Red Blood Cells’ Area Deformation as the Origin of the Photoplethysmography Signal
Abstract
:1. Introduction
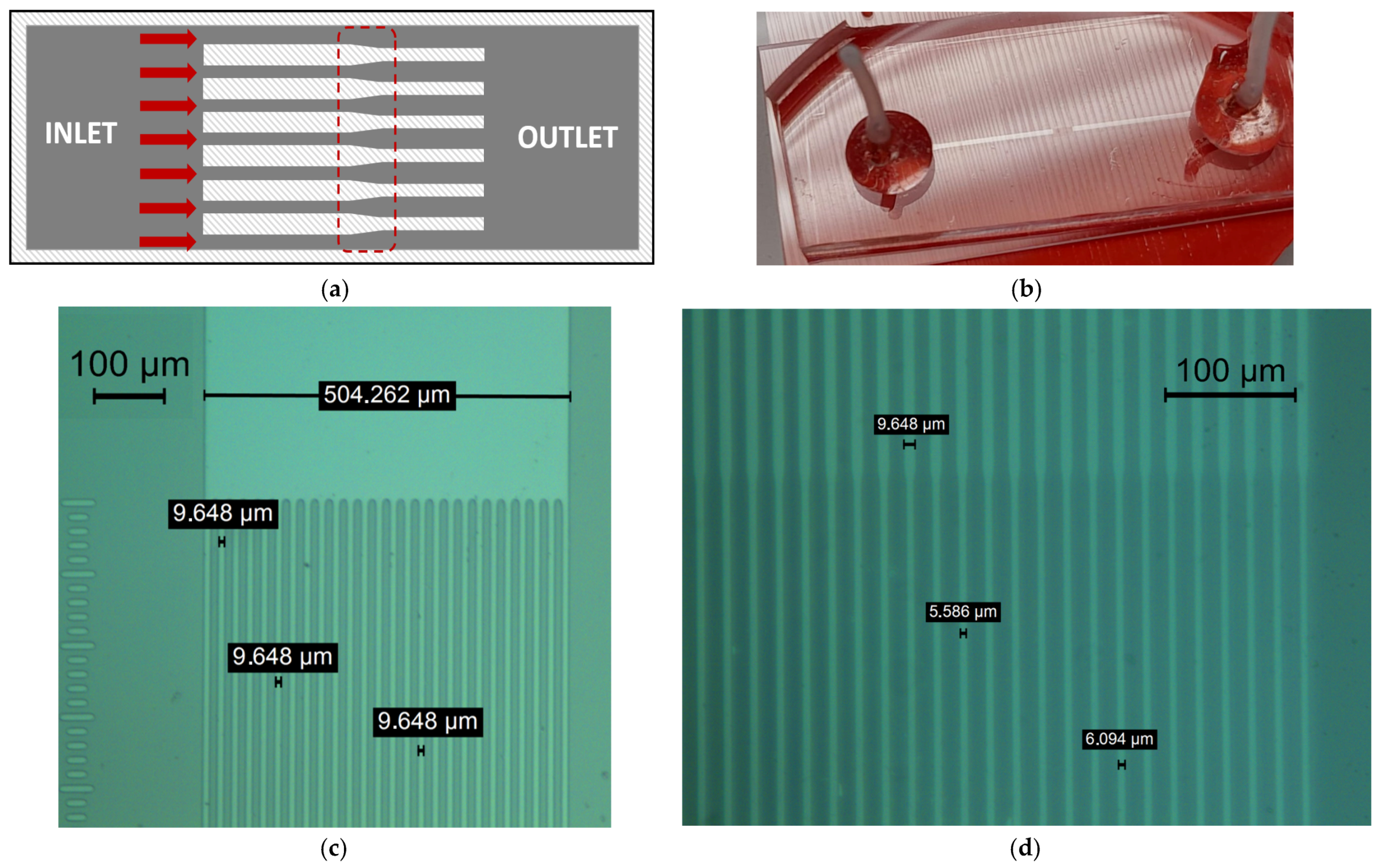
2. Materials and Methods
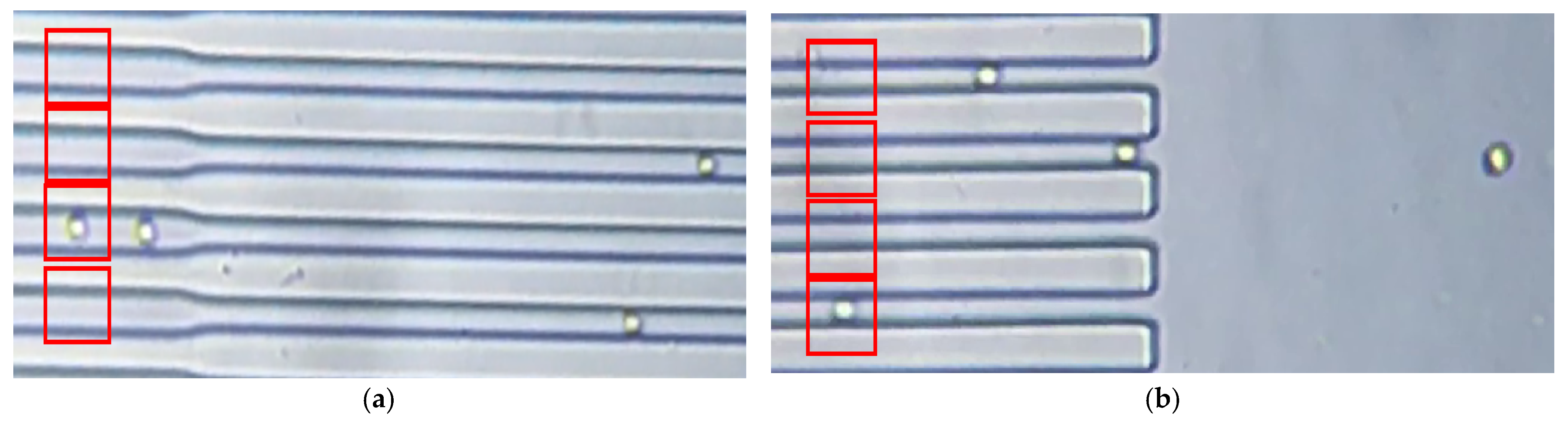
3. Results
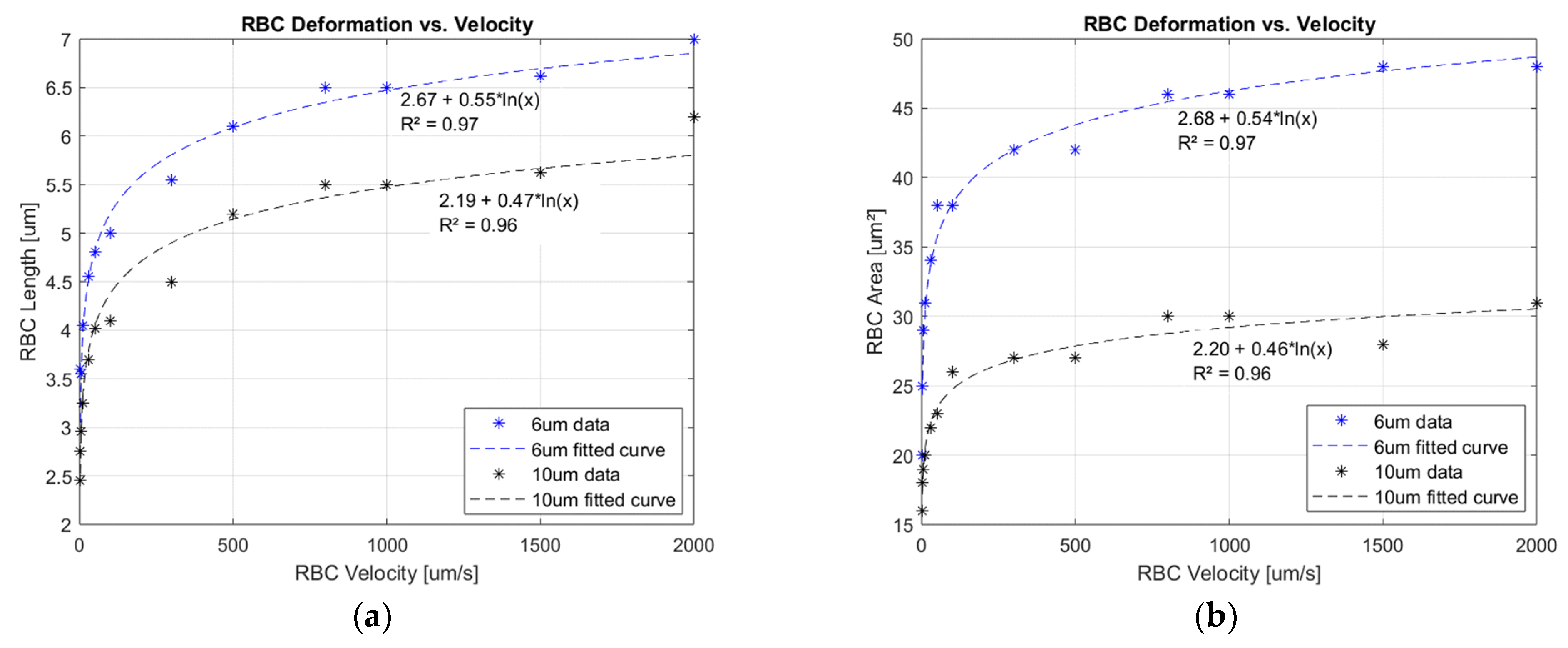
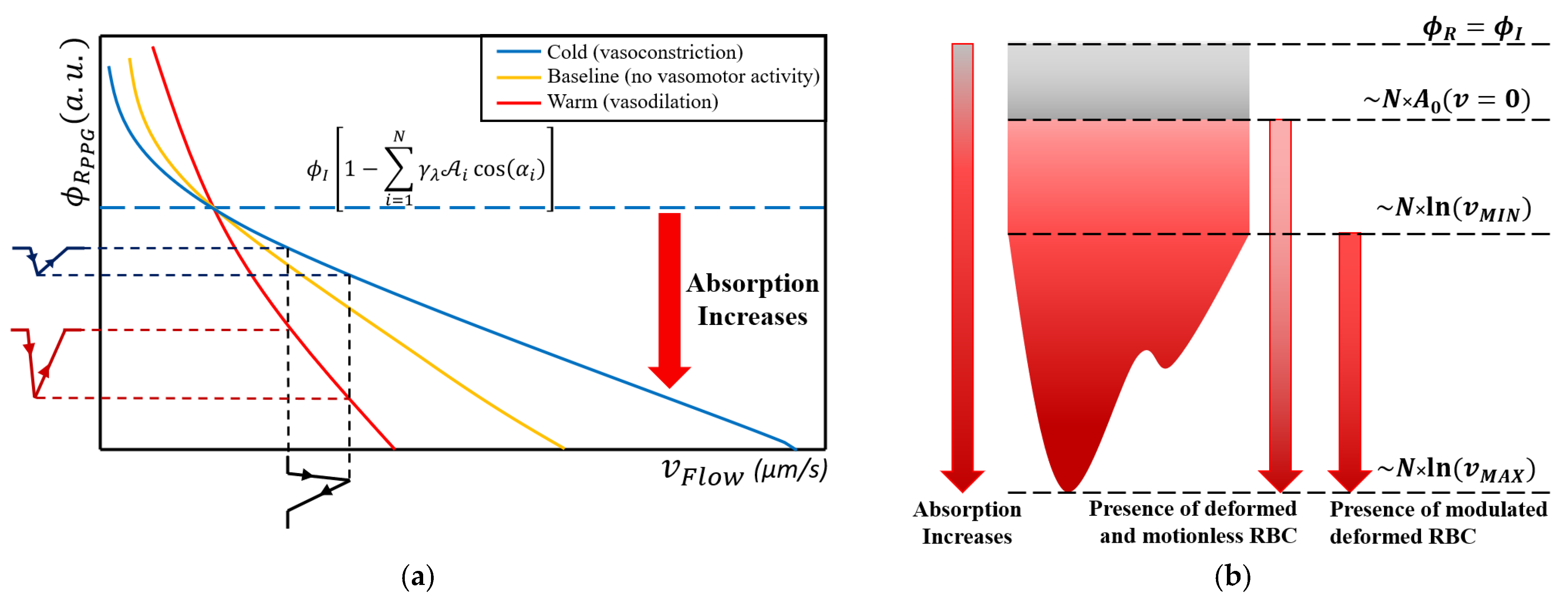
3.1. Deformation Experimental Data
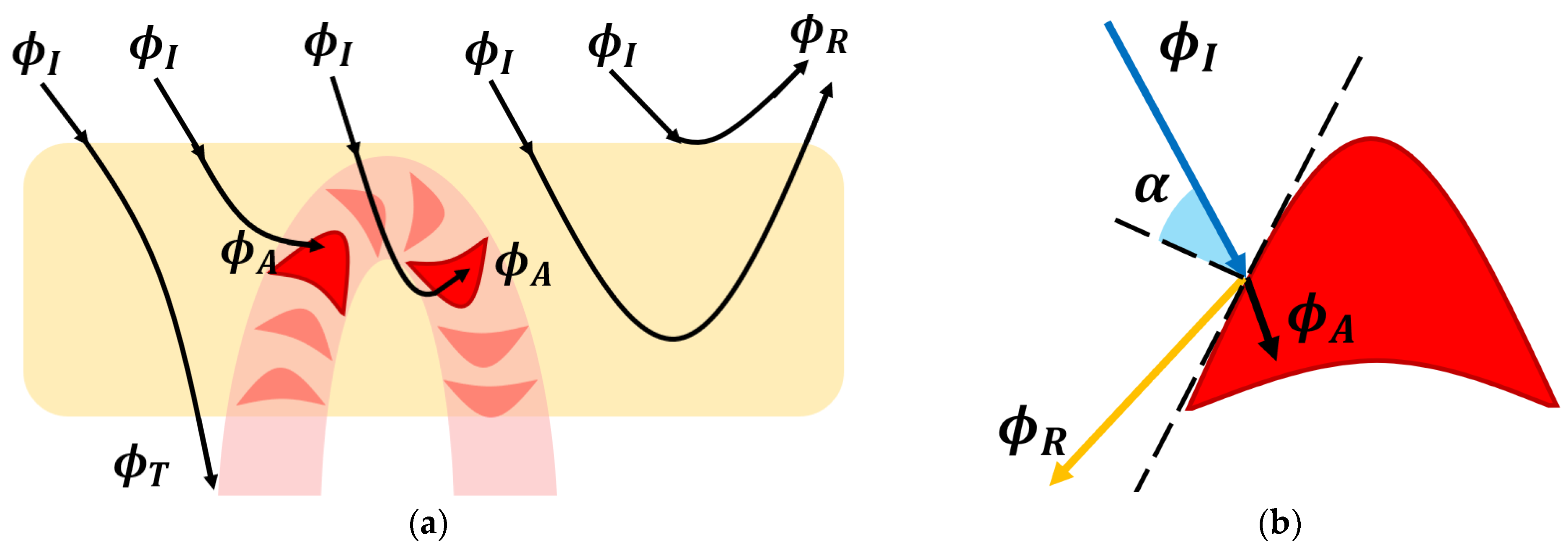
3.2. The Photoplethysmography Mathematical Model
- The dermis layer is treated as a diffuse reflector. Therefore, the received radiant power is just scattered. This approximation is provided by the collagen fiber network, which possesses this property [45];
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonsmann, M.R. Blutdruckversuche an der Maus und Ratte mittels Photozelle. Naunyn-Schmiedebergs Arch. Exp. Pathol. Und Pharmakol. 1934, 176, 460–467. [Google Scholar] [CrossRef]
- Hertzman, A.B. Photoelectric Plethysmography of the Fingers and Toes in Man. Exp. Biol. Med. 1937, 37, 529–534. [Google Scholar] [CrossRef]
- Hertzman, A.B.; Dillon, J.B. Distinction between arterial, venous and flow components in photoelectric plethysmography. Am. Physiol. Soc. 1940, 130, 177–185. [Google Scholar] [CrossRef]
- Miyasaka, K.; Shelley, K.; Takahashi, S.; Kubota, H.; Ito, K.; Yoshiya, I.; Yamanishi, A.; Cooper, J.B.; Steward, D.J.; Nishida, H.; et al. Tribute to Dr. Takuo Aoyagi, inventor of pulse oximetry. J. Anesth. 2021, 35, 671–709. [Google Scholar] [CrossRef]
- Alian, A.A. Photoplethysmography. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 395–406. [Google Scholar] [CrossRef]
- Kamal, A.A.R.; Harness, J.B.; Irving, G.; Mearns, A.J. Skin photoplethysmography—A review. Comput. Methods Programs Biomed. 1989, 28, 257–269. [Google Scholar] [CrossRef]
- Shelley, K.H. Photoplethysmography: Beyond the Calculation of Arterial Oxygen Saturation and Heart Rate. Anesth. Analg. 2007, 105, S31–S36. [Google Scholar] [CrossRef]
- Smith, V.; Thevissen, K.; Trombetta, A.C.; Pizzorni, C.; Ruaro, B.; Piette, Y.; Paolino, S.; Keyser, F.; Sulli, A.; Melsens, K.; et al. Nailfold Capillaroscopy and Clinical Applications in Systemic Sclerosis. Microcirculation 2016, 23, 364–372. [Google Scholar] [CrossRef]
- Tavakol, M.E.; Fatemi, A.; Karbalaie, A.; Emrani, Z.; Erlandsson, B.E. Nailfold Capillaroscopy in Rheumatic Diseases: Which Parameters Should Be Evaluated? J. BioMed Res. 2015, 2015, 974530. [Google Scholar]
- Zambach, S.A.; Cai, C.; Helms, H.C.C.; Hald, B.O.; Dongd, Y.; Fordsmann, J.C.; Nielsen, R.M.; Hud, J.; Lønstrup, M.; Brodin, B.; et al. Regulation of brain microvascular perfusion by contractile pericytes at precapillary sphincters and first-order capillaries. Biol. Sci. 2021, 118, e2023749118. [Google Scholar]
- Sakai, T.; Hosoyamada, Y. Are the precapillary sphincters and metarterioles universal components of the microcirculation? An historical review. J. Physiol. Sci. 2013, 63, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Wiedeman, M.P.; Tuma, R.F.; Mayrovitz, H.N. Defining the Precapillary Sphincter. Microvasc. Res. 1976, 12, 71–75. [Google Scholar] [CrossRef]
- Ivanov, K.P.; Kalinina, M.K.; Levkovich, Y.I. Blood Flow Velocity in Capillaries of Brain and Muscles and Its Physiological Significance. Microvasc. Res. 1981, 22, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Butti, P.; Intaglietta, M.; Reimann, H.; Holliger, C.H.; Bollinger, A.; Anliker, M. Capillary Red Blood Cell Velocity Measurements in Human Nailfold by Videodensitometric Method. Microvasc. Res. 1975, 10, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Klyscz, T.; Jünger, M. Synchronous Measurements of Blood Pressure and Red Blood Cell Velocity in Capillaries of Human Skin. J. Investig. Dermatol. 1996, 106, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Zhang, G.; Huang, T.C.; Lin, K.P. Red blood cell velocity measurements of complete capillary in finger nail-fold using optical flow estimation. Microvasc. Res. 2009, 78, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Fagrell, B.; Intaglietta, M.; Stergren, J. Relative Hematocrit in Human Skin Capillaries and Its Relation, to Capillary Blood Flow Velocity. Microvasc. Res. 1980, 20, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuoloa, G.; Lanottea, L.; D’Apolitoa, R.; Cassinesec, A.; Guidoa, S. Microconfined flow behavior of red blood cells. Med. Eng. Phys. 2015, 38, 11–16. [Google Scholar] [CrossRef]
- McWhirter, J.L.; Noguchiab, H.; Gompper, G. Deformation and clustering of red blood cells in microcapillary flows. J. Soft Matter 2011, 7, 10967–10977. [Google Scholar] [CrossRef]
- Boryczko, K.; Dzwinel, W.; Yuen, D.A. Dynamical clustering of red blood cells in capillary vessels. J. Mol. Model. 2003, 9, 16–33. [Google Scholar] [CrossRef]
- Secomb, T.W.; Skalak, R.; Özkaya, N.; Gross, J.F. Flow of axisymmetric red blood cells in narrow capillaries. J. Fluid Mech. 1986, 163, 405–423. [Google Scholar] [CrossRef]
- Secomb, T.W.; Hsu, R. Motion of red blood cells in capillaries with variable cross-sections. Trans. ASME 1996, 118, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Guido, S.; Tomaiuolo, G. Microconfined flow behavior of red blood cells in vitro. Sci. Direct 2009, 10, 751–763. [Google Scholar] [CrossRef]
- Guo, Q.; Duffy, S.P.; Matthewsa, K.; Santoso, A.T.; Scott, M.D.; Maa, H. Microfluidic analysis of red blood cell deformability. J. Biomech. 2014, 47, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, J.; Cui, T.; Shehata, N.; Wang, C.; Sun, Y. Characterization of red blood cell deformability change during blood storage. R. Soc. Chem. 2014, 14, 577–583. [Google Scholar]
- Berezina, T.L.; Zaets, S.B.; Morgan, C.; Spillert, C.R.; Kamiyama, M.; Spolarics, Z.; Deitch, E.A.; Machiedo, G.W. Influence of Storage on Red Blood Cell Rheological Properties. J. Surg. Res. 2002, 102, 6–12. [Google Scholar] [CrossRef]
- Moço, A.; Verkruysse, W. Pulse oximetry based on photoplethysmography imaging with red and green light. J. Clin. Monit. Comput. 2020, 35, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Collins, T.; Woolley, S.I.; Ponnapalli, P.V.S. A Review of Wearable Multi-wavelength Photoplethysmography. IEEE Rev. Biomed. Eng. 2021, 16, 136–151. [Google Scholar] [CrossRef]
- Han, S.; Roh, D.; Park, J.; Shin, H. Design of Multi-Wavelength Optical Sensor Module for Depth-Dependent Photoplethysmography. Sensors 2019, 19, 5441. [Google Scholar] [CrossRef]
- Chen, S.H.; Chuang, Y.C.; Chang, C.C. Development of a Portable All-Wavelength PPG Sensing Device for Robust Adaptive-Depth Measurement: A Spectrometer Approach with a Hydrostatic Measurement Example. Sensors 2020, 20, 6556. [Google Scholar] [CrossRef]
- Binzoni, T.; Tchernin, D.; Hyacinthe, J.N.; Van De Ville, D.; Richiardi, J. Pulsatile blood flow in human bone assessed by laser-Doppler flowmetry and the interpretation of photoplethysmographic signals. Physiol. Meas. 2013, 34, N25. [Google Scholar] [CrossRef] [PubMed]
- Kamshilin, A.A.; Nippolainen, E.; Sidorov, I.S.; Vasilev, P.V.; Erofeev, N.P.; Podolian, N.P.; Roman, V.; Romashko, R.V. A new look at the essence of the imaging photoplethysmography. Sci. Rep. 2015, 5, srep10494. [Google Scholar] [CrossRef] [PubMed]
- Volkov, M.V.; Margaryants, N.B.; Potemkin, A.V.; Volynsky, M.A.; Gurov, I.P.; Mamontov, O.V.; Kamshilin, A.A. Video capillaroscopy clarifies mechanism of the photoplethysmographic waveform appearance. Sci. Rep. 2017, 7, 13298. [Google Scholar] [CrossRef] [PubMed]
- Moço, A.V.; Stuijk, S.; Haan, G. New insights into the origin of remote PPG signals in visible light and infrared. Sci. Rep. 2018, 8, 8501. [Google Scholar] [CrossRef]
- Chatterjee, S.; Budidha, K.; Kyriacou, P.A. Investigating the origin of photoplethysmographyusing a multiwavelength Monte Carlo model. Physiol. Meas. 2020, 41, 084001. [Google Scholar] [CrossRef]
- Lee, H.; Ko, H.; Lee, J. Reflectance pulse oximetry: Practical issues and limitations. ICT Express 2016, 2, 195–198. [Google Scholar] [CrossRef]
- Hakemi, A.; Bender, J.A. Understanding Pulse Oximetry, Advantages, and Limitations. Home Health Care Manag. Pract. 2005, 17, 416–418. [Google Scholar] [CrossRef]
- Chiriac, E.; Bran, A.M.; Voitincu, C.; Balan, C. Experimental Validation of VOF Method in Microchannel Flows. In Proceedings of the 12th International Symposium on Advanced Topics in Electrical Engineering (ATEE), Bucharest, Romania, 25–27 March 2021. [Google Scholar]
- Chiriac, E.; Avram, M.; Balan, C. Investigation of Multiphase Flow in a Trifurcation Microchannel—A Benchmark Problem. Micromachines 2022, 13, 974. [Google Scholar] [CrossRef]
- Radosinska, J.; Vrbjar, N. The Role of Red Blood Cell Deformability and Na,K-ATPase Function in Selected Risk Factors of Cardiovascular Diseases in Humans: Focus on Hypertension, Diabetes Mellitus and Hypercholesterolemia. Physiol. Res. 2016, 65, S43–S54. [Google Scholar] [CrossRef]
- Keymel, S.; Heiss, C.; Kleinbongard, P.; Kelm, M.; Lauer, T. Impaired Red Blood Cell Deformability in Patients with Coronary Artery Disease and Diabetes Mellitus. Horm. Metab. Res. 2011, 43, 760–765. [Google Scholar] [CrossRef]
- Agrawal, R.; Smart, T.; Nobre-Cardoso, J.; Richards, C.; Bhatnagar, R.; Tufail, A.; Shima, D.; Jones, P.H.; Pavesio, C. Assessment of red blood cell deformability in type 2 diabetes mellitus and diabetic retinopathy by dual optical tweezers stretching technique. Sci. Rep. 2016, 6, 15873. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, W.L. Introduction to Radiometry; SPIE-The International Society for Optical Engineering: Bellingham, WA, USA, 1998. [Google Scholar]
- Bukshtab, M. Photometry, Radiometry, and Measurements of Optical Losses, 2nd ed.; Springer Series in Optical Sciences: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Sekar, S.K.V.; Bargigia, I.; Mora, A.D.; Taroni, P.; Ruggeri, A.; Tosi, A.; Pifferi, A.; Farina, A. Diffuse optical characterization of collagen absorption from 500 to 1700 nm. J. Biomed. Opt. 2017, 22, 015006. [Google Scholar] [CrossRef] [PubMed]
- Wojdyla, M.; Raj, S.; Petrov, D. Absorption spectroscopy of single red blood cells in the presence of mechanical deformations induced by optical traps. J. Biomed. Opt. 2012, 17, 0970061–0970068. [Google Scholar] [CrossRef] [PubMed]
- Gienger, J.; Smuda, K.; Müller, R.; Bär, M.; Neukammer, J. Refractive index of human red blood cells between 290 nm and 1100 nm determined by optical extinction measurements. Sci. Rep. 2019, 9, 4623. [Google Scholar] [CrossRef]
- Lin, A.J.; Ponticorvo, A.; Konecky, S.D.; Cui, H.; Rice, T.B.; Choi, B.; Durkin, A.J.; Tromberg, B.J. Visible spatial frequency domain imaging with a digital light microprojector. J. Biomed. Opt. 2013, 18, 096007. [Google Scholar] [CrossRef]
- Cordone, L.; Cupane, A.; Leone, M.; Vitrano, E. Optical absorption spectra of deoxy- and oxyhemoglobin in the temperature range 300-20 K. Relation with protein dynamics. Biophys. Chem. 1986, 24, 259–275. [Google Scholar] [CrossRef]
- Fitzgerald, R.K.; Johnson, A. Pulse oximetry in sickle cell anemia. Pediatr. Crit. Care 2001, 29, 1803–1806. [Google Scholar] [CrossRef]
- Sinex, J.E. Pulse oximetry: Principles and limitations. Am. J. Emerg. Med. 1999, 17, 59–66. [Google Scholar] [CrossRef]
- Tur, E.; Guy, R.H.; Tur, M.; Maibach, H.I. Noninvasive Assessment of Local Nicotinate Pharmacodynamics by Photoplethysmography. J. Investig. Dermatol. 1983, 80, 499–503. [Google Scholar] [CrossRef]
- Tusman, G.; Acosta, C.M.; Pulletz, S.; Böhm, S.H.; Scandurra, A.; Arca, J.M.; Madorno, M.; Sipmann, F.S. Photoplethysmographic characterization of vascular tone mediated changes in arterial pressure: An observational study. J. Clin. Monit. Comput. 2019, 33, 815–824. [Google Scholar] [CrossRef]
- Martínez, G.; Howard, N.; Abbott, D.; Lim, K.; Ward, R.; Elgendi, M. Mohamed. Can Photoplethysmography Replace Arterial Blood Pressure in the Assessment of Blood Pressure? J. Clin. Med. 2018, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Cooper, R.; Hosanee, M.; Welykholowa, K.A.; Kyriacou, P.A.; Zheng, D.; Allen, J.; Abbott, D.; Lovell, N.H.; Fletcher, R.; et al. Multi-Site Photoplethysmography Technology for Blood Pressure Assessment: Challenges and Recommendations. J. Clin. Med. 2019, 8, 1827. [Google Scholar] [CrossRef] [PubMed]
- Evdochim, L.; Dobrescu, D.; Halichidis, S.; Dobrescu, L.; Stanciu, S. Hypertension Detection Based on Photoplethysmography Signal Morphology and Machine Learning Techniques. Appl. Sci. 2022, 12, 8380. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evdochim, L.; Chiriac, E.; Avram, M.; Dobrescu, L.; Dobrescu, D.; Stanciu, S.; Halichidis, S. Red Blood Cells’ Area Deformation as the Origin of the Photoplethysmography Signal. Sensors 2023, 23, 9515. https://doi.org/10.3390/s23239515
Evdochim L, Chiriac E, Avram M, Dobrescu L, Dobrescu D, Stanciu S, Halichidis S. Red Blood Cells’ Area Deformation as the Origin of the Photoplethysmography Signal. Sensors. 2023; 23(23):9515. https://doi.org/10.3390/s23239515
Chicago/Turabian StyleEvdochim, Lucian, Eugen Chiriac, Marioara Avram, Lidia Dobrescu, Dragoș Dobrescu, Silviu Stanciu, and Stela Halichidis. 2023. "Red Blood Cells’ Area Deformation as the Origin of the Photoplethysmography Signal" Sensors 23, no. 23: 9515. https://doi.org/10.3390/s23239515






