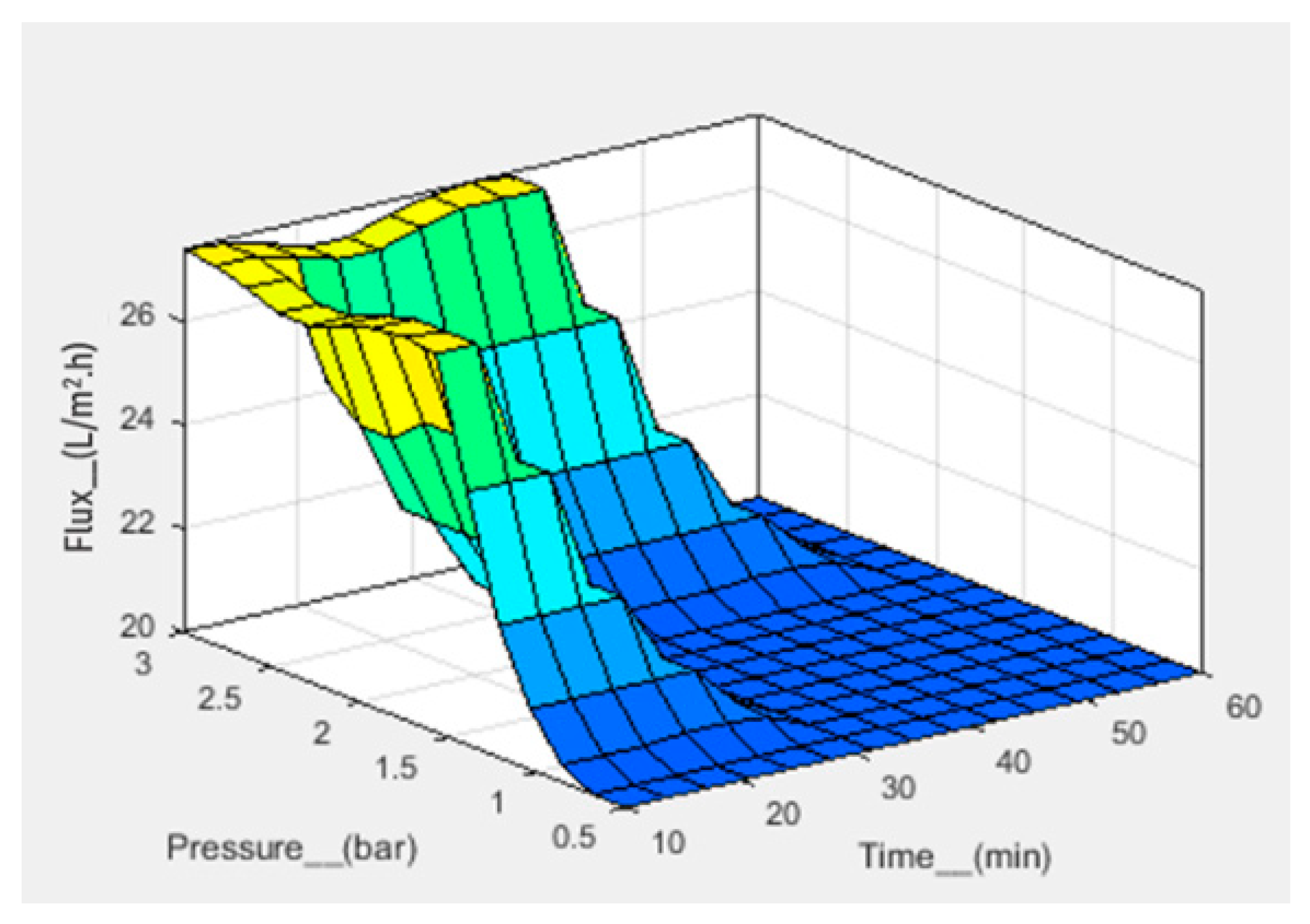

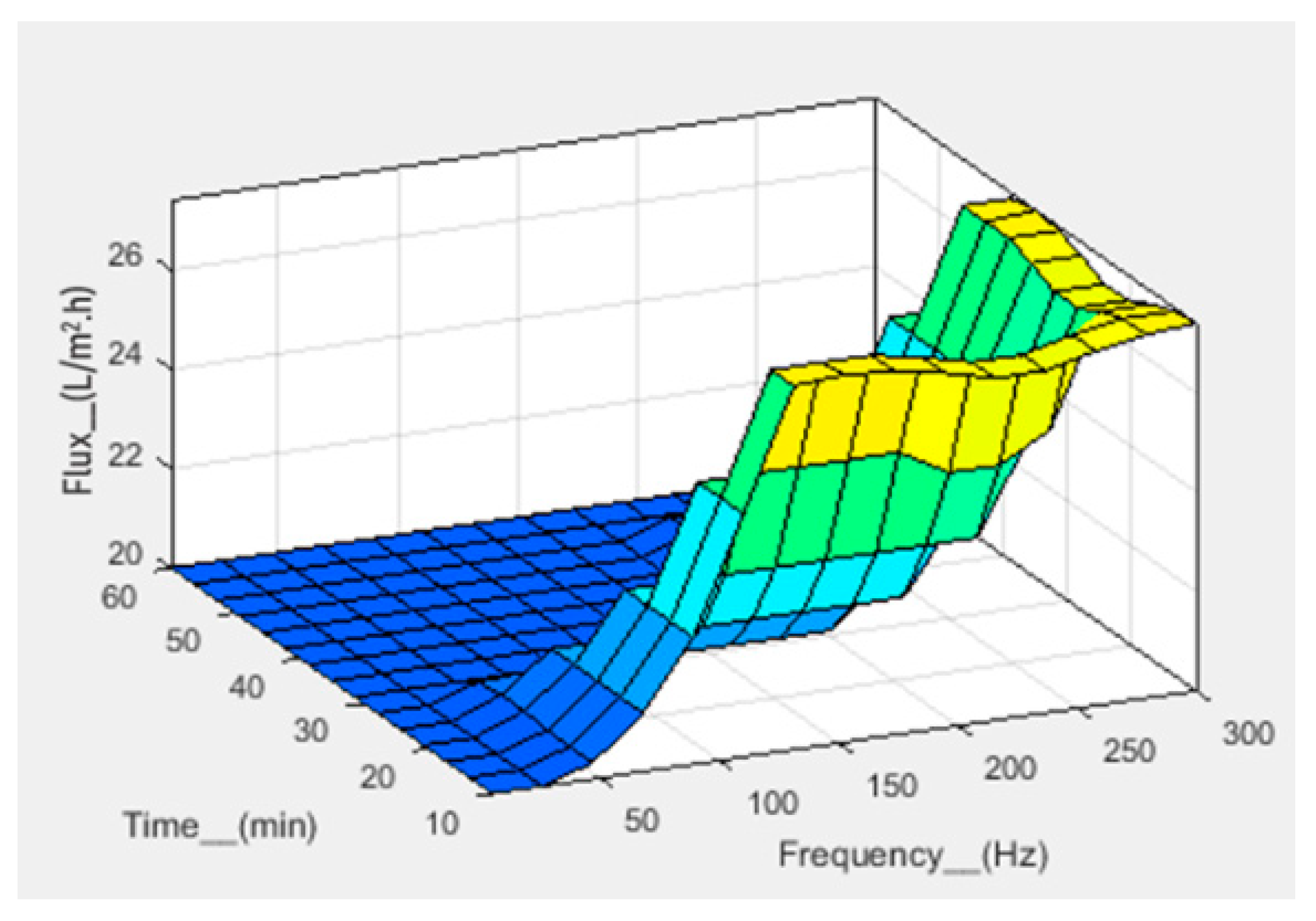
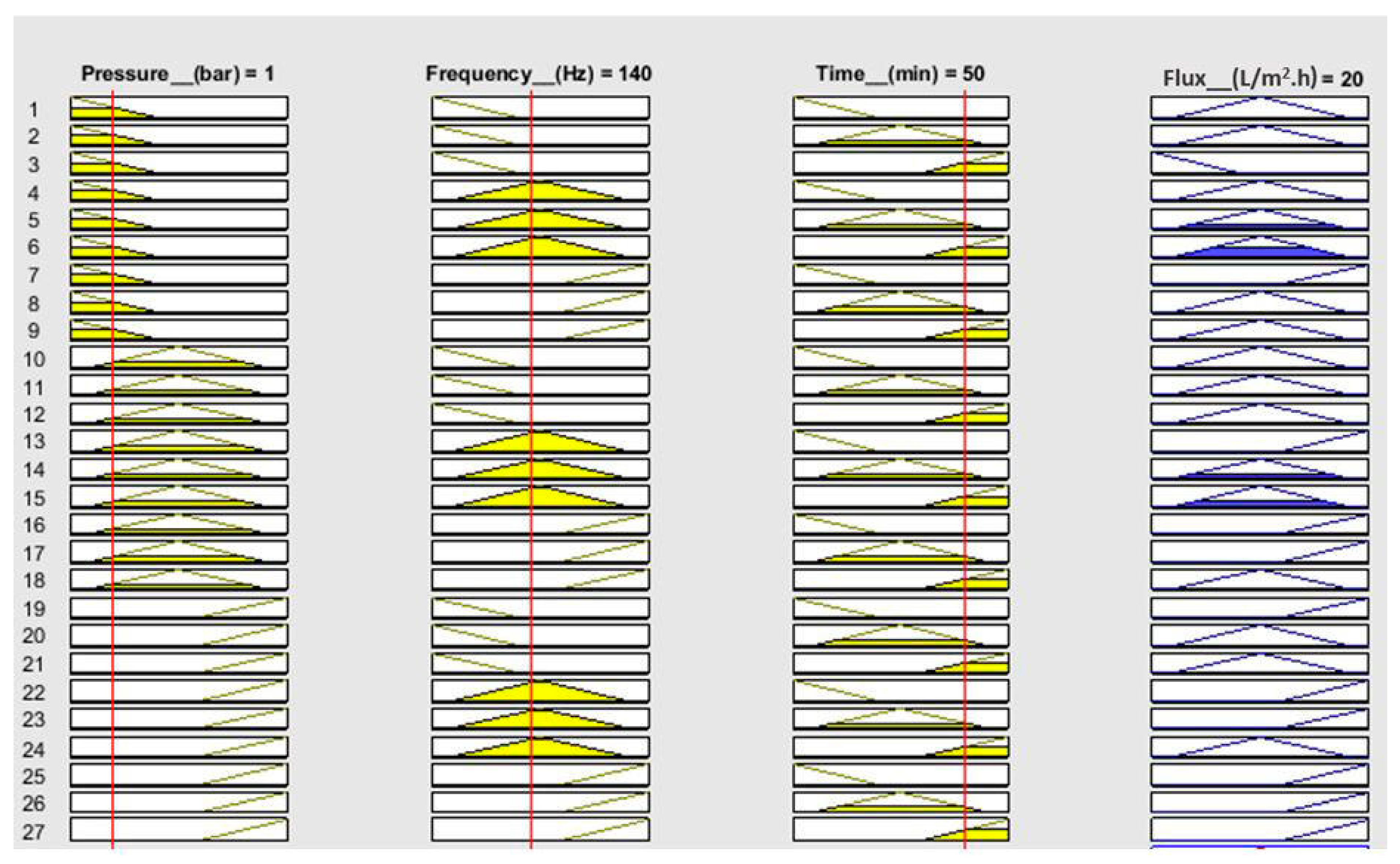
| No. | Pressure (bar) | Frequency (Hz) | Time (min) | Flux (L/m2.h) | ||||
|---|---|---|---|---|---|---|---|---|
| Mf1 | Low | 0.5~1.5 | Low | 5~120 | Low | 10~30 | Low | 10~18 |
| Mf2 | Medium | 0.75~2.75 | Medium | 35~270 | Medium | 15~55 | Medium | 12~28 |
| Mf3 | High | 2~3 | High | 185~300 | High | 40~60 | High | 22~30 |
| Rule no. | Pressure (bar) | Frequency (Hz) | Time (min) | Flux (L/m2.h) | Comparison between Membership Function Value | Min. Value (Mi) | Singleton Value for Flux (Si) | Mi × Si |
|---|---|---|---|---|---|---|---|---|
| R1 | Low | Low | Low | Medium | k1^k3^k5 | 0.17 | 0.2 | 0.034 |
| R2 | Low | Low | Medium | Medium | k1^k3^k6 | 0.53 | 0.2 | 0.106 |
| R3 | Low | Low | High | Low | k1^k4^k5 | 0.17 | 0.1 | 0.017 |
| R4 | Medium | Medium | Medium | Medium | k1^k4^k6 | 0.47 | 0.2 | 0.094 |
| R5 | Medium | Medium | High | Medium | k2^k3^k5 | 0.17 | 0.2 | 0.034 |
| R6 | Medium | High | High | Medium | k2^k3^k6 | 0.33 | 0.2 | 0.066 |
| R7 | High | Medium | High | Medium | k2^k4^k5 | 0.17 | 0.2 | 0.034 |
| R8 | High | High | High | High | k2^k4^k6 | 0.33 | 0.3 | 0.099 |
| Features of AAO Membrane | Filtration Parameters | ||
|---|---|---|---|
| Pore diameter | 80 nm | Pressure/driving force | 1–3 bar |
| Interpore distance | 105 nm | Viscosity | 0.89 mPa.s |
| Porosity | 52% | Density | 940 kg/m3 |
| Thickness | 100 µm | Initial permeance | 63 L/m2.bar.h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoor, S.; Qasim, F.; Ashraf, M.W.; Tayyaba, S.; Tariq, N.; Herrera-May, A.L.; Delgado-Alvarado, E. Simulation and Analysis of Anodized Aluminum Oxide Membrane Degradation. Sensors 2023, 23, 9792. https://doi.org/10.3390/s23249792
Manzoor S, Qasim F, Ashraf MW, Tayyaba S, Tariq N, Herrera-May AL, Delgado-Alvarado E. Simulation and Analysis of Anodized Aluminum Oxide Membrane Degradation. Sensors. 2023; 23(24):9792. https://doi.org/10.3390/s23249792
Chicago/Turabian StyleManzoor, Saher, Faheem Qasim, Muhammad Waseem Ashraf, Shahzadi Tayyaba, Nimra Tariq, Agustín L. Herrera-May, and Enrique Delgado-Alvarado. 2023. "Simulation and Analysis of Anodized Aluminum Oxide Membrane Degradation" Sensors 23, no. 24: 9792. https://doi.org/10.3390/s23249792
APA StyleManzoor, S., Qasim, F., Ashraf, M. W., Tayyaba, S., Tariq, N., Herrera-May, A. L., & Delgado-Alvarado, E. (2023). Simulation and Analysis of Anodized Aluminum Oxide Membrane Degradation. Sensors, 23(24), 9792. https://doi.org/10.3390/s23249792










