1. Introduction
Medical imaging is one of the key achievements of modern medicine, enabling countless lives to be saved every year [
1]. Imaging devices can not only provide an accurate early diagnosis of severe illnesses, and is often the only way of getting one, but can also help to choose appropriate treatment paths, verify treatment efficacy, and guide surgical interventions. Along with advances in imaging techniques, computer vision algorithms are being researched to support radiologists and other physicians in the task of reading and interpreting medical images. Automatic image analysis can also provide a potential solution to essential challenges that medicine faces: interpretation errors that occur during image reading and the growing shortage of medical professionals capable of reading the images.
An important medical imaging field that attracts the attention of many researchers is endoscopy of the human gastrointestinal tract. In modern medicine, endoscopy is still the gold standard in the diagnosis of many diseases of the digestive system, including lethal cancer diseases. It enables the physician to examine the interior of organs and diagnose the early stages of a disease, providing a high chance of successful treatment. Further motivation for research in this field appeared with the introduction of wireless capsule endoscopy (WCE) in 2001, which is less invasive than traditional endoscopy and can potentially acquire images from any part of the gastrointestinal tract.
For both wireless and traditional endoscopy, an important part of the examination is the detection of any occurrence of blood in the gastrointestinal tract, resulting from both active and nonactive bleeding. The diagnostic process requires the cause of any bleeding discovered during the endoscopic examination to be established. Computer vision algorithms are being developed to offer assistance to doctors by providing methods for the automatic detection of bleeding. The task, however, is definitely challenging. Bleeding occurrences are not always clear, and many cannot be recognized by an observer without medical training. Moreover, multiple forms of noise tend to occur in endoscopic images, from natural findings, such as digestive fluid, food debris, other fluids, or bubbles, to technical difficulties that result in blurry images or light-related distortions.
Although many successful algorithms have already been presented for endoscopic bleeding detection, the task remains challenging, and research is constantly being conducted to further improve the accuracy of automatic bleeding detection methods. Current approaches to endoscopic bleeding detection include computer vision methods involving rich feature descriptors, classical machine learning methods that use well-established classifiers, and deep learning methods, based entirely on neural network training in terms of both feature extraction and the classification process. Both approaches have clear benefits and limitations.
This paper introduces a novel method that combines modern convolutional neural network architectures with dedicated high-level features to enhance accuracy in bleeding detection in a scenario of a limited training dataset size, by leveraging insights from the analysis of the blood identification task.
The paper is structured as follows. In
Section 2, a review of the literature is presented. The next sections include a detailed description of the proposed method, the theoretical definition of the identified high-level features, the process of implementing the feature descriptors capturing the features in the form of computer vision algorithms, and the scheme for combining the features with deep neural network models (
Section 3), and the CNN (convolutional neural network) model training procedure (
Section 4). Subsequently, in
Section 5, an experimental verification of the proposed approach is presented by evaluating the impact of extending the models with the proposed high-level features. Finally, a discussion and final remarks are presented in
Section 6.
2. Existing Work
The literature analysis highlights that current bleeding detection methods fall primarily into three categories:
Classical computer vision techniques that rely on image processing and statistical features extracted from images.
Classical machine learning approaches employing well-established algorithms such as SVM, Random Forest, k-NN, PCA, etc.
Deep learning approaches that apply existing convolutional neural network models to endoscopic data.
All three approaches tend to be generic, often employing commonly used image processing techniques that have been successful in various computer vision tasks. However, most of these methods lack interpretability and explainability, which applies both to intermediate results of low-level statistical features and to the operation of deep neural networks.
A comprehensive review of algorithms dedicated to the detection of various pathologies in endoscopic videos, including bleeding, was presented by Liedlgruber and Uhl [
2]. Another review of methods for WCE images taking into account the occurrence of hemorrhages and blood was authored by Musha et al. [
3]. A comprehensive overview of advances regarding upper gastrointestinal tract lesions was also recently presented by Vania et al. [
4]. Several systematic reviews have also been published in the area of deep learning approaches: Du et al. [
5], Soffer et al. [
6], Piccirelli et al. [
7], and Trasolini and Byrne [
8].
2.1. Classical Computer Vision Approach
A wide range of approaches for the detection of bleeding, as well as for other endoscopic findings and diseases, have been developed on the basis of classic computer vision methods. Approaches of this kind utilize feature descriptors based on image processing and statistical measures, designed to capture specific features of the objects to be detected and represent them in the form of a feature vector, which is then classified using simple machine learning methods, e.g., SVM classifiers or shallow neural networks.
Maroulis [
9] examined colorectal lesions by first applying a 2D discrete wavelet transform to grayscale images. Co-occurrence matrices were then derived from the resulting LH, HL, and HH components, and various statistical measures were computed, including energy, angular second moment, correlation, inverse difference moment, and entropy. The classification was accomplished by employing a neural network. Magoulas [
10] examined tumors using the same algorithm but applied it to 16 × 16 pixel blocks instead of the entire image. Kodogiannis [
11] detected abnormalities by processing RGB and HSV color planes with the NTU transform. For each plane, nine statistics were computed, including standard deviation, variance, skewness, kurtosis, entropy, energy, inverse difference moment, contrast, and covariance. Classification was performed using an adaptive fuzzy logic system. In [
12], Li investigated tumors by processing RGB planes with a 2D discrete wavelet transform. A Local Binary Pattern (LBP) transformation was applied to each of the resulting LH, HL, and HH components, and 10-bin histograms were generated. Classification was performed using support vector machines (SVMs) with an RBF kernel. Yuan and Meng [
13] achieved a bleeding detection accuracy of 95% with a sensitivity of 98% and a specificity of 93% using a saliency map in conjunction with an SVM on images of size 256 × 256. Zhao [
14] analyzed polyps using several types of features. For color features, the image was converted into a proposed opponent color space, and a normalized 2D histogram was computed, followed by proposed color moment invariants, which were claimed to be robust against illumination, affine transformations, and blur. The texture features were extracted using the contourlet transform, LBP, and LLE algorithm. Classification was accomplished using an SVM classifier. Kumar [
15] examined Crohn’s disease and extracted features using MPEG-7 descriptors. Edge histogram descriptors were used for edge features (in 16 image blocks), dominant color descriptors for color features, and homogeneous texture descriptors for texture features. Classification was performed using an SVM classifier.
In the classical computer vision approaches, most attention is paid to the feature descriptors. In the context of endoscopic bleeding detection, most methods address the color features of the images, which is strongly justified by the nature of the typical appearance of blood. Other types of features, including texture, contours, or the use of segmentation techniques are utilized less often. Feature descriptors often take the form of complex mathematical transforms that capture specific statistics of the image, which can be considered as low-level image features.
Classical approaches delivered fairly successful results in the area of endoscopic bleeding detection, especially at a time when only small datasets of endoscopic images were available. The big advantage of the classic approach was, in fact, the possibility of developing algorithms even on the basis of small collections of images. However, classic approaches face several difficulties. The development of hand-crafted feature descriptors is typically a hard and time-consuming process. The adequacy of the low-level features with the characteristic visual properties of the detected object is a challenging problem and has to be solved for every new detected object. The high complexity of the computation in the evaluation of the features, as well as the nature of common classifiers, make it difficult for a human to verify the intermediate results and the entire process. This often eliminates the possibility of identifying the reasons for the wrong predictions generated by the system. Therefore, it is difficult to include new features to improve the accuracy of an algorithm.
2.2. Classical Machine Learning Approach
A variety of methods for endoscopic image analysis were developed based on classical machine learning algorithms, where more emphasis is placed on employing classification methods and techniques to facilitate their training. Popular classifiers include shallow artificial neural networks, support vector machines, decision trees, and k-nearest neighbor classifiers.
Giritharan et al. [
16] employed a set of support vector machine classifiers together with training data balancing based on oversampling and preprocessing using an averaging filter to remove random noise. Khun et al. [
17] combined an artificial neural network with a support vector machine for color features by dividing the image into nine non-overlapping blocks. Poh et al. [
18] employed an artificial neural network classifier for each cell and each block, merging the results using rule-based decision making and dividing the image into 4 × 4 cells. Yeh et al. [
19] combined a C4.5 decision tree, multilayer perceptron (NLP), and a support vector machine, with feature selection using SVM feature elimination, OneR, and RELIEF algorithms. Szczypinski et al. [
20] used a support vector machine classifier with an RBF kernel and reduced an extensive feature set using analysis of variance (ANOVA), sequential floating forward selection (SFFS), and the vector-supported convex hull method (VSCH). The image was divided into overlapping, circular regions. Wang and Yang [
21] proposed a Bayesian classifier in which feature vectors are classified by estimating their probability of belonging to each class using the Bayesian formula and selecting the maximum score. The formula incorporates posterior probabilities for each class, derived by estimating the training dataset through the multivariate Gaussian probability density function.
2.3. Deep Learning Approach
Deep learning is a modern area of machine learning focused on training deep neural network models using large collections of data. Neural networks are inspired by the structure and function of the human brain, where interconnected layers of artificial neurons, organized into networks, learn to recognize patterns and make decisions. Commonly used neural network architectures are convolutional neural networks (CNNs), recurrent neural networks (RNNs), and Transformers.
The introduction of deep learning models, especially in the form of convolutional neural networks, offered a completely different approach to computer vision tasks. The feature extraction step is taken over by neural networks, which have the ability to learn features directly from images, eliminating the need for manual design and implementation. This leads to a very simple and elegant approach, where all of the processing steps, from the feature extraction to the classification step, are conducted by a single mechanism in the form of a neural network.
The rapidly growing popularity of convolutional neural networks led to the development of a variety of neural network architectures that consequently improved the accuracy for multiple computer vision tasks. For the field of endoscopic bleeding detection, a series of methods based on deep learning models was also proposed.
The majority of deep-learning-based approaches, including the work of Li et al. [
22,
23], Tsuboi et al. [
24], Aoki et al. [
25], Caroppo [
26], Ghosh [
27], Saraiva et al. [
28], and Garbaz et al. [
29], rely on existing CNN architectures including VGG [
30], Resnet [
31], Inception [
32], and SSD networks [
33] and their direct utilization for training on an endoscopic dataset of annotated blood images. Some of the approaches acknowledge the problem of a limited training data size, which is addressed by applying a transfer learning technique, typically using models trained on the Imagenet dataset [
34].
A notable approach to endoscopic bleeding detection was presented by Kim et al. [
35]. Although the authors only utilized an existing CNN architecture as their classification method (InceptionResnetV2 architecture), the presented approach used an outstandingly large dataset including 656,591 endoscopic images (out of which 164,713 were blood images), which enabled them to achieve high classification accuracy for multiple endoscopic findings, including bleeding. Moreover, 256,591 of the images were treated as unseen images and used by the authors in order to perform external validation of the acquired model. The localization capabilities of the model were also presented by applying class activation maps [
36].
In the context of this paper, an interesting approach was proposed by Jia and Meng [
37]. The authors proposed a method of combining CNN networks with hand-crafted features of blood regions. However, the hand-crafted features proposed by Jia and Meng are relatively simple low-level features that include only a histogram obtained from k-means-clustered CIE Lab color space. The features are represented as a feature vector of 50 values and are eventually concatenated with CNN activation before the classification layer. In contrast, the approach proposed in this paper uses high-level features that produce a more expressive output in the form of spatial feature activation maps. Furthermore, by passing the feature maps to the input of a CNN network (see
Section 3.3), the proposed approach allows the neural network to utilize the content of the feature maps during training and prediction, while in [
37], only the last classification layer can access the information contained in the features. Moreover, Jia and Meng utilized only a very small, eight-layer CNN network.
Analysis of the literature therefore shows that multiple, yet relatively simple, bleeding detection algorithms based on deep learning models have been proposed. Some of them also focus on addressing the problem of low training dataset size. Importantly, however, none of the approaches presented utilize a method equivalent to the method proposed in this work, which involves a set of high-level features dedicated for bleeding detection based on domain knowledge.
The availability of deep learning models enabled a great improvement in terms of the accuracy of computer vision algorithms. However, they also introduced a major difficulty related to the requirement of a very large training dataset size. Meanwhile, the size of available endoscopic image datasets is still not sufficient, and even though techniques including transfer learning are utilized, the application of deep learning models remains limited in the area of endoscopic bleeding detection.
3. The Proposed Approach
In order to partially address the challenges presented in the previous section, a new approach is proposed, including a combination of deep learning models and a set of proposed hand-crafted high-level visual features. The proposed features have the form of 2D feature maps, which enables them to be appended to the regular color channels of the input image as additional image channels, and therefore, for them to be passed to a regular convolutional neural network to perform the actual classification. An illustration of the proposed approach is presented in
Figure 1.
The purpose of the proposed features is to provide initial clues regarding the visual characteristics of the blood to the neural network, which is expected to simplify the task of recognizing blood using the deep learning model, especially in a scenario with a limited training dataset.
The proposed approach is an extension for deep learning models that can potentially be applied in combination with other techniques to improve model accuracy, such as transfer learning or image augmentation. Therefore, it can be incorporated into deep learning approaches as another improvement to achieve greater classification performance gain.
3.1. Visual Features’ Definitions
The proposed high-level features were designed based on domain knowledge, as well as on the analysis of the literature in the field. The identification of the features was an iterative process including multiple proposals of candidate feature sets, assessments of their quality, and adding improvements. The design of the features was discussed with an experienced gastroenterologist in terms of the validity of the visual properties of blood that are assumed to be captured by the proposed features.
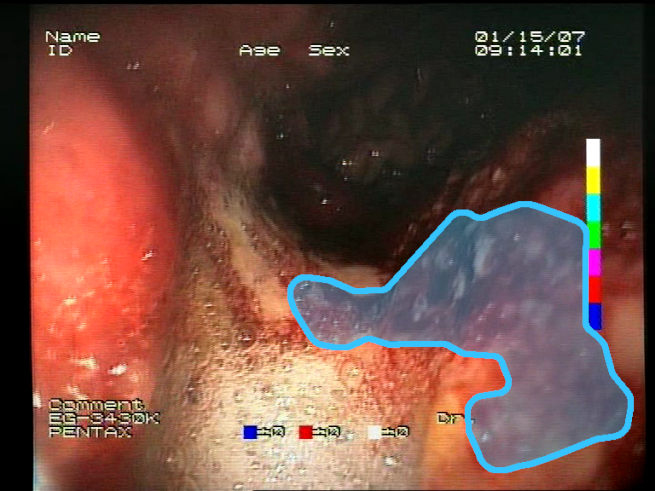
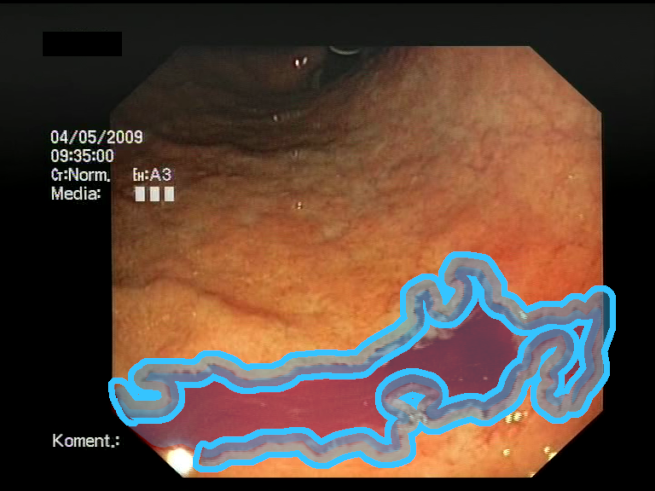
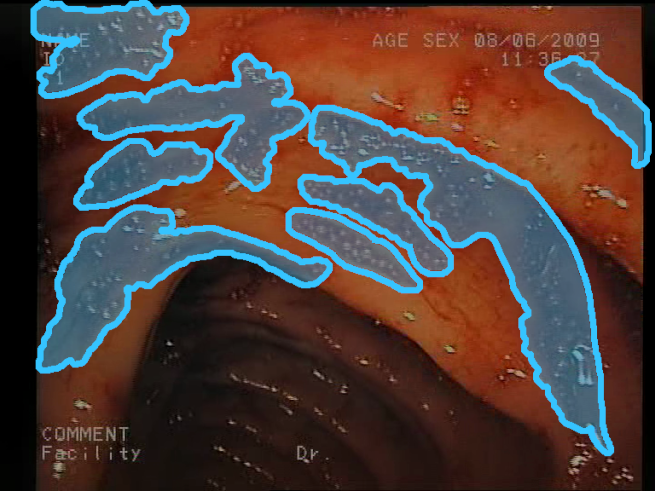
As a result of the design process, nine high-level features (denoted F1–F9) were defined. The features are proposed with simple definitions that also include exemplary images, presented in
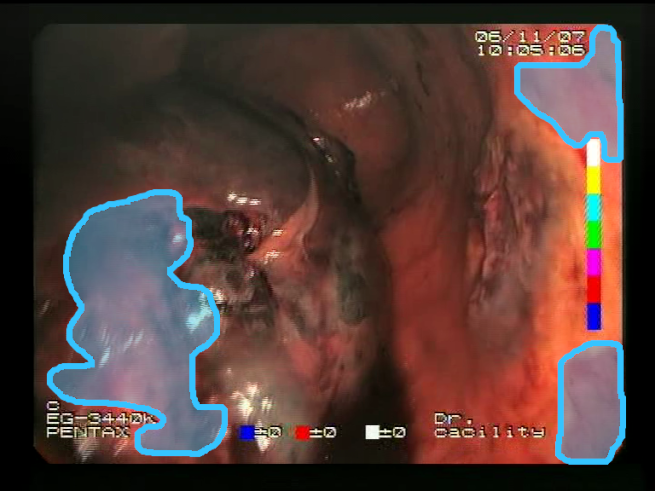
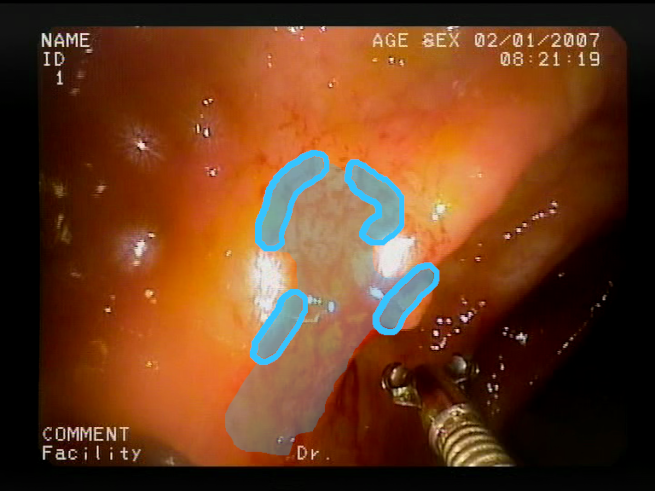
Table 1. For each image, a blue annotation is presented, illustrating the image regions where the feature is hypothetically present.
3.2. Feature Descriptors’ Implementation
For each of the proposed features, a feature descriptor was implemented with the objective of matching definitions of the particular feature as closely as possible. Each feature descriptor was implemented in the form of a simple computer vision algorithm that transforms the input color image into a feature activation map in the form of a single-channel 2D image matching the size of the original image, where pixel intensities of the activation map reflect the strength of a given feature’s appearance in the respective part of the image. The feature descriptors were designed with an assumption of using fairly simple implementations and with a focus on vectorized image processing operations (enabling efficient execution on multicore systems). The implementations were designed using array processing methods dedicated for image processing in Python, using OpenCV and Numpy libraries. The algorithms were also tuned in terms of the choice of image processing operations and their parameters (such as kernel sizes, normalization ranges, etc.) using a novel computer-vision-oriented support tool, CVLab
https://github.com/cvlab-ai/cvlab (accessed on 7 December 2023). An example of a pseudocode for the implementation of a feature descriptor (descriptor F1) is presented in Algorithm 1. The source code for the implementation of the feature descriptors is available on Github
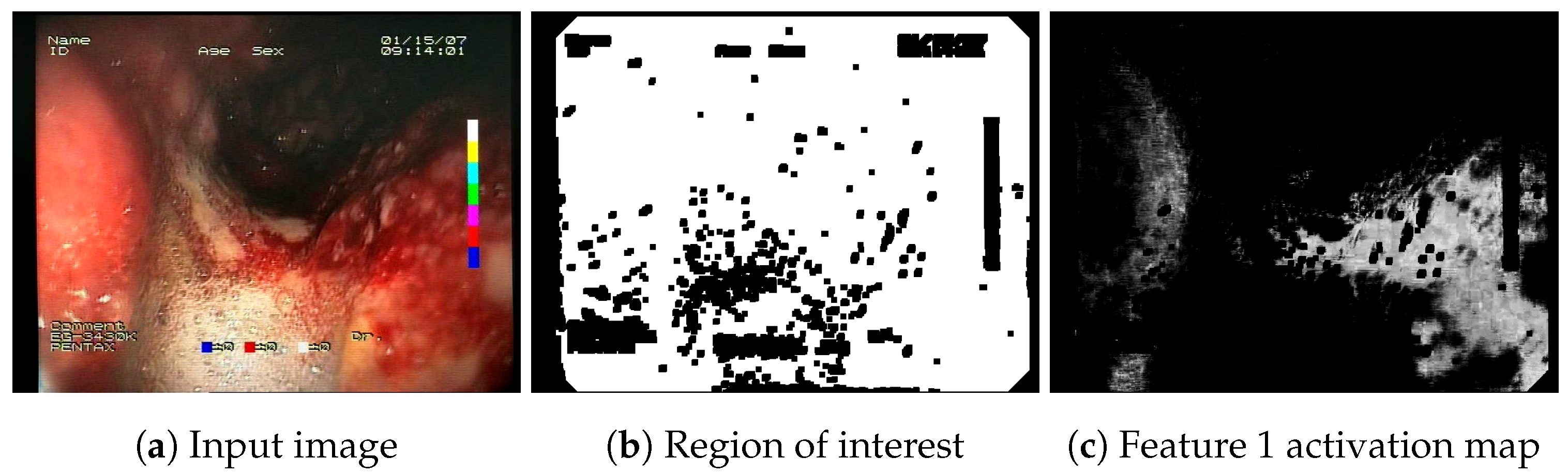
https://github.com/ambrzeski/endoblood-features (accessed on 7 December 2023). An example of a feature activation map generated by the F1 descriptor is presented in
Figure 2.
3.3. Combining the Features with CNNs
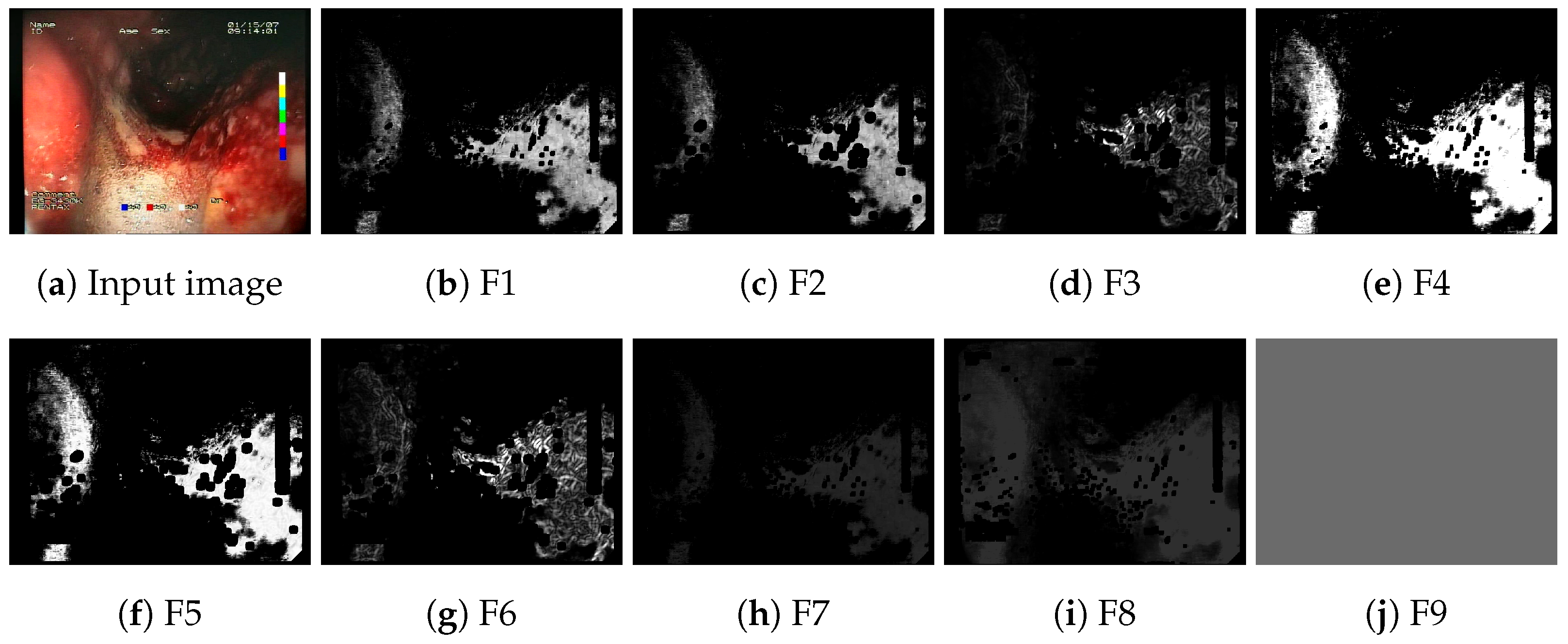
The implemented feature descriptors generate nine single-channel feature activation maps for a given input image. An example of a set of generated feature maps is presented in
Figure 3. The feature maps are subsequently appended to the regular R, G, B channels of the original image, forming a 12-channel image in place of the typical 3-channel input image. The outline of the process of appending feature maps to the input of the neural network is presented in
Figure 1. The resulting 12-channel image is passed as input to a regular convolutional neural network (CNN). The only modification required in the CNN architecture is the change in input size. This results only in a change in the depth dimension of the convolutional filters of the first layer of the neural network, which is modified from 3 to 12. All the remaining layers of the network remain unmodified.
The process of appending feature activation maps is identical for both model training and inference. During training of the model, for each training image, the feature maps are generated and appended to the image before passing to the network as a training data point. Similarly, in the model inference process, the feature maps need to be generated and appended to each input image.
| Algorithm 1: Pseudocode for the blood color region feature descriptor (F1). RGB color channels are first extracted as r, g, and b arrays. Next, two coefficients are introduced to reflect color values typical for bleeding. The first coefficient () measures the excess of red component intensities over the blue and green components by measuring the minimum value of the and ratios, which is similar to the approach presented by Fu et al. [38,39]. The second coefficient () measures the proximity of the blue and green components, which also appears to be typical for blood regions. The two coefficients are combined by pixel-wise multiplication with weights applied (power of 5 applied to ). Finally, pixels with a low red component are cleared from the activation, as well as the area outside the detected region of interest. All parameters of the algorithm, including the and norm arguments, factor (power of 5), were manually tuned using the CVLab tool. |
- 1:
function Descriptor_F1(Array , Array ) - 2:
Array r, g, b← extract_channels_bytes() - 3:
Array g← max(g, 1) - 4:
Array b← max(b, 1) - 5:
- 6:
Array ← min(, )/255 - 7:
← max(, 0) - 8:
← norm(, min=0.003, max=0.01) - 9:
- 10:
Array ← 1 - abs(b - g)/255 - 11:
- 12:
Array ← - 13:
← norm(, min=0.02, max=1.1) - 14:
← 0 - 15:
← 0 - 16:
- 17:
return
|
4. CNN Model Training
Model training was conducted on a set of four neural network architectures:
VGG19 [
30]—uses a simple structure consisting of only 3 × 3 convolutions, pooling, and fully connected layers.
Resnet50 [
31]—uses the concepts of residual connections and bottlenecks.
Resnet152 [
31]—a larger variant of Resnet50 architecture.
Inception-v3 [
32]—uses the concepts of grouped and factorized convolutions.
The choice of architectures was based on their high accuracy achieved in various computer vision tasks, which has also resulted in their high popularity in both research and commercial projects [
40], as well as bleeding detection algorithms (see
Section 2.1). The four architectures originally gained widespread recognition after their successful application in notable ILSVRC [
41] challenges. The architectures are still widely used, although they tend to be extended with additional improvements, such as the squeeze and excitation module [
42], especially in the case of Resnet networks. Mixtures of these architectures are also presented in the literature, e.g., Inception-Resnet [
43]. The selected convolutional neural network architectures also differ significantly from each other, not only in their size, but also as they utilize different conceptions of layers to form the architecture (except the Resnet50 and Resnet152 pair, which differ mostly in the number of layers).
For each of the architectures, a collection of models was trained in a feature-extended variant and a baseline variant that does not utilize the proposed features, to enable comparison between the variants and assessment of the impact of the proposed features.
4.1. Training Procedure
Training of the baseline and feature-extended models was performed using five-fold cross-validation on the training part of the dataset (details in
Section 4.2). For each of the folds, a series of models was trained along with the hyperparameters’ optimization process. The optimization process was designed to find optimal values for a set of important training parameters, including learning rate, momentum, learning rate decay, batch size, and balancing the proportion of positive and negative samples, to acquire the highest possible accuracy of the model.
Separate optimizations were performed for the baseline and feature-extended models, following identical protocols and for an identical number of iterations. For both types of models, the process was conducted in two stages. The first stage included a broad hyperparameter search, in which 30 separate models per fold were trained for 5 epochs using random values of hyperparameters drawn from specified parameter ranges. In the second stage, the top six models per fold from stage one were selected and used as starting points for a set of 18 final models per fold trained in stage two training, where the training was continued until the stop condition, set to 20 epochs, without validation loss improvement.
Training was performed using the SGD (Stochastic Gradient Descent) with a momentum optimizer [
44]. The learning rate was reduced in the validation loss plateau. The training batches were populated with positive and negative samples in a balanced manner following the ratio set by a hyperparameter. The experiments were carried out using Keras framework with TensorFlow backend. The training was conducted on 4 Nvidia Titan Xp GPUs.
4.2. Dataset
The dataset used in the experiments was collected and annotated by physicians at the Medical University of Gdańsk within the ERS project [
45]. The images had been acquired with several types of analog endoscopes, digitized with a video capture device, and stored in a PAL resolution of 720 × 576 with interlacing. The recordings were captured during both gastroscopy and colonoscopy examinations. During the annotation process, a medical doctor reviewed the examinations and annotated samples in the form of short video clips. Multiple types of lesions were annotated in the project, including bleeding. In the context of blood, each sample was assigned to one of two possible classes: blood (presenting blood), considered as positive, or non-blood (no blood presence), considered as negative. More than one sample could be annotated in each examination.
The overall dataset size is 54,130 images, with 29,457 blood images and 24,673 non-blood images presenting an organ without a blood presence. Video frames were extracted from 517 different samples annotated over 372 endoscopic examinations. The dataset was split into training and test subsets using a 3/1 ratio. The statistics of the subsets are presented in
Table 2. The training set was later split into five random folds to perform the cross-validation procedure, ensuring that all images from any given examination were assigned to the same fold.
4.2.1. Dataset Balancing
It is a desired feature of the dataset to contain approximately balanced positive samples (presenting blood) in terms of the number of images. This would make samples similarly important during the training process. Imbalanced samples, in turn, where some blood cases include significantly more images than other cases, will have a significantly greater impact on the training and validation of the model, resulting in a strong bias towards positive samples of a large size. Unfortunately, the presented dataset suffers from a strong imbalance in the number of images per sample: almost 30% of the samples are represented just by a single image, while more than 10% of samples include more than thousand images. A slightly lower imbalance can be observed in non-blood images.
To address this issue, balancing of the samples in the blood class was applied by duplicating images in the small samples so that each sample contained at least 100 images. In this way, the disproportion in significance between the samples was reduced. Balancing was not performed, however, for the non-blood class samples.
4.2.2. Data Augmentation
For the purpose of the training of the model, the images were also subjected to a set of augmentation transformations, including rotation, flip, skew, and perspective transformations, and blur, noise, and color variance transformations: hue distortion, saturation distortion, and PCA color augmentation. Following the common practice used in the field of machine learning, the magnitude of each transformation was randomly selected from predefined value ranges during each execution of the augmentation process.
5. Evaluation
Models trained for VGG19, Resnet50, Resnet152, and Inception v3 architectures were evaluated in order to compare the classification performance of the standard baseline neural network architecture operating on the standard RGB (red, green, blue) channels with the same network operating on the proposed feature channels appended to the RGB channels.
The evaluation was carried out in four scenarios designed to measure different aspects of model accuracy, the capability of localizing blood areas, and computation performance, and is presented in detail in
Section 5.1–
Section 5.4. The accuracies of models using different architectures and variants (baseline or feature-extended) are compared in a set of predefined model configurations including ensembles of groups of models. Model ensembling is performed by averaging the outputs of the models included in the ensemble. The four evaluation scenarios are presented below.
Best single ensemble comparison—the best models for both baseline and feature-extended variants are selected and compared. The best single ensemble (denoted as #1) is an ensemble of best-performing models from the five-fold cross-validation training; one (top) model per each cross-validation fold is used. The results are presented in
Section 5.1.
Statistical analysis of results for multiple single ensembles—for each variant a set of 15 single ensembles (denoted as #1-15) is included and a statistical analysis is performed comparing the mean accuracy metrics values achieved by the ensembles. The results are presented in
Section 5.2.
Blood area localization results—the abilities of both the baseline and feature-extended models to indicate of the location of the actual blood area are evaluated against reference bounding boxes that are available for part of the test set. The results are presented in
Section 5.3.
Processing performance—an evaluation and comparison of processing performance is conducted for the baseline and feature-extended models, including the computational overheads of the features. The results are presented in
Section 5.4.
5.1. Best Single Ensemble Evaluation
In the first evaluation scenario, the best single ensembles (denoted as #1) acquired for each variant are evaluated and compared. The best single ensemble for a given variant is an ensemble of the five best-performing single models acquired for five folds of the five-fold cross-validation training, with one (top) model for each fold. The performances of the best baseline ensembles and the best feature-extended ensembles were evaluated using a set of metrics presented in
Table 3; additional definitions are presented in
Table 4.
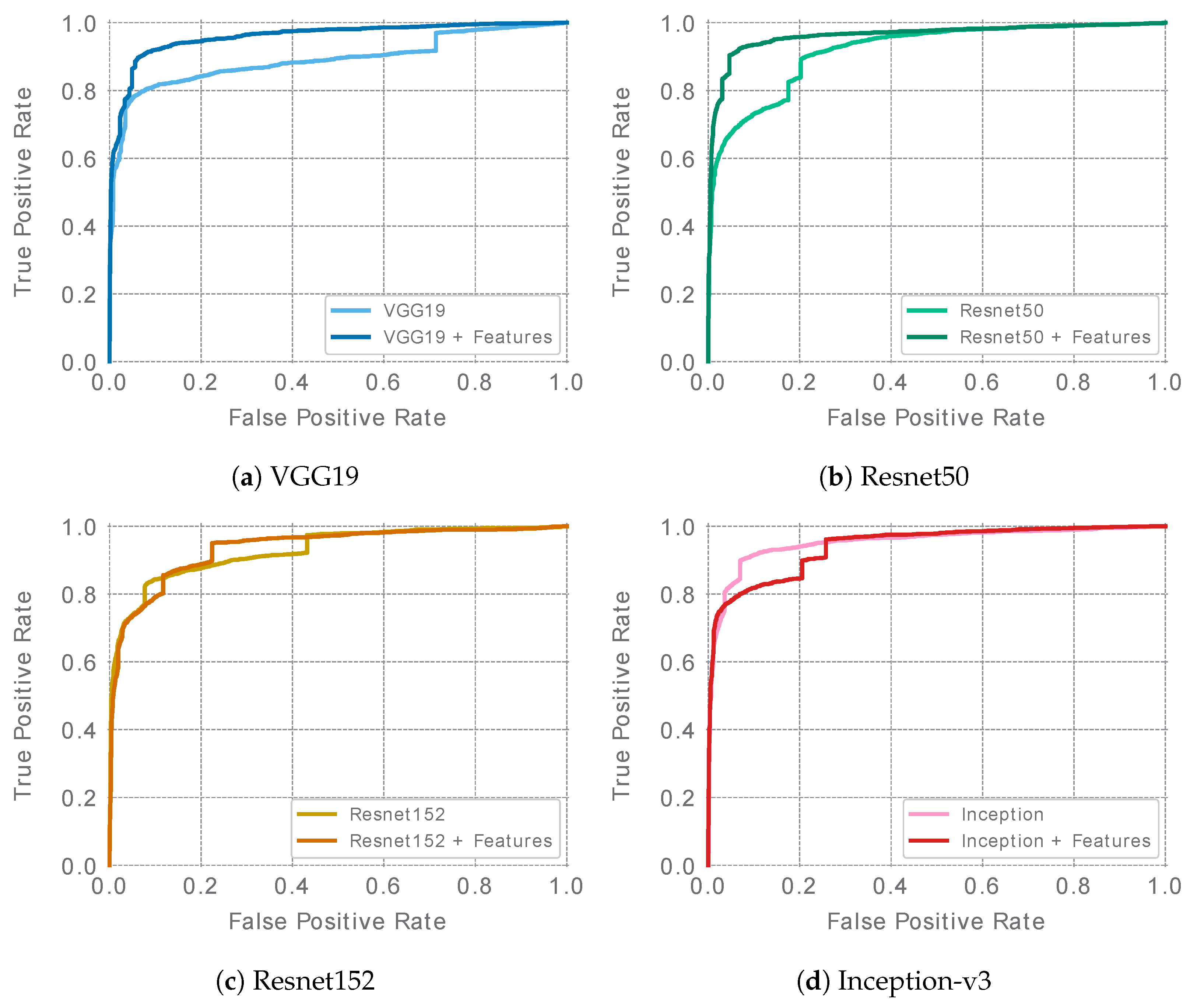
The best single ensemble results for each of the evaluated architectures are presented in
Table 5. ROC curves are presented in
Figure 4. In the case of the Resnet and VGG19 networks, feature-extended ensembles (denoted as “+F” ensembles) achieved higher ROC AUC values than the baseline ensembles. For the Inception v3 architecture, in turn, the baseline ensemble reached a slightly higher ROC AUC. The overall accuracy considering the remaining metrics appears to be similar for the baseline and feature-extended ensembles in the case of the VGG19, Resnet152, and Inception-v3 architectures. However, in the case of Resnet50, a clear advantage of the feature-extended ensemble was observed. It was also the feature-extended Resnet50 that achieved the highest ROC AUC score of all of the best ensembles, reaching a value of 0.963.
High-sensitivity and high-specificity operating points are interesting modes for the potential production application of the models. In particular, two application scenarios of bleeding detection models can be considered. The first scenario is video summarization, where the task is to detect and filter out normal frames while preserving frames containing blood occurrences, therefore resulting in a short summary of an endoscopic video. This scenario requires the model to operate in a strictly high-sensitivity mode. The second potential scenario is a concise (but not necessarily complete) indication of the pathologies detected in the video. Such concise detection results can be appended to a preview of a video in the user interface of the imaging software. Even when not all of the lesions are presented in the detection, some of them could be cases that would be unintentionally missed during the review of the video, so presentation of the lesions decreases the chance of overlooking them. The concise form takes up little space and uses little time to be read by the physician, provided that they do not include irrelevant images that do not include any lesions. Therefore, this scenario prefers high-specificity (low false positive rate) operating points.
The high-sensitivity (
) and high-specificity operating points’ (
) (see
Table 3) results have already been presented in
Table 5. For the
operating point, the Resnet50+F model ensemble achieved 0.855 specificity, which means that for the application in the first scenario described above, the model could potentially remove 85.5% non-bleeding frames while preserving 95% of frames containing bleeding. For the
operating point, the Resnet50+F ensemble achieved 0.122 sensitivity, meaning that in the second application scenario, the model could potentially spot 12.2% of blood-containing frames while misdetecting blood in only 0.01% of the non-blood frames. As expected, based on the ROC curves and AUC results of the models, feature-extended models achieved higher results for most of the operating points presented.
5.2. Statistical Evaluation of Multiple Model Training Runs
The next experiment included a process of using multiple models for the statistical evaluation of the impact of the designed feature descriptors. For each architecture and variant (baseline and feature-extended), a set of 18 single ensembles (in a form of ×5 fold ensembles) were considered. The three ensembles that performed the worst for each variant were excluded (the exclusion rule was formulated before evaluating the models). For the 15 included single ensembles per variant (denoted as #1–15), a statistical analysis was performed by calculating the mean and standard deviation values. The analysis is presented in
Table 6,
Table 7,
Table 8 and
Table 9. In this experiment, the mean values of the
were higher for the feature-extended models for each of the four architectures.
5.3. Bleeding Area Localization
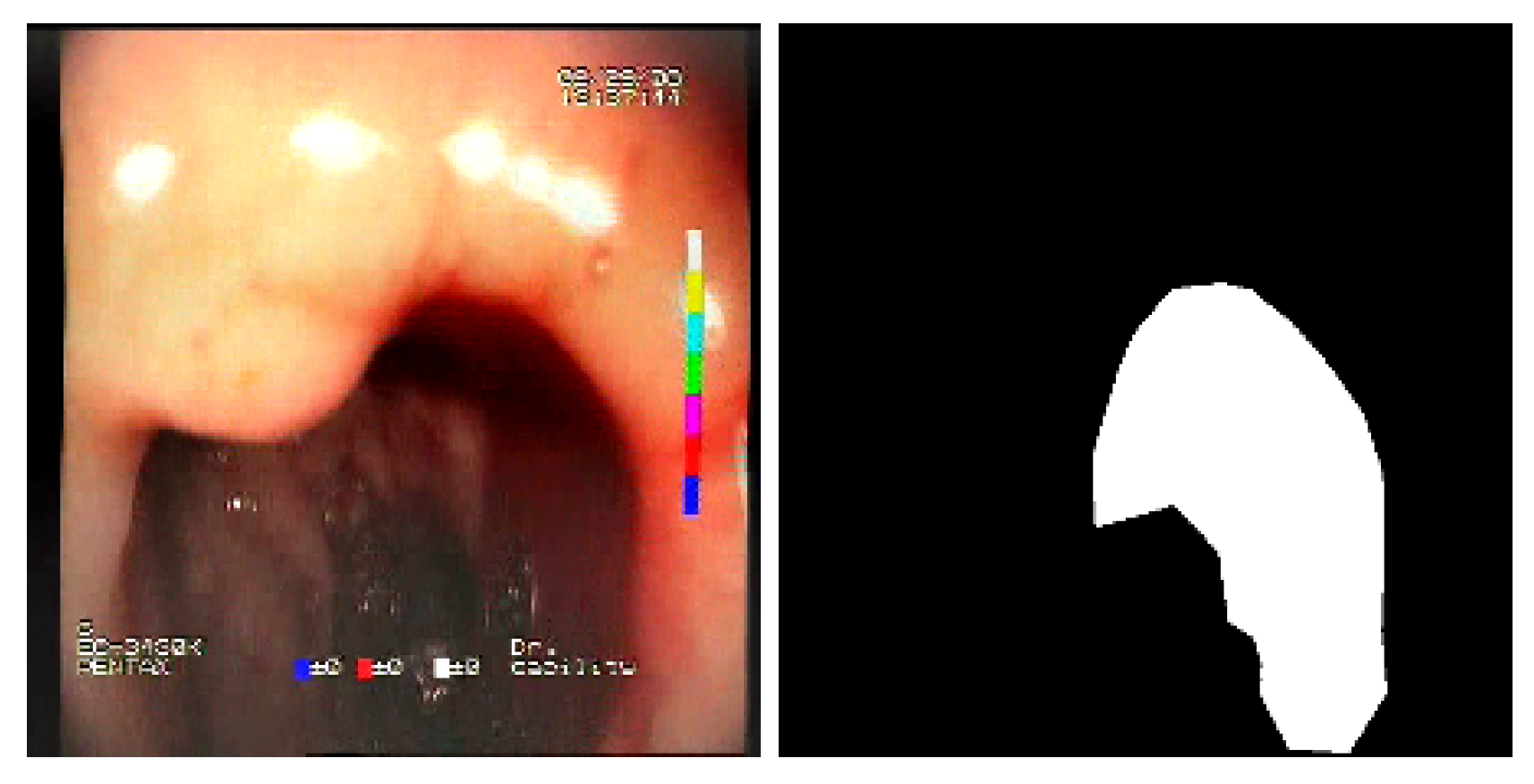
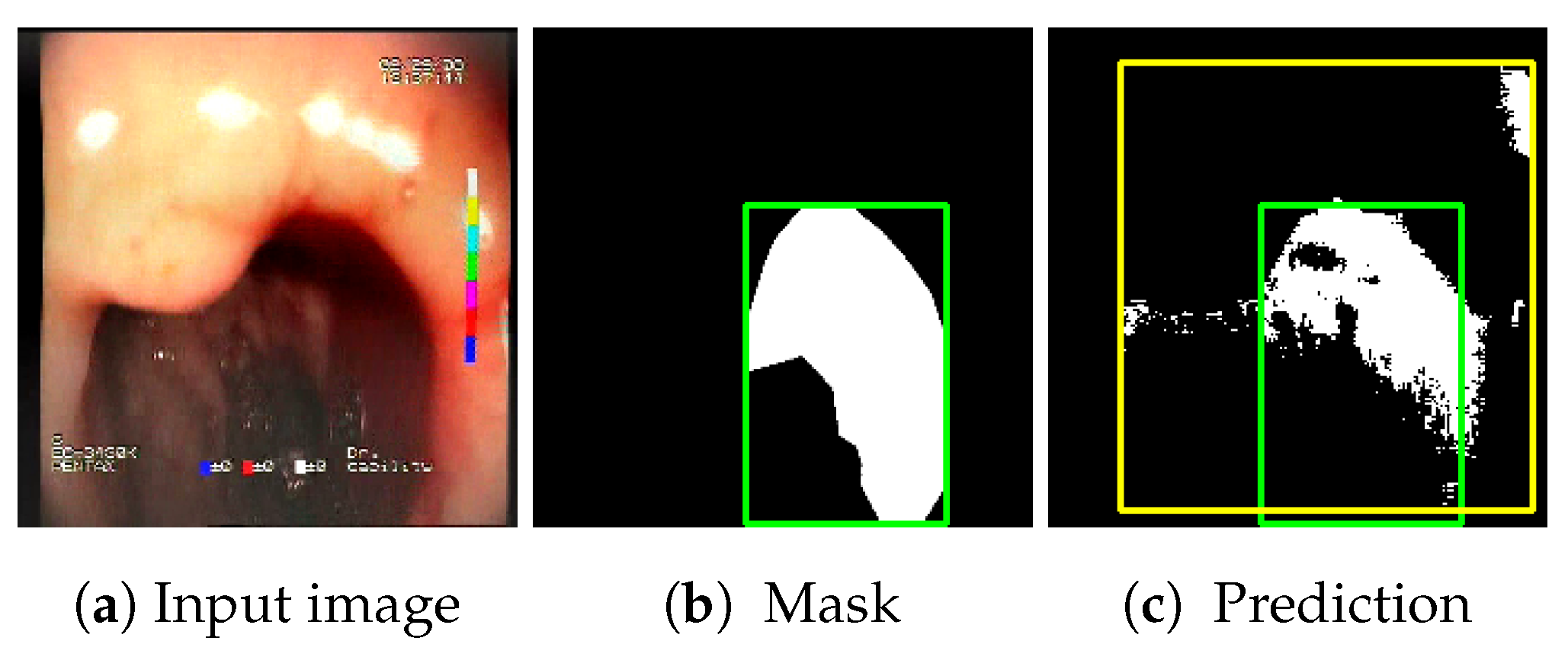
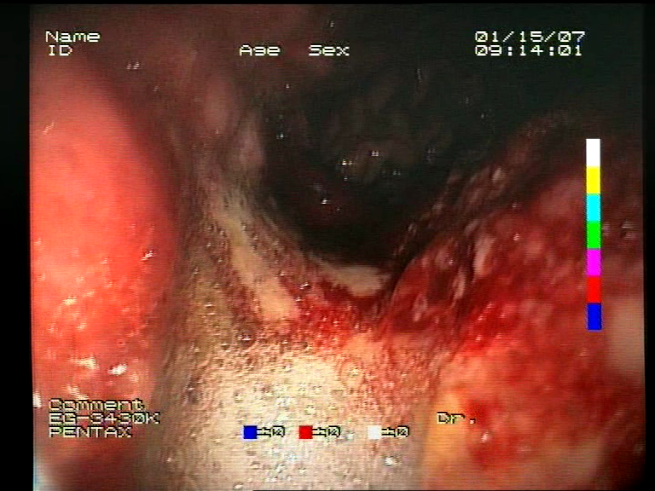
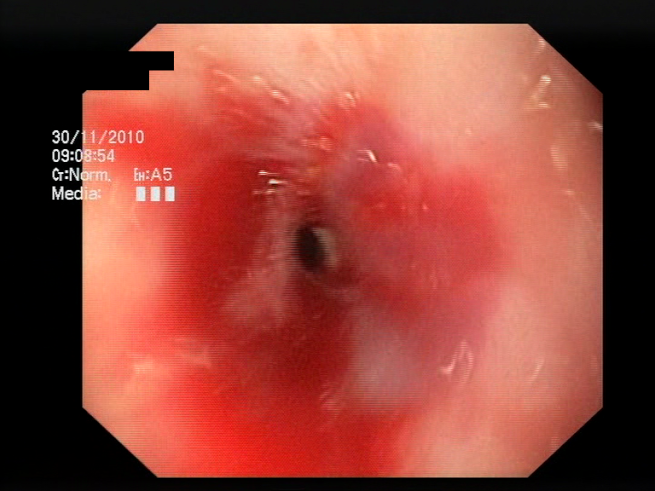
The baseline models and the proposed feature-extended models were also evaluated in terms of providing potential explanations for classification predictions. In this case, the expected form of an explanation is the location of the bleeding areas detected by the classifier. To evaluate the quality of the localizations, a subset of the test set was used, consisting of all images for which blood area masks were available in the dataset. The subset consists of 25 test images with segmentation masks. A sample image with the associated blood area mask is presented in
Figure 5. For each of the 25 test images, localizations were generated for all the evaluated models using a set of approaches and compared against the reference segmentation masks.
5.3.1. Localization Accuracy Metric
The localization accuracy evaluation was performed by comparing the bounding boxes calculated over predicted locations and reference masks measured as the mean intersection over union (IoU), calculated using the standard formula:
where:
is a set of pixels covered by the predicted bounding box region;
is a set of pixels covered by the bounding box of the reference location mask;
is a function that calculates the pixel-wise area of a given region.
The choice to use bounding boxes for calculating the IoU instead of pixel-wise IoU calculation is motivated by the fact that some of the localization methods applied for the models result only in rough location areas with a fairly small resolution (e.g., 13 × 13); hence, pixel-wise evaluation would not be reliable. Bounding boxes are less vulnerable to the low resolution of the localization; therefore, they enable unified evaluation of all of the presented localization methods. An example of bounding boxes for a reference mask and location prediction is presented in
Figure 6.
For each variant evaluated, the mean IoU was calculated on all images in the test set and for three × five models of the given variant, which were the three models that performed the best in each of the five cross-validation folds. For each of the variants, 15 models were evaluated in terms of localization accuracy.
5.3.2. Tested Approaches
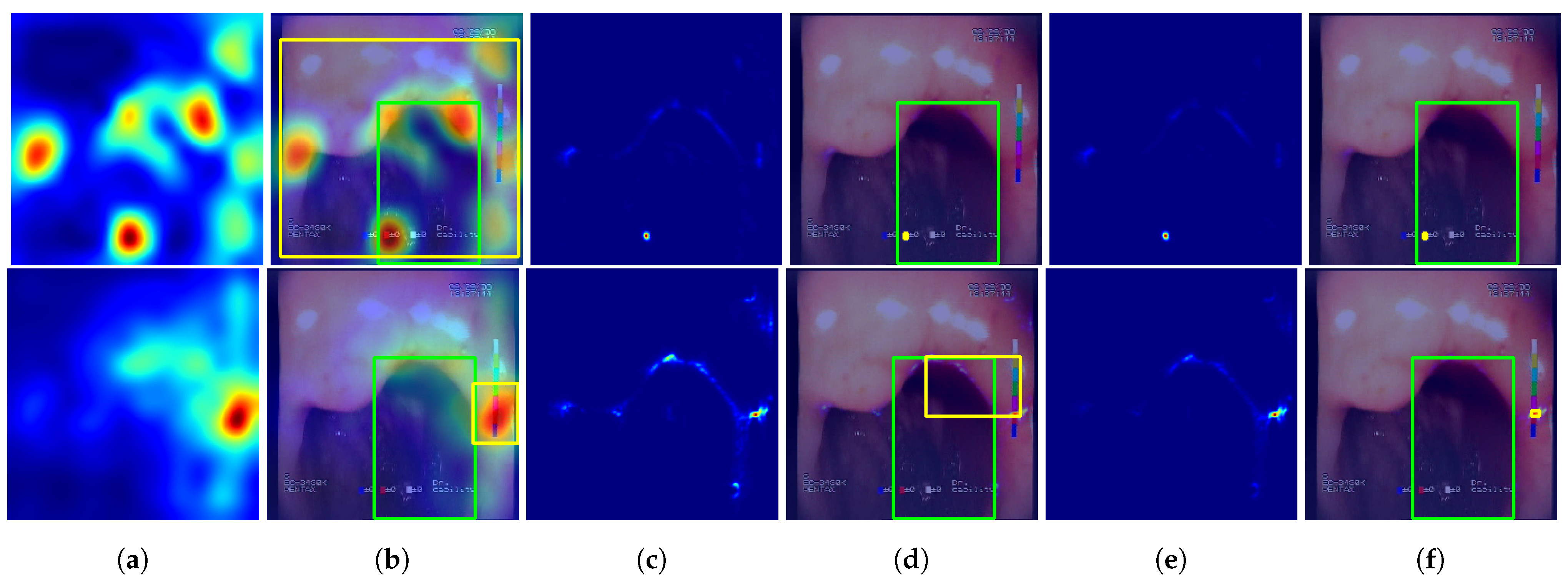
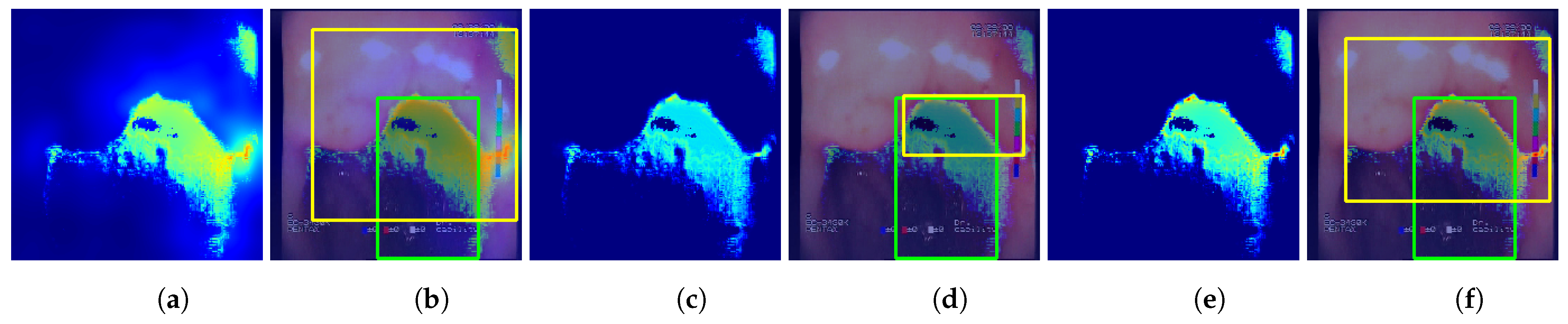
The following methods for generating location maps from the base and feature-extended models were used and evaluated against reference masks and respective bounding boxes. Sample results for the methods are presented in
Figure 7 and
Figure 8.
—Grad-Cam method [
46], postprocessed using function
p that performs normalization of the activation map to 0–1 range and thresholding at 0.5 value.
—Guided Backpropagation method [
47], postprocessed with
p.
—Guided Grad-Cam method [
48], postprocessed with
p.
—location map generated using the proposed features, created by averaging activations of features F1 to F7, postprocessed with p. This method of localization is entirely based on the designed feature descriptors and, in contrast to the GC, GP, and GGC methods, it does not involve inference of the neural network.
—proposed combination of the and methods by averaging the 0-1 normalized results of both methods, with the final result postprocessed with p.
—proposed combination of the and methods by averaging the 0-1 normalized results of both methods, with the final result postprocessed with p.
—proposed combination of the and methods by averaging the 0-1 normalized results of both methods, with the final result postprocessed with p.
5.3.3. Localization Results
The presented localization methods were evaluated for the VGG19, Resnet50, and Inception neural network architectures for both the base and feature-extended model variants. The Resnet152 architecture was excluded due to its incompatibility with the available software libraries for the
,
, and
methods. In contrast to the evaluations presented in the previous sections, where various configurations of model ensembles were used, in this section, single models (each single model is a single convolutional neural network trained for one of the folds of the five-fold cross-validation) are evaluated separately, and the results are averaged over all single models. An evaluation is conducted for the top three single models for each of the five folds of the cross-validation training, resulting in a total of 15 models per architecture and per variant (baseline or feature-extended). The results are presented in
Table 10.
Feature-extended models achieved higher results for localizations, while for and localization, baseline models performed better. However, combining location predictions with improved the results for the three methods. Additionally, the localization itself achieved a mean IoU = 0.517, which is significantly higher than the , , and methods for any of the models evaluated. Finally, the highest mean IoU value, equal to 0.529, was acquired for the method using the VGG19+F models.
5.4. Processing Performance
In the final part of the experiments, the processing performance of the models was evaluated in terms of model inference time. Baseline and feature-extended model variants were evaluated for the four architectures tested. The performance was evaluated for single models by calculating the mean processing time of a single image on all images from the test set (9361 images with a resolution of 720 × 576). To enable performance measurements, a dedicated mechanism was implemented that allowed measurements of the processing time of consecutive stages of the prediction process. Several processing stages, described in
Table 11, were identified and considered in the measurements.
Performance tests were conducted on a system equipped with a Ryzen Threadripper 1920X CPU (12-core/24-thread) 4.0 GHz, 64 GB RAM, Titan Xp GPU. Tests were executed separately for two variants: (1) using the CPU only, and (2) using the GPU for model prediction and the CPU for the remaining part of the processing. The results are presented in
Table 12 (CPU-only variant) and
Table 13 (CPU+GPU variant).
6. Discussion
The presented work investigated the possibility of extending convolutional neural networks with a set of proposed visual features to improve accuracy for the task of endoscopic bleeding detection in color images. A methodical approach was used to theoretically define appropriate visual features and implement computer-vision-based descriptors to capture the respective features. This led to the development of valuable, high-level visual features of endoscopic bleeding. In addition, a simple scheme for combining the features with convolutional neural networks was presented; hence, the proposed features can potentially be applied along with other modifications and improvements of neural network architectures or training algorithms to achieve additional classification accuracy gains. Finally, experiments were conducted to evaluate the actual performance of the feature-extended neural networks on a representative dataset of endoscopic images.
As a result of the study, an efficient and accurate endoscopic bleeding detection algorithm was developed, achieving an ROC AUC of 0.965 in an ensembled configuration. A set of experiments confirmed the positive impact of the proposed features on the ROC AUC results for three evaluated architectures: VGG19, Resnet50, and Resnet152, and in the case of a larger sample of trained models, also for Inception v3. As well as achieving an improvement in model accuracy in terms of the ROC AUC, advantages in other accuracy metrics were also presented, including F-score metrics and the performance in high-sensitivity and high-specificity operating points. The study also evaluated the ability of the models to localize the blood area in a weakly supervised setting. A potential improvement from the feature extension was demonstrated for one of the localization methods. Furthermore, the proposed visual feature descriptors were found to be accurate predictors of bleeding localization themselves.
The accuracy results acquired in the presented study, even though it was not oriented toward optimizing accuracy but instead focused on evaluating the impact of the proposed features, are competitive in regard to the methods presented in the literature. Although several studies reported significantly higher ROC AUC values, as presented in
Table 15, the direct comparison of methods is, however, difficult due to differences in the datasets used, the images sources (WC or traditional endoscopy), or the actual pathology detected (bleeding, active bleeding, angioectasia). Moreover, all of the listed approaches use private datasets, which are therefore not possible to review. The descriptions of the datasets often lack essential information, e.g., the number of included unique bleeding cases for which the positive images were extracted. A large, high-quality dataset was utilized by Kim et al. [
35], which justifies the high accuracy of the method. For other studies, in turn, the size of the dataset is very low. High-accuracy results reported on small datasets are often not validated reliably enough.
The proposed method of extending deep learning models with additional visual features can be facilitated to create new or improve the accuracy of existing bleeding detection algorithms. Such methods have important clinical applications, as they can be used to develop real-time assistant applications that would support physicians in traditional endoscopy by observing the image displayed by the device as a second reader, notifying them about detected occurrences of blood and therefore reducing the risk of overlooking such lesions. In the area of wireless capsule endoscopy, where long video recordings are reviewed by the physician after examination in a time-consuming and labor-intensive process, accurate automatic detection of blood occurrences is also highly demanded. The approach proposed in the study can also be used to shorten capsule endoscopy videos and therefore reduce the physician’s time required to review examinations, although the best results would be obtained when combined with algorithms dedicated to detecting other pathologies of the gastrointestinal tract.
Author Contributions
Conceptualization, A.B. and H.K.; methodology, A.B.; software, A.B.; validation, A.B.; formal analysis, A.B. and H.K; investigation, A.B.; resources, A.B. and T.D.; data curation, A.B. and T.D.; writing—original draft preparation, A.B. and T.D.; writing—review and editing, A.B., T.D. and H.K.; visualization, A.B.; supervision, H.K.; project administration, A.B.; funding acquisition, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by Pomorskie Voivodeship Regional Operational Program for 2014–2020, grant RPPM.01.02.00-22-0001/17, CK STOS.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of POIG.02.03.03-00-008/08 – “MAYDAY EURO 2012 Supercomputer platform for contextual analysis of multimedia data streams to identify specified objects or dangerous events”.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ciccarelli, E.; Jacobs, A.; Berman, P. Looking Back on the Millennium in Medicine. N. Engl. J. Med. 2000, 342, 42–49. [Google Scholar] [CrossRef]
- Liedlgruber, M.; Uhl, A. Computer-aided decision support systems for endoscopy in the gastrointestinal tract: A review. IEEE Rev. Biomed. Eng. 2011, 4, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Musha, A.; Hasnat, R.; Mamun, A.A.; Ping, E.P.; Ghosh, T. Computer-Aided Bleeding Detection Algorithms for Capsule Endoscopy: A Systematic Review. Sensors 2023, 23, 7170. [Google Scholar] [CrossRef] [PubMed]
- Vania, M.; Tama, B.A.; Maulahela, H.; Lim, S. Recent Advances in Applying Machine Learning and Deep Learning to Detect Upper Gastrointestinal Tract Lesions. IEEE Access 2023, 11, 66544–66567. [Google Scholar] [CrossRef]
- Du, W.; Rao, N.; Liu, D.; Jiang, H.; Luo, C.; Li, Z.; Gan, T.; Zeng, B. Review on the applications of deep learning in the analysis of gastrointestinal endoscopy images. IEEE Access 2019, 7, 142053–142069. [Google Scholar] [CrossRef]
- Soffer, S.; Klang, E.; Shimon, O.; Nachmias, N.; Eliakim, R.; Ben-Horin, S.; Kopylov, U.; Barash, Y. Deep learning for wireless capsule endoscopy: A systematic review and meta-analysis. Gastrointest. Endosc. 2020, 92, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Piccirelli, S.; Milluzzo, S.M.; Bizzotto, A.; Cesaro, P.; Pecere, S.; Spada, C. Small Bowel Capsule Endoscopy and artificial intelligence: First or second reader? Best Pract. Res. Clin. Gastroenterol. 2021, 52, 101742. [Google Scholar] [CrossRef]
- Trasolini, R.; Byrne, M.F. Artificial intelligence and deep learning for small bowel capsule endoscopy. Dig. Endosc. 2021, 33, 290–297. [Google Scholar] [CrossRef]
- Maroulis, D.E.; Iakovidis, D.K.; Karkanis, S.A.; Karras, D.A. CoLD: A versatile detection system for colorectal lesions in endoscopy video-frames. Comput. Methods Programs Biomed. 2003, 70, 151–166. [Google Scholar] [CrossRef]
- Magoulas, G.D.; Plagianakos, V.P.; Vrahatis, M.N. Neural network-based colonoscopic diagnosis using on-line learning and differential evolution. Appl. Soft Comput. 2004, 4, 369–379. [Google Scholar] [CrossRef][Green Version]
- Kodogiannis, V.; Boulougoura, M. An adaptive neurofuzzy approach for the diagnosis in wireless capsule endoscopy imaging. Int. J. Inf. Technol. 2007, 13, 127. [Google Scholar]
- Li, B.; Meng, M.H. Computer-Aided Detection of Bleeding Regions for Capsule Endoscopy Images. Biomed. Eng. IEEE Trans. 2009, 56, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Meng, M.Q.H. Automatic bleeding frame detection in the wireless capsule endoscopy images. In Proceedings of the 2015 IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, 26–30 May 2015; pp. 1310–1315. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, M.H. Polyp detection in wireless capsule endoscopy images using novel color texture features. In Proceedings of the Intelligent Control and Automation (WCICA), 2011 9th World Congress, Taipei, Taiwan, 21–25 June 2011; pp. 948–952. [Google Scholar]
- Kumar, R.; Zhao, Q.; Seshamani, S.; Mullin, G.; Hager, G.; Dassopoulos, T. Assessment of crohn’s disease lesions in wireless capsule endoscopy images. Biomed. Eng. IEEE Trans. 2012, 59, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Giritharan, B.; Yuan, X.; Liu, J.; Buckles, B.; Oh, J.; Tang, S.J. Bleeding detection from capsule endoscopy videos. In Proceedings of the Engineering in Medicine and Biology Society, 2008: EMBS 2008, Vancouver, BC, Canada, 20–25 August 2008; pp. 4780–4783. [Google Scholar]
- Khun, P.C.; Zhang, Z.; Liang, Z.; Li, L.; Liu, J. Feature selection and classification for Wireless Capsule Endoscopic frames. In Proceedings of the 2009 Biomedical and Pharmaceutical Engineering, Singapore, 2–4 December 2009; pp. 1–6. [Google Scholar] [CrossRef]
- Poh, C.K.; Htwe, T.M.; Li, L.; Shen, W.; Liu, J.; Lim, J.H.; Chan, K.L.; Tan, P.C. Multi-level local feature classification for bleeding detection in wireless capsule endoscopy images. In Proceedings of the Cybernetics and Intelligent Systems (CIS), Singapore, 28–30 June 2010; pp. 76–81. [Google Scholar]
- Yeh, J.Y.; Wu, T.H.; Tsai, W.J. Bleeding and Ulcer Detection Using Wireless Capsule Endoscopy Images. J. Softw. Eng. Appl. 2014, 7, 422. [Google Scholar] [CrossRef]
- Szczypiński, P.; Klepaczko, A.; Pazurek, M.; Daniel, P. Texture and color based image segmentation and pathology detection in capsule endoscopy videos. Comput. Methods Programs Biomed. 2014, 113, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, D. Computer-Assisted Diagnosis of Digestive Endoscopic Images Based on Bayesian Theory. In Proceedings of the Information Engineering and Computer Science, Wuhan, China, 19–20 December 2009; pp. 1–4. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.; Gao, F.; Wan, L.; Yu, J. Convolutional neural networks for intestinal hemorrhage detection in wireless capsule endoscopy images. In Proceedings of the 2017 IEEE International Conference on Multimedia and Expo (ICME), Hong Kong, China, 10–14 July 2017; pp. 1518–1523. [Google Scholar]
- Li, X.; Zhang, H.; Zhang, X.; Liu, H.; Xie, G. Exploring transfer learning for gastrointestinal bleeding detection on small-size imbalanced endoscopy images. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 1994–1997. [Google Scholar]
- Tsuboi, A.; Oka, S.; Aoyama, K.; Saito, H.; Aoki, T.; Yamada, A.; Matsuda, T.; Fujishiro, M.; Ishihara, S.; Nakahori, M.; et al. Artificial intelligence using a convolutional neural network for automatic detection of small-bowel angioectasia in capsule endoscopy images. Dig. Endosc. 2020, 32, 382–390. [Google Scholar] [CrossRef]
- Aoki, T.; Yamada, A.; Kato, Y.; Saito, H.; Tsuboi, A.; Nakada, A.; Niikura, R.; Fujishiro, M.; Oka, S.; Ishihara, S.; et al. Automatic detection of various abnormalities in capsule endoscopy videos by a deep learning-based system: A multicenter study. Gastrointest. Endosc. 2021, 93, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Caroppo, A.; Leone, A.; Siciliano, P. Deep transfer learning approaches for bleeding detection in endoscopy images. Comput. Med. Imaging Graph. 2021, 88, 101852. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Chakareski, J. Deep transfer learning for automated intestinal bleeding detection in capsule endoscopy imaging. J. Digit. Imaging 2021, 34, 404–417. [Google Scholar] [CrossRef]
- Saraiva, M.M.; Ribeiro, T.; Afonso, J.; Ferreira, J.P.; Cardoso, H.; Andrade, P.; Parente, M.P.; Jorge, R.N.; Macedo, G. Artificial intelligence and capsule endoscopy: Automatic detection of small bowel blood content using a convolutional neural network. GE-Port. J. Gastroenterol. 2022, 29, 331–338. [Google Scholar]
- Garbaz, A.; Lafraxo, S.; Charfi, S.; El Ansari, M.; Koutti, L. Bleeding classification in wireless capsule endoscopy images based on inception-resnet-v2 and cnns. In Proceedings of the 2022 IEEE Conference on Computational Intelligence in Bioinformatics and Computational Biology (CIBCB), Ottawa, ON, Canada, 15–17 August 2022; pp. 1–6. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the inception architecture for computer vision. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 2818–2826. [Google Scholar]
- Liu, W.; Anguelov, D.; Erhan, D.; Szegedy, C.; Reed, S.; Fu, C.Y.; Berg, A.C. Ssd: Single shot multibox detector. In Proceedings of the Computer Vision–ECCV 2016: 14th European Conference, Amsterdam, The Netherlands, 11–14 October 2016; pp. 21–37. [Google Scholar]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. In Proceedings of the Advances in Neural Information Processing Systems, Lake Tahoe, CA, USA, 3–8 December 2012; pp. 1097–1105. [Google Scholar]
- Kim, S.H.; Hwang, Y.; Oh, D.J.; Nam, J.H.; Kim, K.B.; Park, J.; Song, H.J.; Lim, Y.J. Efficacy of a comprehensive binary classification model using a deep convolutional neural network for wireless capsule endoscopy. Sci. Rep. 2021, 11, 17479. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Khosla, A.; Lapedriza, A.; Oliva, A.; Torralba, A. Learning deep features for discriminative localization. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 2921–2929. [Google Scholar]
- Jia, X.; Meng, M.Q.H. Gastrointestinal bleeding detection in wireless capsule endoscopy images using handcrafted and CNN features. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 3154–3157. [Google Scholar]
- Fu, Y.; Zhang, W.; Mandal, M.; Meng, M.H. Computer-Aided Bleeding Detection in WCE Video. Biomed. Health Inform. IEEE J. 2014, 18, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Mandal, M.; Guo, G. Bleeding region detection in WCE images based on color features and neural network. In Proceedings of the Circuits and Systems (MWSCAS), 2011 IEEE 54th International Midwest Symposium, Seoul, Republic of Korea, 7–10 August 2011; pp. 1–4. [Google Scholar] [CrossRef]
- Canziani, A.; Paszke, A.; Culurciello, E. An analysis of deep neural network models for practical applications. arXiv 2017, arXiv:1605.07678. [Google Scholar]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. ImageNet Large Scale Visual Recognition Challenge. Int. J. Comput. Vis. (IJCV) 2015, 115, 211–252. [Google Scholar] [CrossRef]
- Hu, J.; Shen, L.; Sun, G. Squeeze-and-excitation networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 7132–7141. [Google Scholar]
- Szegedy, C.; Ioffe, S.; Vanhoucke, V.; Alemi, A. Inception-v4, inception-resnet and the impact of residual connections on learning. In Proceedings of the AAAI Conference on Artificial Intelligence, San Francisco, CA, USA, 4–9 February 2017; Volume 31. [Google Scholar]
- Qian, N. On the momentum term in gradient descent learning algorithms. Neural Netw. 1999, 12, 145–151. [Google Scholar] [CrossRef]
- Cychnerski, J.; Dziubich, T.; Brzeski, A. ERS: A novel comprehensive endoscopy image dataset for machine learning, compliant with the MST 3.0 specification. arXiv 2022, arXiv:2201.08746. [Google Scholar]
- Selvaraju, R.R.; Das, A.; Vedantam, R.; Cogswell, M.; Parikh, D.; Batra, D. Grad-CAM: Why did you say that? arXiv 2016, arXiv:1611.07450. [Google Scholar]
- Springenberg, J.T.; Dosovitskiy, A.; Brox, T.; Riedmiller, M. Striving for simplicity: The all convolutional net. arXiv 2014, arXiv:1412.6806. [Google Scholar]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-cam: Visual explanations from deep networks via gradient-based localization. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017; pp. 618–626. [Google Scholar]
Figure 1.
Illustration of the proposed approach including combining feature channels with a convolutional neural network. RGB color input image of 720 × 576 resolution is expected (images of different resolutions are resized, respectively). The input image is processed separately by the 9 proposed feature descriptors, each producing a 2D single-channel feature activation map (720 × 576 resolution). All feature maps combined form a tensor of 720 × 576 × 9 size, which is concatenated with original RGB channels (720 × 576 × 3), resulting in a combined output of a size of 720 × 576 × 12. The spatial resolution of the resulting tensor is finally rescaled to a size of 224 × 224 × 12 to match the typical input size of CNNs and passed as input to the model to perform the prediction (or a training step).
Figure 1.
Illustration of the proposed approach including combining feature channels with a convolutional neural network. RGB color input image of 720 × 576 resolution is expected (images of different resolutions are resized, respectively). The input image is processed separately by the 9 proposed feature descriptors, each producing a 2D single-channel feature activation map (720 × 576 resolution). All feature maps combined form a tensor of 720 × 576 × 9 size, which is concatenated with original RGB channels (720 × 576 × 3), resulting in a combined output of a size of 720 × 576 × 12. The spatial resolution of the resulting tensor is finally rescaled to a size of 224 × 224 × 12 to match the typical input size of CNNs and passed as input to the model to perform the prediction (or a training step).
Figure 2.
Example of a feature activation map generated by the F1 feature descriptor, along with the automatically generated ROI (region of interest) mask.
Figure 2.
Example of a feature activation map generated by the F1 feature descriptor, along with the automatically generated ROI (region of interest) mask.
Figure 3.
Feature activation maps generated for a sample image.
Figure 3.
Feature activation maps generated for a sample image.
Figure 4.
ROC curves of the best single ensembles (ensembles of 5 models—one per cross-validation fold) for each of the evaluated architectures.
Figure 4.
ROC curves of the best single ensembles (ensembles of 5 models—one per cross-validation fold) for each of the evaluated architectures.
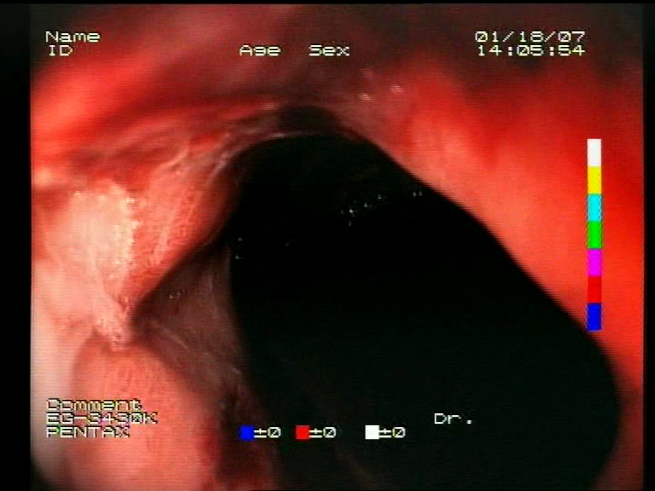
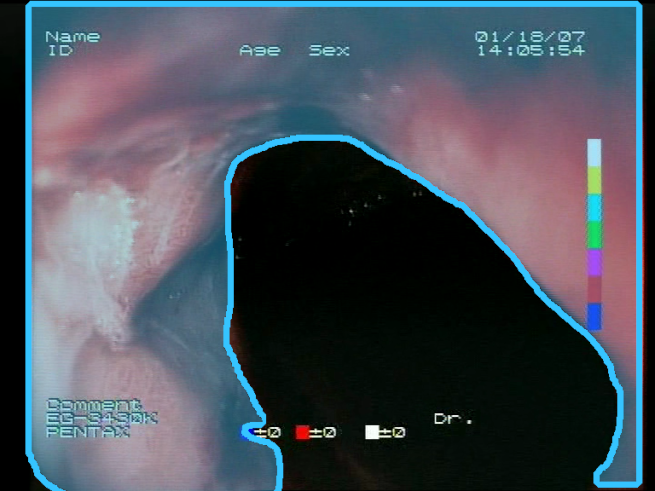
Figure 5.
Sample test set image and the associated blood area mask.
Figure 5.
Sample test set image and the associated blood area mask.
Figure 6.
Sample prediction of blood area location acquired from the proposed feature descriptors (): (a) input image; (b) reference mask with its bounding box (green); (c) location prediction with its bounding box (yellow) and reference mask bounding box (green). Resulting IoU is 0.327.
Figure 6.
Sample prediction of blood area location acquired from the proposed feature descriptors (): (a) input image; (b) reference mask with its bounding box (green); (c) location prediction with its bounding box (yellow) and reference mask bounding box (green). Resulting IoU is 0.327.
Figure 7.
Sample predictions of the evaluated localization methods. First row—VGG19 #1 (single fold) model results, second row—VGG19+F #1 (single fold) results. Columns: (a) non-postprocessed output; (b) non-postprocessed output visualized over original image, with yellow rectangle presenting the bounding box of final result (postprocessed) and green rectangle presenting the bounding box of the reference mask; (c) non-postprocessed output; (d) non-postprocessed output visualized over original image with bounding boxes; (e) non-postprocessed output; (f) non-postprocessed output visualized over original image with bounding boxes.
Figure 7.
Sample predictions of the evaluated localization methods. First row—VGG19 #1 (single fold) model results, second row—VGG19+F #1 (single fold) results. Columns: (a) non-postprocessed output; (b) non-postprocessed output visualized over original image, with yellow rectangle presenting the bounding box of final result (postprocessed) and green rectangle presenting the bounding box of the reference mask; (c) non-postprocessed output; (d) non-postprocessed output visualized over original image with bounding boxes; (e) non-postprocessed output; (f) non-postprocessed output visualized over original image with bounding boxes.
Figure 8.
Sample predictions of the localization methods utilizing the for the VGG19+F #1 model (single fold). Columns: (a) non-postprocessed output; (b) non-postprocessed output visualized on original image with bounding boxes; (c) non-postprocessed output; (d) non-postprocessed output visualized on original image with bounding boxes; (e) non-postprocessed output; (f) non-postprocessed output visualized over original image with bounding boxes.
Figure 8.
Sample predictions of the localization methods utilizing the for the VGG19+F #1 model (single fold). Columns: (a) non-postprocessed output; (b) non-postprocessed output visualized on original image with bounding boxes; (c) non-postprocessed output; (d) non-postprocessed output visualized on original image with bounding boxes; (e) non-postprocessed output; (f) non-postprocessed output visualized over original image with bounding boxes.
Table 1.
Definitions of the nine proposed visual features of endoscopic bleeding. For each image, a blue annotation is presented, which illustrates the area of the image where the feature is hypothetically present.
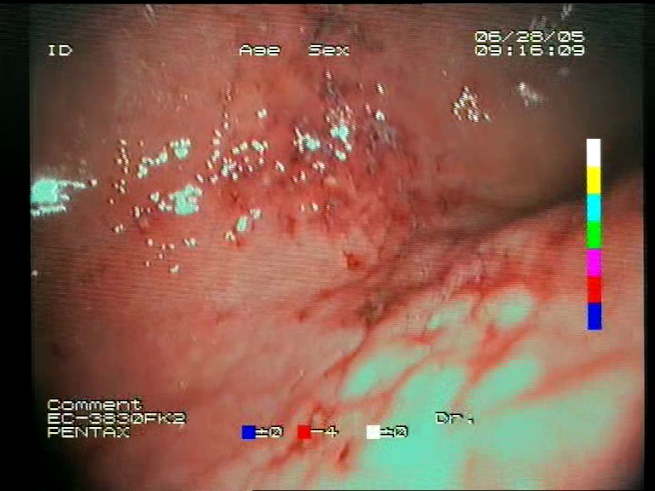
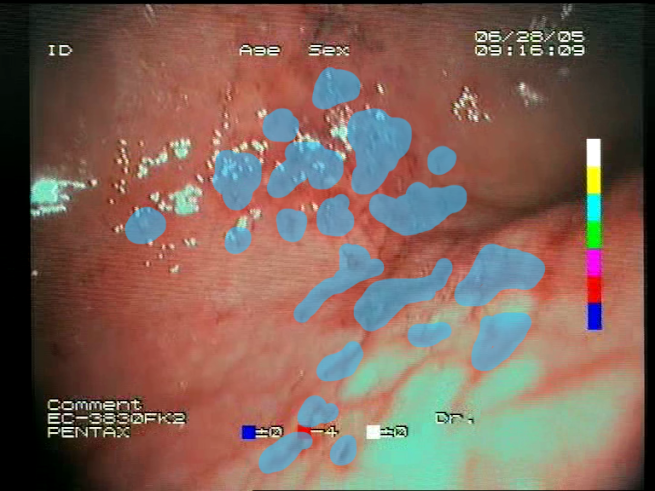
Table 1.
Definitions of the nine proposed visual features of endoscopic bleeding. For each image, a blue annotation is presented, which illustrates the area of the image where the feature is hypothetically present.
| Id | Image | Feature Area | Definition |
|---|
| F1 | ![Sensors 23 09717 i001 Sensors 23 09717 i001]() | ![Sensors 23 09717 i002 Sensors 23 09717 i002]() | Blood color region—Most of the bleeding cases appear as regions of characteristic shades of red color typical of intensive bleeding or fresh blood. Therefore, color information is the strongest clue to detect bleeding. Blood color can be defined as a narrow range in a certain color space that is often present in bleeding regions and, at the same time, rarely occurs in non-blood images. The detected area must be of significant size, exceeding approximately 1% of the clearly visible part of the image. |
| F2 | ![Sensors 23 09717 i003 Sensors 23 09717 i003]() | ![Sensors 23 09717 i004 Sensors 23 09717 i004]() | Blood color region with a smooth surface—In the case where the blood region is present, the occurrence of a significant amount of blood is probable. In that case, blood can fully cover the surface of the organ. This results in the appearance of a compact, smooth blood surface. The feature is considered present if at least one of the regions detected by the blood region feature has a smooth surface. |
| F3 | ![Sensors 23 09717 i005 Sensors 23 09717 i005]() | ![Sensors 23 09717 i006 Sensors 23 09717 i006]() | The blood region has a clear boundary—A fairly common characteristic of blood regions is a clear and sharp boundary separating the blood area from the normal tissue. The feature is considered present if at least a certain part of the blood region’s boundary has a clear edge separating it from the adjacent area. |
| F4 | ![Sensors 23 09717 i007 Sensors 23 09717 i007]() | ![Sensors 23 09717 i008 Sensors 23 09717 i008]() | The red color region—Some of the bleeding cases appear as regions of a shade of red, which is less typical for blood and also appears commonly in non-blood images. To capture these cases, the feature is supposed to identify red regions similarly to the blood region feature, but with a wider range of shades of red being accepted. |
| F5 | ![Sensors 23 09717 i009 Sensors 23 09717 i009]() | ![Sensors 23 09717 i010 Sensors 23 09717 i010]() | Red color region with a smooth surface—The feature is related to the previous feature and has a similar motivation as the smooth blood region feature (F2). The parameters of the expected descriptor might, however, be changed as a result of tuning towards specificity of red regions. |
| F6 | ![Sensors 23 09717 i011 Sensors 23 09717 i011]() | ![Sensors 23 09717 i012 Sensors 23 09717 i012]() | The red region has a clear boundary—The feature is the equivalent of a clear boundary feature (F3) for the red color region feature. Similarly to the previous feature, some descriptor parameters may differ due to adjustments made to the red regions. |
| F7 | ![Sensors 23 09717 i013 Sensors 23 09717 i013]() | ![Sensors 23 09717 i014 Sensors 23 09717 i014]() | Dispersed blood color—Small amounts of blood can appear as small, spread areas, spots, or thin blood streams. In contrast to the blood region feature, large regions are ignored here, and the color is validated on a pixel level. However, some larger areas of blood contaminated by other fluids or bubbles can also be detected because they do not form continuous areas of blood color. Again, a narrow range of red color needs to be defined to describe blood. |
| F8 | ![Sensors 23 09717 i015 Sensors 23 09717 i015]() | ![Sensors 23 09717 i016 Sensors 23 09717 i016]() | Red color domination—An additional clue for analyzing the image is provided by the assessment of a dominant color in the image. The feature is considered present if the dominant color of the image is red; that is, the red color covers more than 50% of the image, excluding unclear parts of the image resulting from highlights or darkness. The red color domination is considered to be an excess of the red component in the RGB color space, allowing a wide range of shades of red. |
| F9 | ![Sensors 23 09717 i017 Sensors 23 09717 i017]() | ![Sensors 23 09717 i018 Sensors 23 09717 i018]() | Image is blurred—Blurriness significantly affects image analysis, hindering the identification of bleeding areas. It also affects the evaluation of the remaining features. Therefore, the blurriness of the image is also evaluated as a feature. Blurriness can be evaluated by detecting edges in the image and measuring the variance in the colors in the image. High variance with a low number of edges is an indication of possible blurriness. |
Table 2.
Statistics of the ERS dataset used in the experiments, including number of endoscopic examinations videos, number of samples extracted from the videos, and total images available in all the extracted samples.
Table 2.
Statistics of the ERS dataset used in the experiments, including number of endoscopic examinations videos, number of samples extracted from the videos, and total images available in all the extracted samples.
| Set | Class | Endoscopic Examinations | Endoscopic Samples | Endoscopic Images |
|---|
| Training | blood | 56 | 58 | 22,545 |
| non-blood | 226 | 331 | 17,212 |
| Test | blood | 19 | 19 | 6912 |
| non-blood | 76 | 113 | 7461 |
| Total | blood | 75 | 77 | 29,457 |
| non-blood | 302 | 444 | 24,673 |
Table 3.
Classification accuracy metrics used in the evaluation.
Table 3.
Classification accuracy metrics used in the evaluation.
| Metric | hlDefinition |
|---|
| area under the receiver operating characteristic curve |
| |
| |
| |
| |
| |
| |
| calculated for the classification threshold resulting in value equal to 0.01 |
| for equal to 0.001 |
| for equal to 0.0001 |
| for equal to 0.95 |
| for equal to 0.99 |
| for equal to 0.999 |
Table 4.
Additional terms used in the evaluation metrics.
Table 4.
Additional terms used in the evaluation metrics.
| Metric | Definition |
|---|
| number of correctly classified blood cases, calculated at 0.5 classification threshold |
| number of incorrectly classified blood cases at the 0.5 threshold |
| number of correctly classified normal cases at the 0.5 threshold |
| number of incorrectly classified normal cases at the 0.5 threshold |
| equivalent to |
| equivalent to |
| |
Table 5.
The best single ensemble (denoted as #1—ensembles of 5 folds in each case) results for baseline (base) and feature-extended variants (+F). For the VGG19 and Resnet152 architectures, the feature-extended ensembles reached a higher ROC AUC. Overall accuracy was also better, although the advantage is not clear. For Resnet50, the feature-extended ensemble reached a significantly higher ROC AUC and also had a clearly higher overall accuracy. In the case of the Inception architecture, the baseline ensemble achieved a higher ROC AUC than the feature-extended ensemble, and the overall accuracy was higher for the baseline ensemble.
Table 5.
The best single ensemble (denoted as #1—ensembles of 5 folds in each case) results for baseline (base) and feature-extended variants (+F). For the VGG19 and Resnet152 architectures, the feature-extended ensembles reached a higher ROC AUC. Overall accuracy was also better, although the advantage is not clear. For Resnet50, the feature-extended ensemble reached a significantly higher ROC AUC and also had a clearly higher overall accuracy. In the case of the Inception architecture, the baseline ensemble achieved a higher ROC AUC than the feature-extended ensemble, and the overall accuracy was higher for the baseline ensemble.
| Metric | VGG19 | Resnet50 | Resnet152 | Inception |
|---|
| ×5 Folds #1 | ×5 Folds #1 | ×5 Folds #1 | ×5 Folds #1 |
| Base | +F | Base | +F | Base | +F | Base | +F |
| 0.891 | 0.959 | 0.918 | 0.963 | 0.929 | 0.936 | 0.954 | 0.942 |
| 0.762 | 0.755 | 0.684 | 0.865 | 0.758 | 0.720 | 0.797 | 0.776 |
| 0.785 | 0.854 | 0.713 | 0.888 | 0.751 | 0.801 | 0.863 | 0.786 |
| 0.801 | 0.936 | 0.734 | 0.904 | 0.746 | 0.867 | 0.914 | 0.794 |
| 0.923 | 0.862 | 0.895 | 0.953 | 0.943 | 0.862 | 0.903 | 0.936 |
| 0.726 | 0.633 | 0.640 | 0.829 | 0.770 | 0.615 | 0.707 | 0.759 |
| 0.898 | 0.877 | 0.862 | 0.943 | 0.903 | 0.863 | 0.906 | 0.907 |
| 0.561 | 0.622 | 0.513 | 0.667 | 0.591 | 0.522 | 0.583 | 0.606 |
| 0.341 | 0.226 | 0.138 | 0.251 | 0.131 | 0.171 | 0.184 | 0.198 |
| 0.068 | 0.010 | 0.025 | 0.122 | 0.005 | 0.094 | 0.020 | 0.004 |
| 0.286 | 0.772 | 0.649 | 0.855 | 0.568 | 0.775 | 0.764 | 0.743 |
| 0.088 | 0.295 | 0.254 | 0.215 | 0.331 | 0.155 | 0.191 | 0.327 |
| 0.008 | 0.028 | 0.007 | 0.003 | 0.012 | 0.017 | 0.034 | 0.063 |
Table 6.
Mean metric values calculated for 15 base VGG19 and 15 feature-extended single ensembles.
Table 6.
Mean metric values calculated for 15 base VGG19 and 15 feature-extended single ensembles.
| Metric | VGG19 ×5 Folds #1–15 | VGG19+F ×5 Folds #1–15 |
|---|
| Mean | STD | Mean | STD |
| 0.923 | 0.018 | 0.962 | 0.004 |
| 0.778 | 0.014 | 0.801 | 0.022 |
| 0.783 | 0.014 | 0.870 | 0.009 |
| 0.787 | 0.018 | 0.924 | 0.006 |
| 0.940 | 0.008 | 0.902 | 0.018 |
| 0.769 | 0.024 | 0.708 | 0.035 |
| 0.909 | 0.007 | 0.906 | 0.013 |
| 0.579 | 0.039 | 0.627 | 0.027 |
| 0.315 | 0.038 | 0.271 | 0.053 |
| 0.079 | 0.041 | 0.038 | 0.073 |
| 0.566 | 0.171 | 0.815 | 0.037 |
| 0.146 | 0.050 | 0.323 | 0.053 |
| 0.027 | 0.022 | 0.053 | 0.039 |
Table 7.
Mean metric values calculated for 15 base Resnet50 and 15 feature-extended single ensembles.
Table 7.
Mean metric values calculated for 15 base Resnet50 and 15 feature-extended single ensembles.
| Metric | Resnet50 ×5 Folds #1–15 | Resnet50+F ×5 Folds #1–15 |
|---|
| Mean | STD | Mean | STD |
| 0.913 | 0.012 | 0.953 | 0.007 |
| 0.705 | 0.033 | 0.813 | 0.030 |
| 0.751 | 0.021 | 0.824 | 0.032 |
| 0.786 | 0.031 | 0.833 | 0.049 |
| 0.886 | 0.032 | 0.944 | 0.028 |
| 0.643 | 0.061 | 0.800 | 0.063 |
| 0.865 | 0.023 | 0.922 | 0.017 |
| 0.422 | 0.040 | 0.582 | 0.069 |
| 0.141 | 0.066 | 0.195 | 0.062 |
| 0.026 | 0.032 | 0.062 | 0.039 |
| 0.588 | 0.078 | 0.796 | 0.048 |
| 0.211 | 0.088 | 0.182 | 0.085 |
| 0.013 | 0.012 | 0.009 | 0.012 |
Table 8.
Mean metric values calculated for 15 base Resnet152 and 15 feature-extended single ensembles.
Table 8.
Mean metric values calculated for 15 base Resnet152 and 15 feature-extended single ensembles.
| Metric | Resnet152 ×5 Folds #1–15 | Resnet152+F ×5 Folds #1–15 |
|---|
| Mean | STD | Mean | STD |
| 0.935 | 0.013 | 0.946 | 0.007 |
| 0.760 | 0.027 | 0.787 | 0.029 |
| 0.785 | 0.026 | 0.813 | 0.034 |
| 0.803 | 0.038 | 0.833 | 0.055 |
| 0.920 | 0.024 | 0.927 | 0.029 |
| 0.724 | 0.051 | 0.753 | 0.064 |
| 0.896 | 0.016 | 0.908 | 0.017 |
| 0.508 | 0.052 | 0.616 | 0.058 |
| 0.156 | 0.071 | 0.193 | 0.078 |
| 0.044 | 0.043 | 0.049 | 0.059 |
| 0.641 | 0.142 | 0.747 | 0.062 |
| 0.257 | 0.076 | 0.147 | 0.041 |
| 0.014 | 0.015 | 0.008 | 0.014 |
Table 9.
Mean metric values calculated for 15 base Inception-v3 and 15 feature-extended single ensembles.
Table 9.
Mean metric values calculated for 15 base Inception-v3 and 15 feature-extended single ensembles.
| Metric | Inception ×5 Folds #1–15 | Inception+F ×5 Folds #1–15 |
|---|
| Mean | STD | Mean | STD |
| 0.947 | 0.007 | 0.956 | 0.010 |
| 0.782 | 0.025 | 0.806 | 0.028 |
| 0.784 | 0.042 | 0.817 | 0.033 |
| 0.788 | 0.064 | 0.825 | 0.043 |
| 0.942 | 0.027 | 0.943 | 0.015 |
| 0.785 | 0.067 | 0.789 | 0.044 |
| 0.910 | 0.014 | 0.919 | 0.012 |
| 0.540 | 0.054 | 0.618 | 0.057 |
| 0.185 | 0.074 | 0.226 | 0.076 |
| 0.048 | 0.049 | 0.059 | 0.041 |
| 0.734 | 0.066 | 0.809 | 0.084 |
| 0.294 | 0.055 | 0.312 | 0.064 |
| 0.032 | 0.034 | 0.043 | 0.039 |
Table 10.
Mean IoU values acquired for bleeding area localization for each architecture, variant (baseline—“base”; feature-extended—“+F”) and localization method, averaged over all 25 images from the test set and 15 (top 3 × 5 folds) models. “-” indicates that the given method was not evaluated. Mean IoU value of localization map itself achieved the value of 0.517.
Table 10.
Mean IoU values acquired for bleeding area localization for each architecture, variant (baseline—“base”; feature-extended—“+F”) and localization method, averaged over all 25 images from the test set and 15 (top 3 × 5 folds) models. “-” indicates that the given method was not evaluated. Mean IoU value of localization map itself achieved the value of 0.517.
| Method | VGG19 | Resnet50 | Inception |
|---|
| Base | +F | Base | +F | Base | +F |
| 0.423 | 0.396 | 0.369 | 0.369 | 0.381 | 0.334 |
| - | 0.529 | - | 0.497 | - | 0.485 |
| 0.203 | 0.266 | 0.223 | 0.231 | 0.176 | 0.249 |
| - | 0.387 | - | 0.394 | - | 0.398 |
| 0.154 | 0.148 | 0.182 | 0.179 | 0.156 | 0.217 |
| - | 0.298 | - | 0.340 | - | 0.374 |
Table 11.
Processing stages of the complete algorithm evaluated in the performance tests.
Table 11.
Processing stages of the complete algorithm evaluated in the performance tests.
| Stage | Definition |
|---|
| Preparation of the input image for the actual processing, including deinterlacing and resizing using cubic interpolation. |
| Additional preprocessing step required by the proposed feature descriptors, including detection of the area of the image presenting the actual organ (that is, excluding black border and overlay information printed in the image by the endoscopic device). |
| – | Calculation of each of the F1–F9 feature planes using the corresponding feature descriptor. |
| Total processing time of all feature descriptors (excluding detection). |
| Inference process of the neural network model. |
| Total processing time of a single endoscopic image. |
Table 12.
Single model inference mean processing time in milliseconds for a CPU-only system. “-” denotes stages that are not present in the baseline (“base”) variants.
Table 12.
Single model inference mean processing time in milliseconds for a CPU-only system. “-” denotes stages that are not present in the baseline (“base”) variants.
| Stage | VGG19 | Resnet50 | Resnet152 | Inception |
|---|
| Base | +F | Base | +F | Base | +F | Base | +F |
| 1.14 | 1.42 | 1.18 | 1.43 | 1.17 | 1.43 | 1.28 | 1.45 |
| - | 13.75 | - | 14.18 | - | 14.15 | - | 14.29 |
| - | 3.95 | - | 4.04 | - | 4.36 | - | 4.26 |
| - | 1.74 | - | 1.78 | - | 1.82 | - | 1.80 |
| - | 1.83 | - | 1.84 | - | 1.89 | - | 1.86 |
| - | 3.82 | - | 3.83 | - | 3.91 | - | 4.01 |
| - | 1.71 | - | 1.72 | - | 1.78 | - | 1.72 |
| - | 1.82 | - | 1.81 | - | 1.87 | - | 1.84 |
| - | 0.13 | - | 0.14 | - | 0.15 | - | 0.14 |
| - | 0.77 | - | 0.78 | - | 0.80 | - | 0.78 |
| - | 1.34 | - | 1.34 | - | 1.39 | - | 1.34 |
| - | 31.30 | - | 31.90 | - | 32.56 | - | 32.46 |
| 181.84 | 183.18 | 87.82 | 89.52 | 231.13 | 233.41 | 57.82 | 57.94 |
| 183.25 | 217.71 | 89.31 | 124.90 | 232.60 | 269.29 | 59.41 | 93.86 |
Table 13.
Single model inference mean processing time in miliseconds for a CPU+GPU system, where stage is performed using GPU, while the remaining stages are performed on CPU. “-” denotes stages that are not present in the baseline (“base”) variants.
Table 13.
Single model inference mean processing time in miliseconds for a CPU+GPU system, where stage is performed using GPU, while the remaining stages are performed on CPU. “-” denotes stages that are not present in the baseline (“base”) variants.
| Stage | VGG19 | Resnet50 | Resnet152 | Inception |
|---|
| Base | +F | Base | +F | Base | +F | Base | +F |
| 1.35 | 1.44 | 1.30 | 1.45 | 1.22 | 1.48 | 1.30 | 1.58 |
| - | 13.52 | - | 13.90 | - | 13.83 | - | 14.11 |
| - | 30.68 | - | 31.47 | - | 31.53 | - | 31.73 |
| 9.03 | 11.34 | 19.13 | 20.75 | 51.30 | 55.30 | 29.74 | 31.34 |
| 10.67 | 45.16 | 20.73 | 55.47 | 52.82 | 90.07 | 31.34 | 66.52 |
Table 14.
Relative overhead in inference time of feature-extended models in comparison to baseline models for different model and system variants.
Table 14.
Relative overhead in inference time of feature-extended models in comparison to baseline models for different model and system variants.
| Model Variant | System Variant | VGG19 | Resnet50 | Resnet152 | Inception |
|---|
| single model | cpu | +18.8% | +39.9% | +15.8% | +58.0% |
| ×5 fold | +5.7% | +12.5% | +5.0% | +16.4% |
| ensemble ×3 | +2.4% | +5.4% | +2.3% | +5.6% |
| single model | cpu+gpu | +323.1% | +167.6% | +70.5% | +112.3% |
| ×5 fold | +120.0% | +55.3% | +25.5% | +36.1% |
| ensemble ×3 | +57.6% | +24.2% | +13.7% | +15.7% |
Table 15.
Comparison of the presented study results of ROC AUC values reported in the literature.
Table 15.
Comparison of the presented study results of ROC AUC values reported in the literature.
| Method | Year | Dataset | Total Positive (Blood) Images | Image Source | Lesion Type | ROC AUC |
|---|
| Li et al. [23] | 2017 | Private dataset | 185 | WCE | Bleeding | 0.991 |
| Tsuboi et al. [24] | 2019 | Private dataset | 2725 | WCE | Angioectasia | 0.998 |
| Aoki et al. [25] | 2021 | Private dataset | 2237 + 29 videos | WCE | Angioectasia | 0.871 |
| Kim et al. [35] | 2021 | Private dataset | 164,713 | WCE | Bleeding | 0.922–0.998 |
| This study | 2023 | ERS [45] | 29,457 | Traditional endoscopy | Bleeding | 0.965 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).