Effects of Artificially Induced Leg Length Discrepancy on Treadmill-Based Walking and Running Symmetry in Healthy College Students: A Lab-Based Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Musculoskeletal Assessment
2.3. Testing Procedure
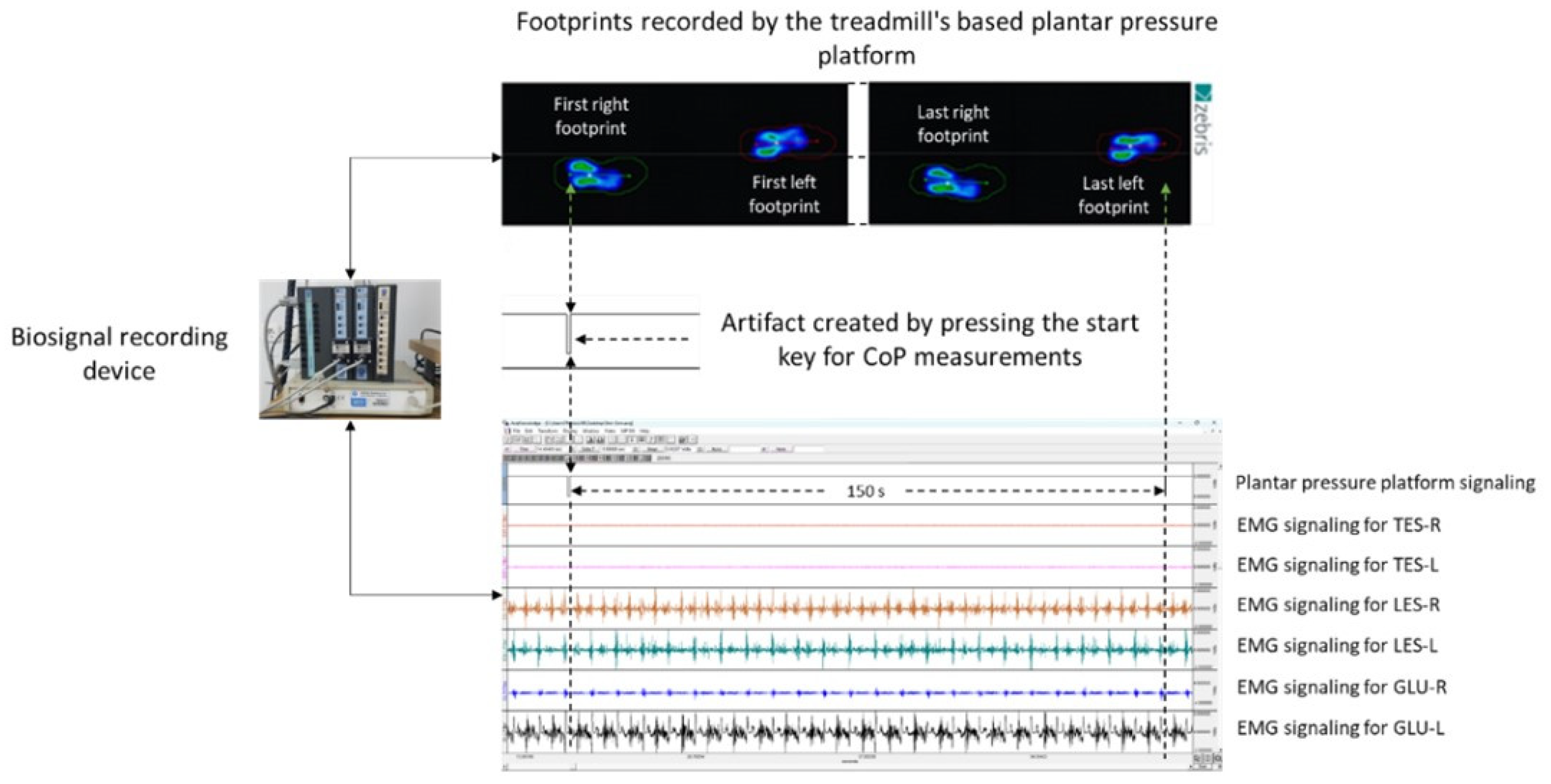
2.4. Spatiotemporal and Ground Reaction Forces Measurements
2.5. EMG Activity Measurements
2.5.1. Recording, Processing, and Analysis of the Signal
2.5.2. Normalization of the EMG Activity
2.6. Heart Rate and Perceived Exertion Measurements
2.7. Statistical Analysis
3. Results
3.1. Spatiotemporal Parameters of Walking and Running
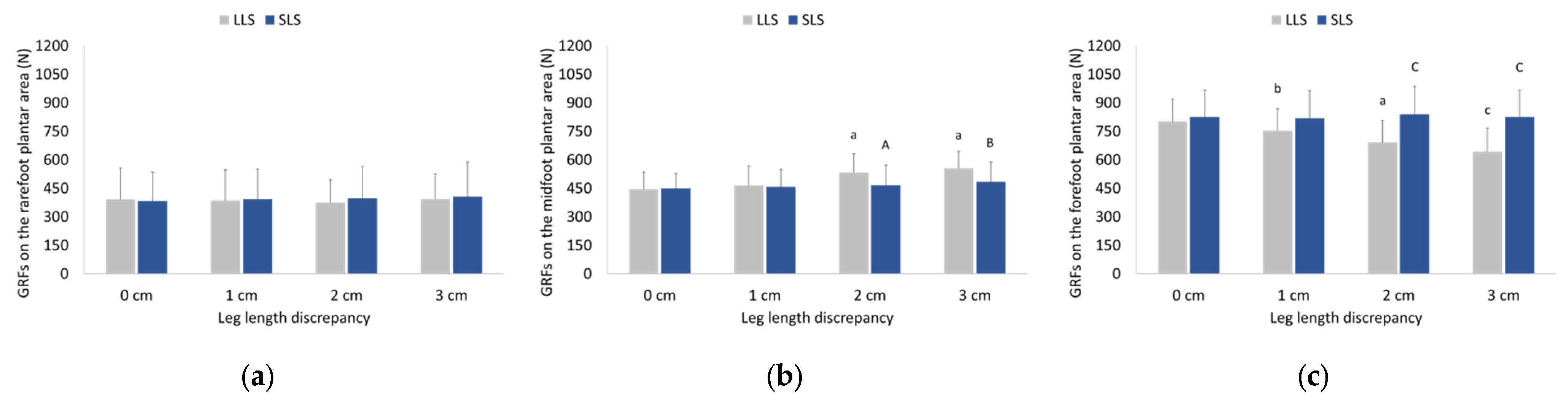
3.2. Ground Reaction Forces Exerted during Walking and Running
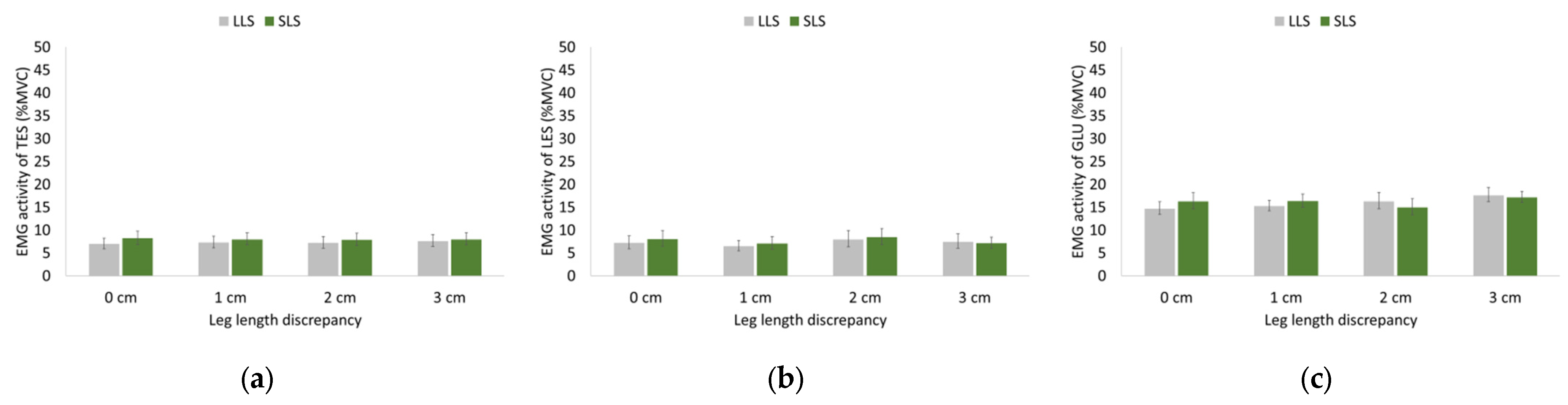
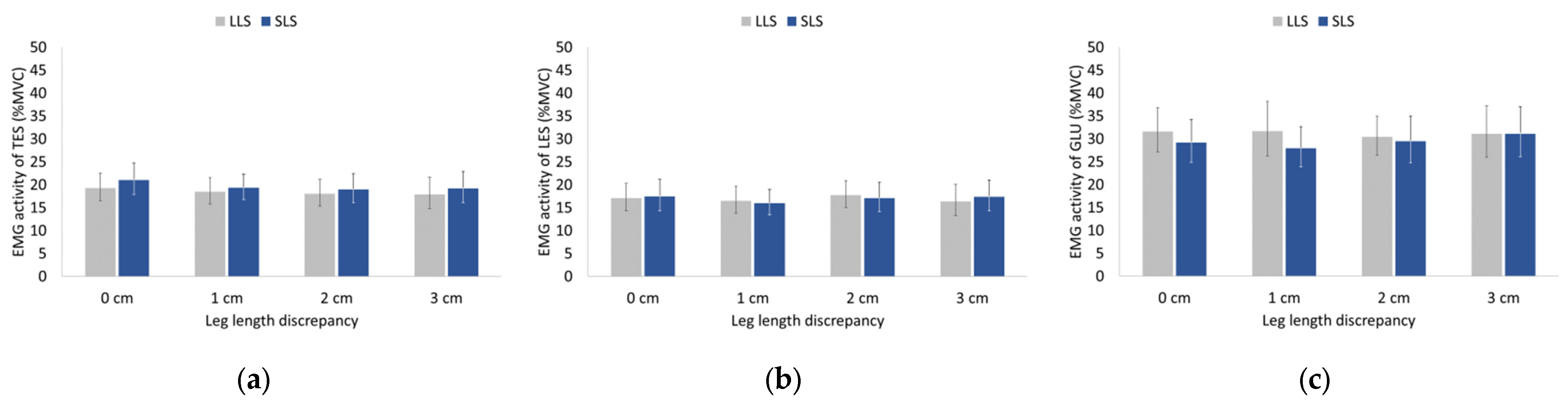
3.3. EMG Activity of Hip and Trunk Muscles during Walking and Running
3.4. Heart Rate and Perceived Exertion
4. Discussion
4.1. Effects of LLD on Spatiotemporal Gait Parameters
4.2. Effects of LLD on GRFs
4.3. Effects of LLD on EMG of Trunk and Hip Musculature
4.4. Clinical Implications
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knutson, G.A. Anatomic and Functional Leg-Length Inequality: A Review and Recommendation for Clinical Decision-Making. Part I, Anatomic Leg-Length Inequality: Prevalence, Magnitude, Effects and Clinical Significance. Chiropr. Osteopat. 2005, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Knutson, G.A. Anatomic and Functional Leg-Length Inequality: A Review and Recommendation for Clinical Decision-Making. Part II, the Functional or Unloaded Leg-Length Asymmetry. Chiropr. Osteopat. 2005, 13, 12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gross, R.H. Leg Length Discrepancy: How Much Is Too Much? Orthopedics 1978, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.J.; Azari, M.F. Leg Length Discrepancy and Osteoarthritis in the Knee, Hip and Lumbar Spine. J. Can. Chiropr. Assoc. 2015, 59, 226–237. [Google Scholar] [PubMed]
- Murray, K.J.; Molyneux, T.; Le Grande, M.R.; Castro Mendez, A.; Fuss, F.K.; Azari, M.F. Association of Mild Leg Length Discrepancy and Degenerative Changes in the Hip Joint and Lumbar Spine. J. Manip. Physiol. Ther. 2017, 40, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Pittala, K.A. Etiologic Factors Associated with Selected Running Injuries. Med. Sci. Sports Exerc. 1988, 20, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Huffman, L.K.; Harris, J.G. Limb-Length Discrepancy as a Cause of Plantar Fasciitis. J. Am. Podiatr. Med. Assoc. 2010, 100, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Applebaum, A.; Nessim, A.; Cho, W. Overview and Spinal Implications of Leg Length Discrepancy: Narrative Review. CiOS Clin. Orthop. Surg. 2021, 13, 127–134. [Google Scholar] [CrossRef]
- Sheha, E.D.; Steinhaus, M.E.; Kim, H.J.; Cunningham, M.E.; Fragomen, A.T.; Rozbruch, S.R. Leg-Length Discrepancy, Functional Scoliosis, and Low Back Pain. JBJS Rev. 2018, 6, e6. [Google Scholar] [CrossRef]
- Wretenberg, P. Hip Joint Load in Relation to Leg Length Discrepancy. Med. Devices Evid. Res. 2008, 1, 13–18. [Google Scholar] [CrossRef]
- Betsch, M.; Wild, M.; Große, B.; Rapp, W.; Horstmann, T. The Effect of Simulating Leg Length Inequality on Spinal Posture and Pelvic Position: A Dynamic Rasterstereographic Analysis. Eur. Spine J. 2012, 21, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Balci, A.; Kocahan, T.; Akinoglu, B.; Yilmaz, A.E.; Hasanoglu, A. The Immediate Effect of Simulating Leg-Length Discrepancy on Spinal Posture and Pelvic Position: A Cross-over Designed Study. Res. Sports Med. 2022, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cooperstein, R.; Lew, M. The Relationship between Pelvic Torsion and Anatomical Leg Length Inequality: A Review of the Literature. J. Chiropr. Med. 2009, 8, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Specht, D.L.; De Boer, K.F. Anatomical Leg Length Inequality, Scoliosis and Lordotic Curve in Unselected Clinic Patients. J. Manip. Physiol. Ther. 1991, 14, 368–375. [Google Scholar]
- Buyukaslan, A.; Abul, K.; Berk, H.; Yilmaz, H. Leg Length Discrepancy and Adolescent Idiopathic Scoliosis: Clinical and Radiological Characteristics. Spine Deform. 2022, 10, 307–314. [Google Scholar] [CrossRef] [PubMed]
- White, S.C.; Gilchrist, L.A.; Wilk, B.E. Asymmetric Limb Loading with True or Simulated Leg-Length Differences. Clin. Orthop. Relat. Res. 2004, 421, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Pereiro-Buceta, H.; Becerro-De-bengoa-vallejo, R.; Losa-Iglesias, M.E.; López-López, D.; Navarro-Flores, E.; Martínez-Jiménez, E.M.; Martiniano, J.; Calvo-Lobo, C. The Effect of Simulated Leg-Length Discrepancy on the Dynamic Parameters of the Feet during Gait—Cross-Sectional Research. Healthcare 2021, 9, 932. [Google Scholar] [CrossRef] [PubMed]
- Zabri, S.W.K.A.; Basaruddin, K.S.; Salleh, A.F.; Rusli, W.M.R.; Basah, S.N. Leg Length Inequality Effects on Ground and Lower Extremity Joint Reaction Forces during Walking. J. Telecommun. Electron. Comput. Eng. 2018, 10, 141–145. [Google Scholar]
- Perttunen, J.R.; Anttila, E.; Södergård, J.; Merikanto, J.; Komi, P.V. Gait Asymmetry in Patients with Limb Length Discrepancy. Scand. J. Med. Sci. Sports 2004, 14, 49–56. [Google Scholar] [CrossRef]
- Azizan, N.A.; Basaruddin, K.S.; Salleh, A.F.; Sulaiman, A.R.; Safar, M.J.A.; Rusli, W.M.R. Leg Length Discrepancy: Dynamic Balance Response during Gait. J. Healthc. Eng. 2018, 10, 7815451. [Google Scholar] [CrossRef]
- Giles, L.G.F.; Taylor, J.R. Low-Back Pain Associated with Leg Length Inequality. Spine 1981, 6, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.F. Association of Leg-Length Inequality with Knee Osteoarthritis. Ann. Intern. Med. 2010, 152, 287. [Google Scholar] [CrossRef] [PubMed]
- Gurney, B. Leg Length Discrepancy. Gait Posture 2002, 15, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Vink, P.; Huson, A. Lumbar Back Muscle Activity during Walking with a Leg Inequality. Acta Morphol. Neerl. Scand. 1987, 25, 261–271. [Google Scholar] [PubMed]
- Gurney, J.K.; Kersting, U.G.; Rosenbaum, D. Between-Day Reliability of Repeated Plantar Pressure Distribution Measurements in a Normal Population. Gait Posture 2008, 27, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Seeley, M.K.; Umberger, B.R.; Clasey, J.L.; Shapiro, R. The Relation between Mild Leg-Length Inequality and Able-Bodied Gait Asymmetry. J. Sports Sci. Med. 2010, 9, 572–579. [Google Scholar] [PubMed]
- Pereira, C.S.; Sacco, I.D.C.N. Is Structural and Mild Leg Length Discrepancy Enough to Cause a Kinetic Change in Runners’ Gait? Acta Ortop. Bras. 2008, 16, 28–31. [Google Scholar] [CrossRef]
- O’Toole, G.C.; Makwana, N.K.; Lunn, J.; Harty, J.; Stephens, M.M. The Effect of Leg Length Discrepancy on Foot Loading Patterns and Contact Times. Foot Ankle Int. 2003, 24, 256–259. [Google Scholar] [CrossRef]
- Bhave, A.; Paley, D.; Herzenberg, J.E. Improvement in Gait Parameters after Lengthening for the Treatment of Limb-Length Discrepancy. J. Bone Jt. Surg. 1999, 81, 529–534. [Google Scholar] [CrossRef]
- Kaufman, K.; Miller, L.; Sutherland, D.H. Gait Asymmetry in Patients with Limb-Length Inequality. J. Pediatr. Orthop. Issue 1996, 16, 144–150. [Google Scholar] [CrossRef]
- Gurney, A.B.; Mermier, C.; Rivero, D.; Gibson, A. Effects of Leg Length Discrepancy on Gait Economy and Lower Extremity Muscle Activity in Older Adults. J. Geriatr. Phys. Ther. 2001, 24, 24. [Google Scholar] [CrossRef]
- Bird, A.R.; Bendrups, A.P.; Payne, C.B. The Effect of Foot Wedging on Electromyographic Activity in the Erector Spinae and Gluteus Medius Muscles during Walking. Gait Posture 2003, 18, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Khamis, S.; Carmeli, E. Relationship and Significance of Gait Deviations Associated with Limb Length Discrepancy: A Systematic Review. Gait Posture 2017, 57, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A. Assessing Lateral Stability of the Hip and Pelvis. Man. Ther. 2011, 16, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Mattila, M.; Zhdanov, A.; Kulmala, J.-P. Patients with Scoliosis Have Dysfunctional Spinal Muscles, Preliminary Study. Gait Posture 2023, 106, S120. [Google Scholar] [CrossRef]
- Rinaldi, V.G.; Prill, R.; Jahnke, S.; Zaffagnini, S.; Becker, R. The Influence of Gluteal Muscle Strength Deficits on Dynamic Knee Valgus: A Scoping Review. J. Exp. Orthop. 2022, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.J.; Lack, S.; Malliaras, P.; Morrissey, D. Gluteal Muscle Activity and Patellofemoral Pain Syndrome: A Systematic Review. Br. J. Sports Med. 2013, 47, 207–214. [Google Scholar] [CrossRef]
- Subotnick, S.I. Limb Length Discrepancies of the Lower Extremity (the Short Leg Syndrome). J. Orthop. Sports Phys. Ther. 1981, 3, 11–16. [Google Scholar] [CrossRef]
- Menez, C.; L’Hermette, M.; Coquart, J. Orthotic Insoles Improve Gait Symmetry and Reduce Immediate Pain in Subjects with Mild Leg Length Discrepancy. Front. Sports Act. Living 2020, 2, 579152. [Google Scholar] [CrossRef]
- Shi, Y.; Pang, H.; Xu, H.; Li, X.; Cao, Y.; Merryweather, A.; Zheng, P.; Xiang, J. Effects of Orthotic Insole on Gait Patterns in Children with Mild Leg Length Discrepancy. Gait Posture 2022, 93, 191–197. [Google Scholar] [CrossRef]
- Moharrami, A.; Mirghaderi, P.; Hoseini Zare, N.; Moazen-Jamshidi, M.M.; Ebrahimian, M.; Mortazavi, S.M.J. Slight Pelvic Obliquity Is Normal in a Healthy Population: A Cross-Sectional Study. J. Exp. Orthop. 2023, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Redmond, A.C.; Crosbie, J.; Ouvrier, R.A. Development and Validation of a Novel Rating System for Scoring Standing Foot Posture: The Foot Posture Index. Clin. Biomech. 2006, 21, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, W.P. An Objective Criterion for Scoliosis Screening. J. Bone Jt. Surg. Am. 1984, 66, 1381–1387. [Google Scholar] [CrossRef]
- Mandalidis, D.; Glakousakis, G.; Kalatzis, P. An Anthropometric-Based Method for the Assessment of Pelvis Position in Three-Dimensional Space. MethodsX 2022, 9, 101616. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.A.; Cole, J.; Twomey, L. An Alternative Method for the Measurement of Pelvic Skeletal Asymmetry (PSA) Using an Asymmetry Ratio (AR). J. Man. Manip. Ther. 1999, 7, 11–19. [Google Scholar] [CrossRef]
- Alfuth, M.; Fichter, P.; Knicker, A. Leg Length Discrepancy: A Systematic Review on the Validity and Reliability of Clinical Assessments and Imaging Diagnostics Used in Clinical Practice. PLoS ONE 2021, 16, e0261457. [Google Scholar] [CrossRef] [PubMed]
- Stefanouli, V.; Kapreli, E.; Anastasiadi, E.; Nakastsis, A.; Strimpakos, N. Validity and Reliability of the Greek Version of Modified Baecke Questionnaire. Public Health 2022, 203, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Pols, M.A.; Peeters, P.H.M.; Bueno-de-Mesquita, H.B.; Ocke, M.C.; Wentink, C.A.; Kemper, H.C.G.; Collette, H.J.A. Validity and Repeatability of a Modified Baecke Questionnaire on Physical Activity. Int. J. Epidemiol. 1995, 24, 381–388. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. [Google Scholar]
- Lohman, E.B.; Balan Sackiriyas, K.S.; Swen, R.W. A Comparison of the Spatiotemporal Parameters, Kinematics, and Biomechanics between Shod, Unshod, and Minimally Supported Running as Compared to Walking. Phys. Ther. Sport 2011, 12, 151–163. [Google Scholar] [CrossRef]
- Paillard, T. Effects of General and Local Fatigue on Postural Control: A Review. Neurosci. Biobehav. Rev. 2012, 36, 162–176. [Google Scholar] [CrossRef]
- Callaghan, J.P.; Gunning, J.L.; McGill, S.M. The Relationship Between Lumbar Spine Load and Muscle Activity During Extensor Exercises. Phys. Ther. 1998, 78, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Danneels, L.A.; Cagnie, B.J.; Cools, A.M.; Vanderstraeten, G.G.; Cambier, D.C.; Witvrouw, E.E.; de Cuyper, H.J. Intra-Operator and Inter-Operator Reliability of Surface Electromyography in the Clinical Evaluation of Back Muscles. Man. Ther. 2001, 6, 145–153. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A.V. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.; Grant, S.W.; Takkenberg, J.J.M.; Mokhles, M.M. Statistical Primer: How to Deal with Missing Data in Scientific Research? Interact. Cardiovasc. Thorac. Surg. 2018, 27, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Kim, K.W.; Kim, C.H. Effect of Leg Length Discrepancy on Gait and Cobb’s Angle. Korean J. Sport Biomech. 2016, 26, 101–113. [Google Scholar] [CrossRef]
- Wünnemann, M.; Klein, D.; Rosenbaum, D. Effects of the Twin Shoe (Darco) to Compensate Height Differences in Normal Gait. Gait Posture 2011, 33, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, I.; Ohta, M.; Tamari, M. Effect of Leg Length Discrepancy on Dynamic Gait Stability. Prog. Rehabil. Med. 2023, 8, 20230013. [Google Scholar] [CrossRef]
- Swaminathan, V.; Cartwright-Terry, M.; Moorehead, J.D.; Bowey, A.; Scott, S.J. The Effect of Leg Length Discrepancy upon Load Distribution in the Static Phase (Standing). Gait Posture 2014, 40, 561–563. [Google Scholar] [CrossRef]
- Osaka, H.; Fujita, D.; Kobara, K.; Suehiro, T. Immediate Effect of Restricted Knee Extension on Ground Reaction Force and Trunk Acceleration during Walking. Rehabil. Res. Pract. 2021, 2021, 8833221. [Google Scholar] [CrossRef]
- Cook, T.M.; Farrell, K.P.; Carey, I.A.; Gibbs, J.M.; Wiger, G.E. Effects of Restricted Knee Flexion and Walking Speed on the Vertical Ground Reaction Force During Gait. J. Orthop. Sports Phys. Ther. 1997, 25, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Hillstrom, H.J.; Song, J.; Kraszewski, A.P.; Hafer, J.F.; Mootanah, R.; Dufour, A.B.; Chow, B.S.; Deland, J.T. Foot Type Biomechanics Part 1: Structure and Function of the Asymptomatic Foot. Gait Posture 2013, 37, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Osaka, H.; Hanayama, K.; Fujita, D.; Kobara, K.; Yoshimura, Y.; Suehiro, T.; Asada, Y.C. Immediate Effect of Restricted Ankle Dorsiflexion on Ground Reaction Force and Trunk Acceleration during Walking. Ann. Phys. Rehabil. Med. 2018, 61, e449. [Google Scholar] [CrossRef]
- Othman, N.F.; Basaruddin, K.S.; Som, M.H.M.; Majid, M.S.A.; Sulaiman, A.R. The Effect of Leg Length Inequality on Joint Contact Forces of Lower Limbs during Walking. Acta Bioeng. Biomech. 2019, 21, 54–62. [Google Scholar] [CrossRef]
- Vink, P.; Kamphuisen, H.A.C. Leg Length Inequality, Pelvic Tilt and Lumbar Back Muscle Activity during Standing. Clin. Biomech. 1989, 4, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Drerup, B.; Ellger, B.; Meyer zu Bentrup, F.M.; Hierholzer, E. Functional Rasterstereographic Images. A New Method for Biomechanical Analysis of Skeletal Geometry. Orthopade 2001, 30, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Bangerter, C.; Romkes, J.; Lorenzetti, S.; Krieg, A.H.; Hasler, C.C.; Brunner, R.; Schmid, S. What Are the Biomechanical Consequences of a Structural Leg Length Discrepancy on the Adolescent Spine during Walking? Gait Posture 2019, 68, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Connolly, P.; Jenkinson, A.; O’brien, T. Leg Length Discrepancy-an Experimental Study of Compensatory Changes in Three Dimensions Using Gait Analysis. Gait Posture 2000, 12, 156–161. [Google Scholar] [CrossRef]
- Kakushima, M.; Miyamoto, K.; Shimizu, K. The Effect of Leg Length Discrepancy on Spinal Motion during Gait. Spine 2003, 28, 2472–2476. [Google Scholar] [CrossRef]
- Indahl, A.; Kaigle, A.; Reikerås, O.; Holm, S. Sacroiliac Joint Involvement in Activation of the Porcine Spinal and Gluteal Musculature. J. Spinal Disord. 1999, 12, 325–330. [Google Scholar] [CrossRef]
- Allum, J.H.J.; Honegger, F. Interactions between Vestibular and Proprioceptive Inputs Triggering and Modulating Human Balance-Correcting Responses Differ across Muscles. Exp. Brain Res. 1998, 121, 478–494. [Google Scholar] [CrossRef]
- Korpelainen, R.; Orava, S.; Karpakka, J.; Siira, P.; Hulkko, A. Risk Factors for Recurrent Stress Fractures in Athletes. Am. J. Sports Med. 2001, 29, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Friberg, O. Leg Length Asymmetry in Stress Fractures. A Clinical and Radiological Study. J. Sports Med. Phys. Fit. 1982, 22, 485–488. [Google Scholar]
- Marras, W.S.; Jorgensen, M.J.; Granata, K.P.; Wiand, B. Female and Male Trunk Geometry: Size and Prediction of the Spine Loading Trunk Muscles Derived from MRI. Clin. Biomech. 2001, 16, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.F.; Sparrey, C.J.; Been, E.; Kramer, P.A. Morphological and Postural Sexual Dimorphism of the Lumbar Spine Facilitates Greater Lordosis in Females. J. Anat. 2016, 229, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Mitteroecker, P. Allometry and Sexual Dimorphism in the Human Pelvis. Anat. Rec. 2017, 300, 698–705. [Google Scholar] [CrossRef]
- Chung, M.J.; Wang, M.J. Gender and Walking Speed Effects on Plantar Pressure Distribution for Adults Aged 20–60 Years. Ergonomics 2012, 55, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Bruening, D.A.; Frimenko, R.E.; Goodyear, C.D.; Bowden, D.R.; Fullenkamp, A.M. Sex Differences in Whole Body Gait Kinematics at Preferred Speeds. Gait Posture 2015, 41, 540–545. [Google Scholar] [CrossRef]
- Sardroodian, M.; Hosseinzadeh, M. Gender Differences in the Spatial–Temporal Variability between Walking and Running. Sport. Sci. Health 2020, 16, 123–127. [Google Scholar] [CrossRef]
- Desai, A.S.; Dramis, A.; Board, T.N. Leg Length Discrepancy after Total Hip Arthroplasty: A Review of Literature. Curr. Rev. Musculoskelet. Med. 2013, 6, 336–341. [Google Scholar] [CrossRef]
- Assogba, T.F.; Boulet, S.; Detrembleur, C.; Mahaudens, P. The Effects of Real and Artificial Leg Length Discrepancy on Mechanical Work and Energy Cost during the Gait. Gait Posture 2018, 59, 147–151. [Google Scholar] [CrossRef]
- Dahl, M.T. Limb Length Discrepancy. Pediatr. Clinincs N. Am. 1996, 43, 849–865. [Google Scholar] [CrossRef]
- O’Leary, K.; Vorpahl, K.A.; Heiderscheit, B. Effect of Cushioned Insoles on Impact Forces during Running. J. Am. Podiatr. Med. Assoc. 2008, 98, 36–41. [Google Scholar] [CrossRef]
- Zhang, S.; Clowers, K.G.; Powell, D. Ground Reaction Force and 3D Biomechanical Characteristics of Walking in Short-Leg Walkers. Gait Posture 2006, 24, 487–492. [Google Scholar] [CrossRef]



| Gait Parameters | Side | Leg Length Discrepancy | |||
|---|---|---|---|---|---|
| 0 cm | 1 cm | 2 cm | 3 cm | ||
| Step length (cm) | LLS | 73.28 ± 2.84 | 74.21 ± 2.58 a | 75.23 ± 2.71 b,A | 75.85 ± 3.36 b,A |
| SLS | 73.27 ± 2.59 | 73.04 ± 2.41 | 72.46 ± 2.22 c | 72.12 ± 2.47 d | |
| Stride length (cm) | 146.55 ± 5.20 | 147.25 ± 4.72 | 147.69 ± 4.52 | 147.98 ± 5.37 | |
| Step width (cm) | 9.67 ± 2.38 | 9.79 ± 2.53 | 10.06 ± 2.63 | 10.44 ± 2.54 e | |
| Step time (s) | LLS | 0.53 ± 0.02 | 0.52 ± 0.02 c | 0.51 ± 0.02 b,A | 0.51 ± 0.02 b,A |
| SLS | 0.52 ± 0.02 | 0.53 ± 0.02 a | 0.55 ± 0.02 b | 0.55 ± 0.02 f | |
| Stride time (s) | 1.05 ± 0.04 | 1.06 ± 0.03 | 1.06 ± 0.03 | 1.06 ± 0.04 | |
| Stance phase time (s) | LLS | 0.65 ± 0.02 | 0.66 ± 0.02 a | 0.67 ± 0.02 b,A | 0.68 ± 0.02 b,A |
| SLS | 0.66 ± 0.03 | 0.65 ± 0.02 | 0.65 ± 0.02 b | 0.65 ± 0.03 b | |
| Swing phase time (s) | LLS | 0.40 ± 0.02 | 0.39 ± 0.02 c | 0.39 ± 0.02 e,A | 0.39 ± 0.02 e,A |
| SLS | 0.40 ± 0.02 | 0.40 ± 0.01 a | 0.41 ± 0.01 b | 0.42 ± 0.02 f | |
| Cadence (step·min−1) | 114.16 ± 4.09 | 113.66 ± 3.66 | 113.30 ± 3.50 | 113.15 ± 4.14 | |
| Gait Parameters | Side | Leg Length Discrepancy | |||
|---|---|---|---|---|---|
| 0 cm | 1 cm | 2 cm | 3 cm | ||
| Step length (cm) | LLS | 83.09 ± 4.10 | 82.77 ± 3.87 | 82.72 ± 3.60 | 83.14 ± 3.99 |
| SLS | 83.23 ± 3.83 | 83.71 ± 4.03 | 84.24 ± 3.99 | 84.57 ± 4.00 a | |
| Stride length (cm) | 166.32 ± 7.75 | 166.50 ± 7.68 | 166.96 ± 7.21 | 167.72 ± 7.46 | |
| Step width (cm) | 6.84 ± 3.09 | 6.99 ± 3.14 | 7.34 ± 3.03 b | 7.43 ± 2.96 c | |
| Step time (s) | LLS | 0.37 ± 0.02 | 0.37 ± 0.02 | 0.37 ± 0.02 d,A | 0.36 ± 0.02 e,A |
| SLS | 0.37 ± 0.02 | 0.38 ± 0.02 | 0.38 ± 0.02 e | 0.39 ± 0.02 f | |
| Stride time (s) | 0.75 ± 0.03 | 0.75 ± 0.03 | 0.75 ± 0.03 | 0.75 ± 0.03 | |
| Stance phase time (s) | LLS | 0.32 ± 0.03 | 0.33 ± 0.03 g,B | 0.34 ± 0.02 e,A | 0.35 ± 0.02 f,A |
| SLS | 0.32 ± 0.03 | 0.32 ± 0.02 | 0.31 ± 0.02 h | 0.31 ± 0.02 b | |
| Swing phase time (s) | LLS | 0.43 ± 0.03 | 0.42 ± 0.04 b | 0.41 ± 0.03 i,A | 0.41 ± 0.03 d,A |
| SLS | 0.43 ± 0.03 | 0.43 ± 0.04 | 0.44 ± 0.03 j | 0.44 ± 0.04 d | |
| Cadence (step·min−1) | 161.11 ± 7.78 | 161.20 ± 8.37 | 160.40 ± 7.08 | 159.81 ± 7.48 | |
| Parameters | Phase | Leg Length Discrepancy | |||
|---|---|---|---|---|---|
| 0 cm | 1 cm | 2 cm | 3 cm | ||
| Walking | |||||
| HR (beats·min−1) | Start | 82.0 ± 10.0 A | 84.5 ± 12.9 A | 84.3 ± 12.2 A | 85.3 ± 12.3 A |
| End | 99.1 ± 13.7 | 100.2 ± 13.8 | 100.6 ± 13.9 | 102.9 ± 12.4 | |
| PE (score) | Start | 7.4 ± 1.3 B | 7.2 ± 1.1 C | 7.1 ± 1.2 A | 6.8 ± 1.0 a,A |
| End | 7.8 ± 1.7 | 7.7 ± 1.6 | 7.7 ± 1.4 | 7.7 ± 1.6 | |
| Running | |||||
| HR (beats·min−1) | Start | 88.4 ± 11.8 A | 87.6 ± 11.7 A | 89.9 ± 12.0 A | 90.0 ± 8.3 A |
| End | 149.9 ± 17.9 | 148.6 ± 18.4 | 147.8 ± 22.9 | 149.4 ± 21.7 | |
| PE (score) | Start | 8.1 ± 1.5 A | 7.8 ± 1.5 A | 7.5 ± 1.9 A | 8.2 ± 1.8 A |
| End | 10.2 ± 2.6 | 10.4 ± 2.7 | 10.1 ± 2.6 | 10.7 ± 2.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korontzi, M.; Kafetzakis, I.; Mandalidis, D. Effects of Artificially Induced Leg Length Discrepancy on Treadmill-Based Walking and Running Symmetry in Healthy College Students: A Lab-Based Experimental Study. Sensors 2023, 23, 9695. https://doi.org/10.3390/s23249695
Korontzi M, Kafetzakis I, Mandalidis D. Effects of Artificially Induced Leg Length Discrepancy on Treadmill-Based Walking and Running Symmetry in Healthy College Students: A Lab-Based Experimental Study. Sensors. 2023; 23(24):9695. https://doi.org/10.3390/s23249695
Chicago/Turabian StyleKorontzi, Maria, Ioannis Kafetzakis, and Dimitris Mandalidis. 2023. "Effects of Artificially Induced Leg Length Discrepancy on Treadmill-Based Walking and Running Symmetry in Healthy College Students: A Lab-Based Experimental Study" Sensors 23, no. 24: 9695. https://doi.org/10.3390/s23249695
APA StyleKorontzi, M., Kafetzakis, I., & Mandalidis, D. (2023). Effects of Artificially Induced Leg Length Discrepancy on Treadmill-Based Walking and Running Symmetry in Healthy College Students: A Lab-Based Experimental Study. Sensors, 23(24), 9695. https://doi.org/10.3390/s23249695








