A Novel Polymeric Membrane Sensor for Chlorhexidine Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Apparatus and Measurement
2.3. Electrode Construction and Preparation of the Membranes
2.4. Determination of Chlorhexidine in Pharmaceuticals
3. Results
3.1. Composition of Membrane and Electrode Performance
3.2. Selectivity
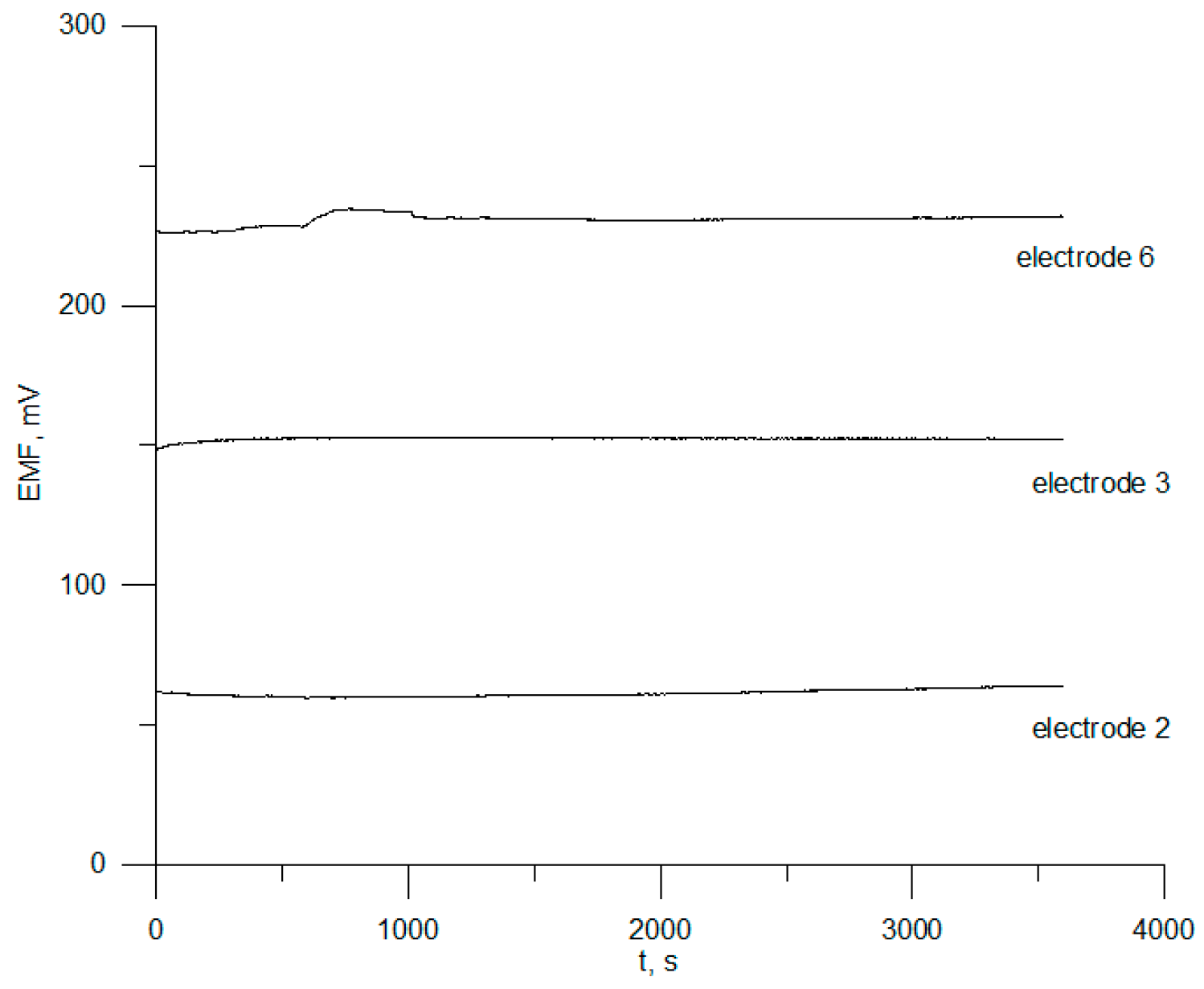
3.3. Reversibility, Response Time, and Electrode Drift
3.4. Working pH Range
3.5. Lifetime
3.6. Analytical Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozbek, O.; Berkel, C.; Isildak, O. Applications of Potentiometric Sensors for the Determination of Drug Molecules in Biological Samples. Crit. Rev. Anal. Chem. 2020, 52, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Isildak, O.; Ozbek, O. Application of Potentiometric Sensors in Real Samples. Crit. Rev. Anal. Chem. 2021, 51, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Derar, A.R.; Ahmed, N.; Hussien, E.M. A new strategy for the determination of the antidiabetics alogliptin, saxagliptin and vildagliptin using all-solid state potentiometric sensors. BMC Chem. 2023, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Kelani, K.M.; Hegazy, M.A.; Tantawy, M.A. Molecular imprinted polymer-based potentiometric approach for the assay of the co-formulated tetracycline HCl, metronidazole and bismuth subcitrate in capsules and spiked human plasma. Anal. Chim. Acta 2023, 1278, 341707. [Google Scholar] [CrossRef] [PubMed]
- Heba, M.; El-Sayed, H.; Ezzat, A.; Amr, M.; Mahmoud, H.; Hendawy, A.M.; Omar, M.; El-Abassy, H. Safinamide detection based on Prussian blue analogue modified Solid-contact potentiometric sensor. Microchem. J. 2023, 191, 108829. [Google Scholar]
- Pospíšilová, E.; Paškanová, N.; Kuchař, M.; Shishkanova, T.V. Potentiometric determination of mephedrone in oral fluids with ion-selective membranes. Electroanalysis 2023, 35, e202200468. [Google Scholar] [CrossRef]
- Zejca, A.; Gorczyca, M. Chemia Leków; PZWL: Warsaw, Poland, 2004; p. 603. [Google Scholar]
- Łukomska-Szymańska, M.; Sokołowski, J.; Łapińska, B. Chlorhexidine—Mechanism of action and its application to dentistry. J. Stomatol. 2017, 70, 405–417. [Google Scholar]
- Flávia, A.; Másquio, F.; Marcos, A.C.; Hérida, R.; Nunes, S. Analytical Methods for the Determination of Chlorhexidine: A Review. Crit. Rev. Anal. Chem. 2010, 40, 89–101. [Google Scholar]
- Vrachas, A.; Gkountanas, K.; Boutsikaris, H.; Dotsikas, Y. Development and Validation of a Novel RP-HPLC Method for the Determination of Cetrimide and Chlorhexidine Gluconate in Antiseptic Solution. Analytica 2022, 3, 79–91. [Google Scholar] [CrossRef]
- Bogdanovska, L.; Saliu, S.; Popovska, M.; Dimitrovska, A.; Ugrinova, L.; Petkovska, R. Development and validation of RP–HPLC assay of chlorhexidine in gingival crevicular fluid. Arh. Farm. 2014, 64, 69–82. [Google Scholar] [CrossRef]
- Cardoso, M.A.; Fa’vero, M.L.D.; Gasparetto, J.C.; Hess, B.S.; Stremel, D.P.; Pontarolo, R. Development and validation of an RP-HPLC method for the determination of chlorhexidine and p-chloroaniline in various pharmaceutical formulations. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1556–1567. [Google Scholar] [CrossRef]
- Golpe, M.C.; Castro, G.; Ramil, M.; Santos, Y.; Rodríguez, I. Chlorhexidine residues in sludge from municipal wastewater treatment plants: Analytical determination and toxicity evaluation. Anal. Bioanal. Chem. 2022, 414, 6571–6580. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.R.; do Nascimento, H.L.; Duarte, G.H.B.; Eberlin, M.N.; Marques, L.A. Liquid Chromatography-Tandem Mass Spectrometry Determination of p-Chloroaniline in Gel and Aqueous Chlorhexidine Products Used in Dentistry. Chromatographia 2016, 79, 841–849. [Google Scholar] [CrossRef]
- Pan, J.; Fair, S.J.; Mao, D. Quantitative analysis of skeletal symmetric chlorhexidine in rat plasma using doubly charged molecular ions in LC-MS/MS detection. Bioanalysis 2011, 3, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.; Nursabah, B.E. Development and Validation of RP-HPLC and Ultraviolet Spectrophotometric Methods of Analysis for the Quantitative Determination of Chlorhexidine Gluconate and Benzydamine Hydrochloride in Pharmaceutical Dosage Forms. Curr. Pharm. Anal. 2011, 7, 167–175. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Naguib, I.A.; Elsayed, M.A.; Zaazaa, H.A. Spectrophotometric Methods for Quantitative Determination of Chlorhexidine Gluconate and its Major Impurity, Metabolite and Degradation Product: Para-chloro-aniline. Anal. Chem. Lett. 2016, 6, 232–248. [Google Scholar] [CrossRef]
- Montes, R.H.O.; Lima, A.P.; dos Santos, V.B.; Vidal, D.T.R.; do Lago, C.L.; Richter, E.M.; Munoz, R.A.A. Carbon-nanotube amperometric sensor for selective determination of 4-chloroaniline in commercial chlorhexidine solutions. Sens. Actuators 2016, 231, 38–44. [Google Scholar] [CrossRef]
- Sousa, C.P.; de Oliveira, R.C.; Freire, T.M.; Fechine, P.B.A.; Salvado, M.A.; Homem-de-Mello, P.; Morais, S.; de Lima-Neto, P.; Correia, A.N. Chlorhexidine digluconate on chitosan-magnetic iron oxide nanoparticles modified electrode: Electroanalysis and mechanistic insights by computational simulation. Sens. Actuators B 2017, 240, 417–442. [Google Scholar] [CrossRef]
- Castro, S.V.F.; Silva, C.V.; Stefano, S.; Richter, E.M.; Munoz, R.A.A. Voltammetric determination of traces of 4-chloroaniline in antiseptic samples on a cathodically-treated boron-doped diamond electrode. J. Electroanal. Chem. 2020, 877, 114500. [Google Scholar] [CrossRef]
- Polish Pharmacopoeia XI; Prezes Urzędu Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych: Warsaw, Poland, 2017; Volume 2, p. 2406.
- Kong, B.; Zeng, J.; Luo, G.; Luo, S.; Wei, W.; Li, J. Layer-by-layer assembled carbon nanotube films with molecule recognition function and lower capacitive background current. Bioelectrochemistry 2009, 74, 289–294. [Google Scholar] [CrossRef]
- Zakaria, A.B.M.; Erick, S.V.; Waltersc, K.B.; Leszczynska, D. Functional holey graphene oxide: A new electrochemically transformed substrate material for dopamine sensing. RSC Adv. 2015, 5, 107–123. [Google Scholar] [CrossRef]
- Suhaimi, N.F.; Baharin, S.N.A.; Jamion, N.A.; Mohd Zain, Z.; Sambasevam, K.P. Polyaniline-chitosan modified on screen-printed carbon electrode for the electrochemical detection of perfluorooctanoic acid. Microchem. J. 2023, 188, 108502. [Google Scholar] [CrossRef]
- Wardak, C.; Pietrzak, K.; Morawska, K.; Grabarczyk, M. Ion-Selective Electrodes with Solid Contact Based on Composite Materials: A Review. Sensors 2023, 23, 5839. [Google Scholar] [CrossRef] [PubMed]
- Magdy, N.; Sobaih, A.E.; Hussein, L.A.; Mahmoud, A.M. Graphene-based Disposable Electrochemical Sensor for Chlorhexidine Determination. Electroanalysis 2022, 35, 2200119. [Google Scholar] [CrossRef]
- Hussien, E.M. Novel PVC-free All-solid-state Ion-selective Electrode for Determination of Chlorhexidine in Pharmaceutical Formulations. Anal. Bioanal. Electrochem. 2014, 6, 138–150. [Google Scholar]
- Lenik, J. Preparation and characterization of a sulindac sensor based on PVC/TOA–SUL membrane. Mater. Sci. Eng. C 2014, 37, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Lenik, J.; Wardak, C. Studies on influence of (2-Hydroxypropyl)-β-cyclodextrin on properties of a new indomethacin electrode. IEEE Sens. J. 2013, 13, 4638–4647. [Google Scholar] [CrossRef]
- Lenik, J. Application of PVC in constructions of ion selective electrodes for pharmaceutical analysis. In Handbook of Polymers for Pharmaceutical Technologies; Processing and Applications; Kumar Thakur, V., Kumar Thakur, M., Eds.; Wiley Scrivener Publishing: Beverly, MA, USA, 2015; Volume 2, pp. 195–227. [Google Scholar]
- Dumkiewicz, R.; Sykut, K.; Wardak, C. Sequence of characteristics of the ion-selective electrode with the pseudoliquid membrane as a function of active substance concentration. Chem. Anal. 2000, 45, 383–394. [Google Scholar]
- Cosofret, V.V.; Buck, R.P. Pharmaceutical Applications of Membrane Sensors; CRC Press: Boca Raton, FL, USA; Ann Arbor, MI, USA; London, UK; Tokyo, Japan, 2000; pp. 66, 79–82. [Google Scholar]
- Ahmed, Y.M.; Badawy, S.S.; Abdel-Haleem, F.M. Dibenzo-18-crown-6-based carbon paste sensors for the nanomolar potentiometric determination of daclatasvir dihydrochloride: An anti-HCV drug and a potential candidate for treatment of SARS-CoV-2. Microchem. J. 2022, 177, 107276. [Google Scholar] [CrossRef]
- Gallardo-González, J.; Baraket, A.; Bonhomme, A.; Zine, N.; Sigaud, M.; Bausells, J.; Errachid, A. Sensitive Potentiometric Determination of Amphetamine with an All-Solid-State Micro Ion-Selective Electrode. Anal. Lett. 2018, 51, 348–358. [Google Scholar] [CrossRef]
- Shamsipur, M.; Pashaba, A. Interrupting the flux of delocalized electrons on a dibenzo-18-crown-6-embedded graphite sheet and its relative counteraction in the presence of potassium ions. Analyst 2016, 141, 4227–4234. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Goswami, A. Effect of Cation Driven Loading of Dibenzo-18-Crown-6 in Nafion-117 Membrane on the Diffusion and Transport Behavior of Alkali Metal Ions. J. Phys. Chem. B 2009, 113, 12958–12963. [Google Scholar] [CrossRef] [PubMed]
- Zolgharnein, J.; Shahmoradi, G.; Zamani, K. Potentiometric study of complexation of phenylaza-15-crown-5, 4-nitrobenzo-15-crown-5 and dibenzopyridino-18-crown-6 and other derivative of 18-crowns-6 with Na+ ion in methanol. J. Incl. Phenom. Macrocycl. Chem. 2007, 59, 99–103. [Google Scholar] [CrossRef]
- Jackson, D.T.; Nelson, P.N.; Booysen, I.N. Lead ion selective electrodes from dibenzo-18-crown-6 derivatives: An exploratory study Author links open overlay panel. J. Mol. Struct. 2021, 1227, 129575. [Google Scholar] [CrossRef]
- Schoeffel, A.C.; Morikava, F.S.; Urban, A.M.; Novatski, A.; Moraes, G.; Matioli, S.G.; Ferrari, P.C.; Neppelenbroek, K.H.; Farago, P.V.; Urban, V.M. Characterization and antifungal activity of chlorhexidine:β-cyclodextrin inclusion complexes. Ther. Deliv. 2023, 14, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, S.V. Ion-selective electrodes in medicinal drug determination. Russ. Chem. Rev. 2007, 76, 361–395. [Google Scholar] [CrossRef]
- Vlascici, D.; Pruneanu, S.; Olenic, L.; Pogacean, F.; Ostafe, V.; Chiriac, V.; Pica, E.M.; Bolundut, L.C.; Nica, L.; Fagadar-Cosma, E. Manganese(III) Porphyrin-based Potentiometric Sensors for Diclofenac Assay in Pharmaceutical Preparations. Sensors 2010, 10, 8850–8864. [Google Scholar] [CrossRef]
- Lenik, J.; Nieszporek, J. Construction of a glassy carbon ibuprofen electrode modified with multi-walled carbon nanotubes and cyclodextrins. Sens. Actuators 2018, 255, 2282–2289. [Google Scholar] [CrossRef]
- Stefan, R.I.; Aboul—Enein, H.Y. Validation criteria for developing ion-selective membrane electrodes for analysis of pharmaceuticals. Accred. Qual. Assur. 1998, 3, 194–196. [Google Scholar] [CrossRef]







| Qualitative and Quantitative Composition of the Outer Layers (mg) | |||||||
|---|---|---|---|---|---|---|---|
| Number of Electrodes | PVC | DOS | o-NPOE | DBC | KTpClBP | HSBβCD | NaFBP |
| 1 | 60 | 130 | - | 6 | 4 | - | - |
| 2 | 60 | - | 130 | 6 | 4 | - | - |
| 3 | 60 | 130 | - | - | 4 | 6 | - |
| 4 | 60 | - | 130 | - | 4 | 6 | - |
| 5 | 60 | 130 | - | - | - | - | 10 |
| 6 | 60 | - | 130 | - | - | - | 10 |
| Electrode No. Membrane Composition | S ± s, mV Decade−1 | E0, mV | R2 | n | Linear Range, mol L−1 | LD, mol L−1 |
|---|---|---|---|---|---|---|
| 1 DBC+KTpClBP+DOS | 22.5 ± 5.8 | 279.6 ± 74.6 | 0.9959 ± 0.0048 | 5 | 1 × 10−5–1 × 10−3 | 3 × 10−6 |
| 2 DBC+KTpClBP+NPOE | 26.7 ± 3.7 | 96.77 ± 77.0 | 0.9948 ± 0.0026 | 5 | 1 × 10−6–1 × 10−3 | 5 × 10−7 |
| 3 βCD+KTpClBP+DOS | 30.4 ± 2.9 | 214.7 ± 40.6 | 0.9970 ± 0.0026 | 6 | 1 × 10−6–1 × 10−3 | 4 × 10−7 |
| 4 βCD+KTpClBP+NPOE | 11.0 ± 3.2 | 477.6 ± 58.9 | 0.9972 ± 0.0027 | 5 | 1 × 10−5–1 × 10−3 | 2 × 10−6 |
| 5 NaFBP+DOS | 16.0 ± 4.0 | 233.3 ± 85.5 | 0.9948 ± 0.0028 | 6 | 1 × 10−5–1 × 10−3 | 8 × 10−6 |
| 6 NaFBP+NPOE | 26.8 ± 3.4 | 266.5 ± 79.0 | 0.9969 ± 0.0025 | 4 | 1 × 10−6–1 × 10−3 | 2.5 × 10−7 |
| Electrode 2 | Electrode 3 | Electrode 6 | [24] | [23] | |
|---|---|---|---|---|---|
| KCl | 8.36 | 1.19 × 10−1 | 7.12 × 10−1 | − | − |
| NaCl | 2.33 | 1.08 | 7.60 × 10−1 | 2.57 | − |
| CaCl2 | 1.46 × 10−5 | 4.03 × 10−5 | 2.28 × 10−7 | 3.02 × 10−3 | 6.81 × 10−5 |
| MgCl2 | 4.84 × 10−5 | 3.98 × 10−5 | 1.28 × 10−3 | 3.02 × 10−3 | 4.28 × 10−4 |
| Citrate | 7.97 × 10−6 | 3.4 × 10−8 | 8.23 × 10−3 | − | − |
| Mannitol | 2.52 × 10−5 | 1.04 × 10−7 | 1.14 × 10−1 | − | − |
| Glucose | 9.39 × 10−5 | 1.05 × 10−7 | 4.56 × 10−2 | − | − |
| SDS | 6.42 × 10−3 | 2.23 × 10−6 | 5.69 × 10−1 | − | 1.36 × 10−6 |
| Glycerine | 1.79 × 10−4 | 1.48 × 10−4 | 1.21 × 10−5 | − | − |
| Glycol | 1.51 × 10−4 | 2.64 × 10−4 | 1.59 × 10−5 | − | − |
| Sucralose | 1.01 × 10−4 | 4.37 × 10−4 | 4.09 × 10−5 | − | − |
| Xylitol | 1.93 × 10−3 | 1.47 × 10−3 | 3.39 × 10−4 | − | − |
| Sample | Method | Taken mol L−1 | Found mol L−1 | Relative Error, % | RSD, % | Confidence Range, mol L−1 |
|---|---|---|---|---|---|---|
| Pure (Alfa Aesar) | Calibration Curve | 1.00 × 10−4 | 1.01 × 10−4 | 0.76 | 1.89 | 1.01 × 10−4 ± 3 × 10−6 |
| Standard Addition | 1.00 × 10−4 | 1.01 × 10−4 | 1.04 | 2.49 | 1.01 × 10−4 ± 6 × 10−6 | |
| Corsodyl (GlaxoSmith) | Calibration Curve | 2.22 × 10−4 | 2.19 × 10−4 | 1.15 | 0.35 | 2.19 × 10−4 ± 2 × 10−6 |
| Standard Addition | 2.22 × 10−4 | 2.09 × 10−4 | 5.81 | 7.60 | 2.09 × 10−4 ± 3.9 × 10−5 | |
| Eludril Extra (Pierre Fabre) | Calibration Curve | 2.22 × 10−4 | 2.29 × 10−4 | 3.19 | 4.87 | 2.29 × 10−4 ± 2.7 × 10−5 |
| Gran’s Method | 2.22 × 10−4 | 2.24 × 10−4 | 1.20 | 1.12 | 2.24 × 10−4 ± 6 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenik, J.; Sokal, K. A Novel Polymeric Membrane Sensor for Chlorhexidine Determination. Sensors 2023, 23, 9508. https://doi.org/10.3390/s23239508
Lenik J, Sokal K. A Novel Polymeric Membrane Sensor for Chlorhexidine Determination. Sensors. 2023; 23(23):9508. https://doi.org/10.3390/s23239508
Chicago/Turabian StyleLenik, Joanna, and Karolina Sokal. 2023. "A Novel Polymeric Membrane Sensor for Chlorhexidine Determination" Sensors 23, no. 23: 9508. https://doi.org/10.3390/s23239508
APA StyleLenik, J., & Sokal, K. (2023). A Novel Polymeric Membrane Sensor for Chlorhexidine Determination. Sensors, 23(23), 9508. https://doi.org/10.3390/s23239508






