A Flexible PDMS-Based Optical Biosensor for Stretch Monitoring in Cardiac Tissue Samples
Abstract
:1. Introduction
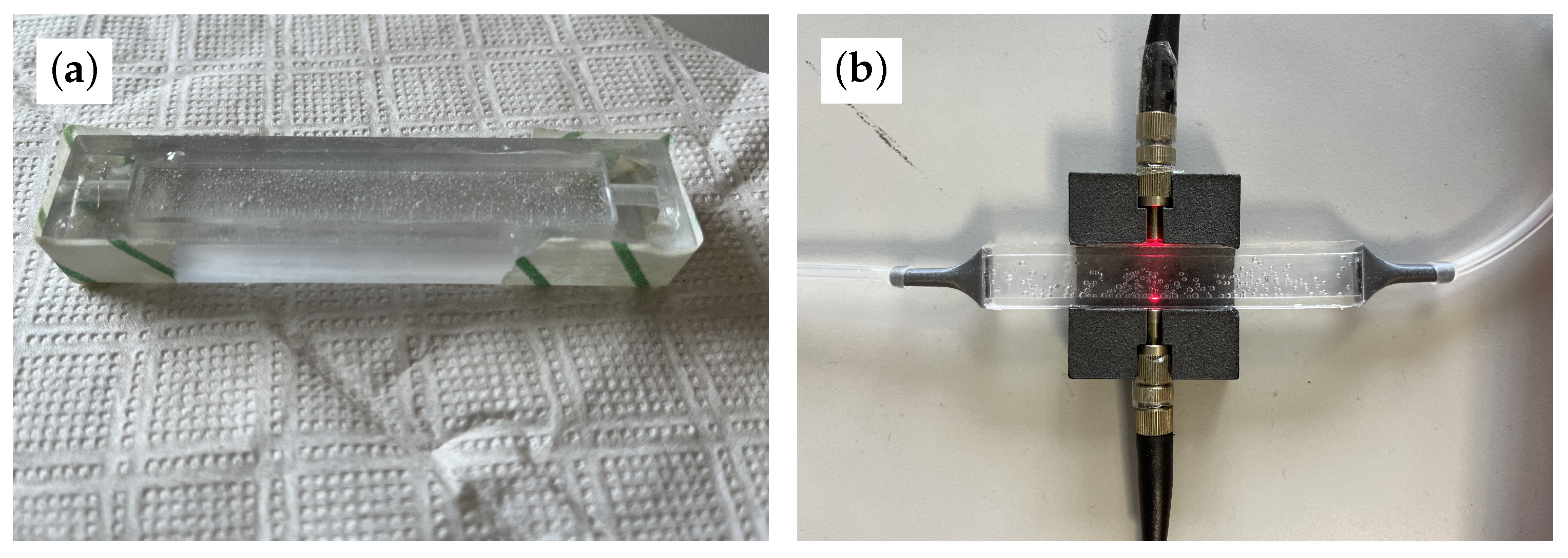
2. Stretch Sensor: Design and Fabrication
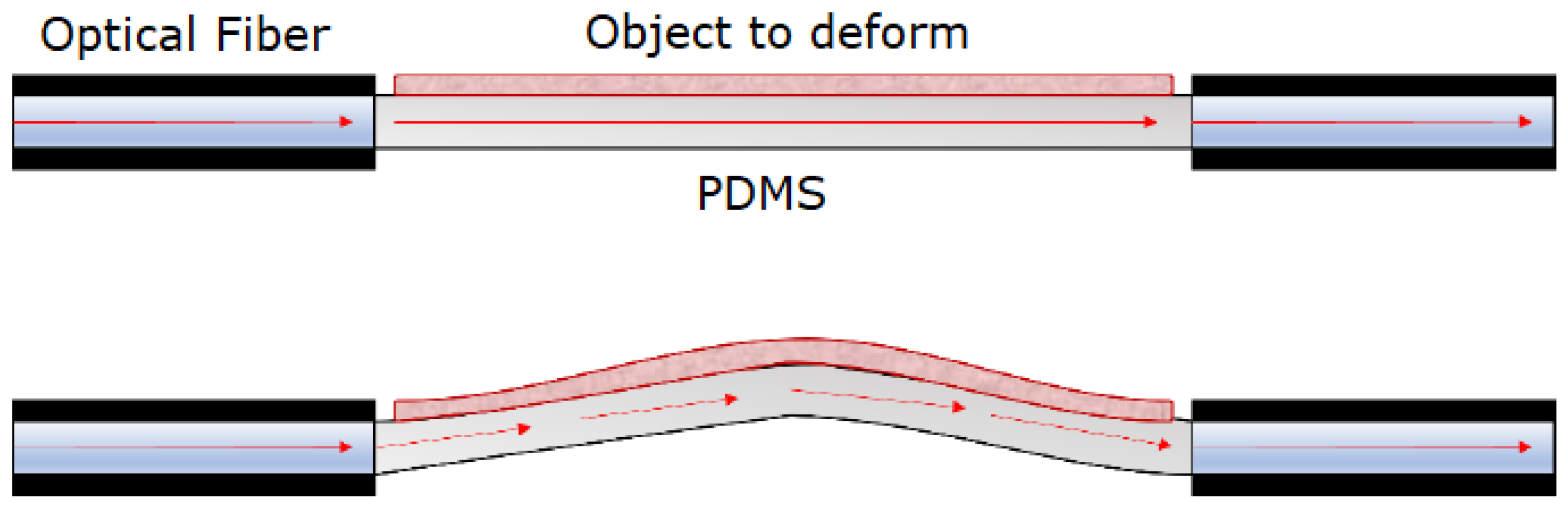
2.1. Stretch Application and Sensing Mechanism
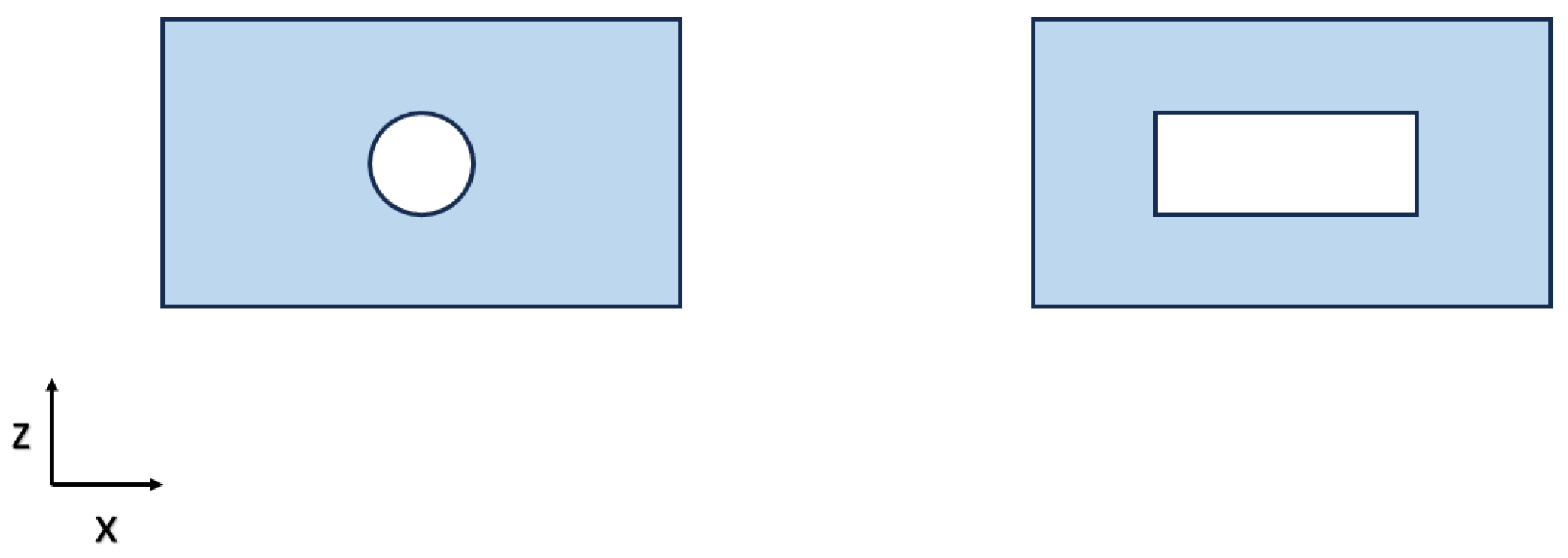
2.2. Sensor Structural Design
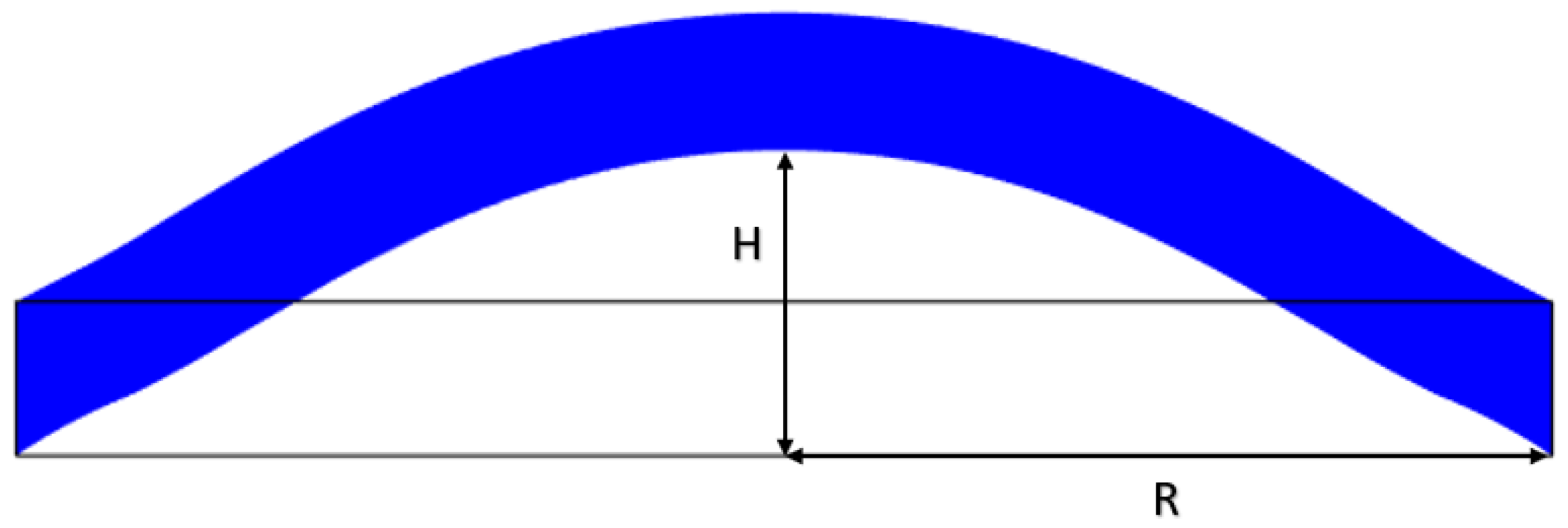
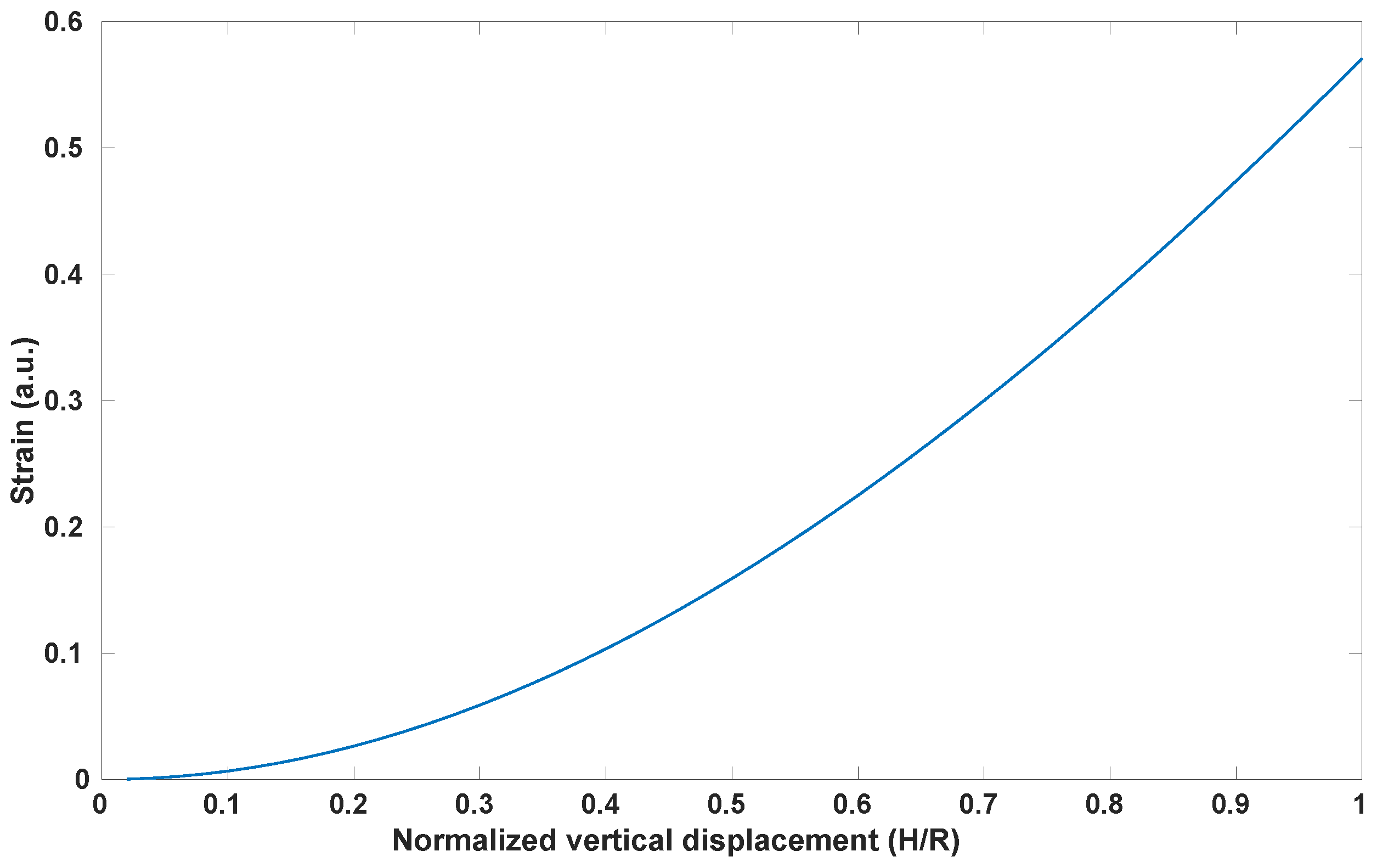
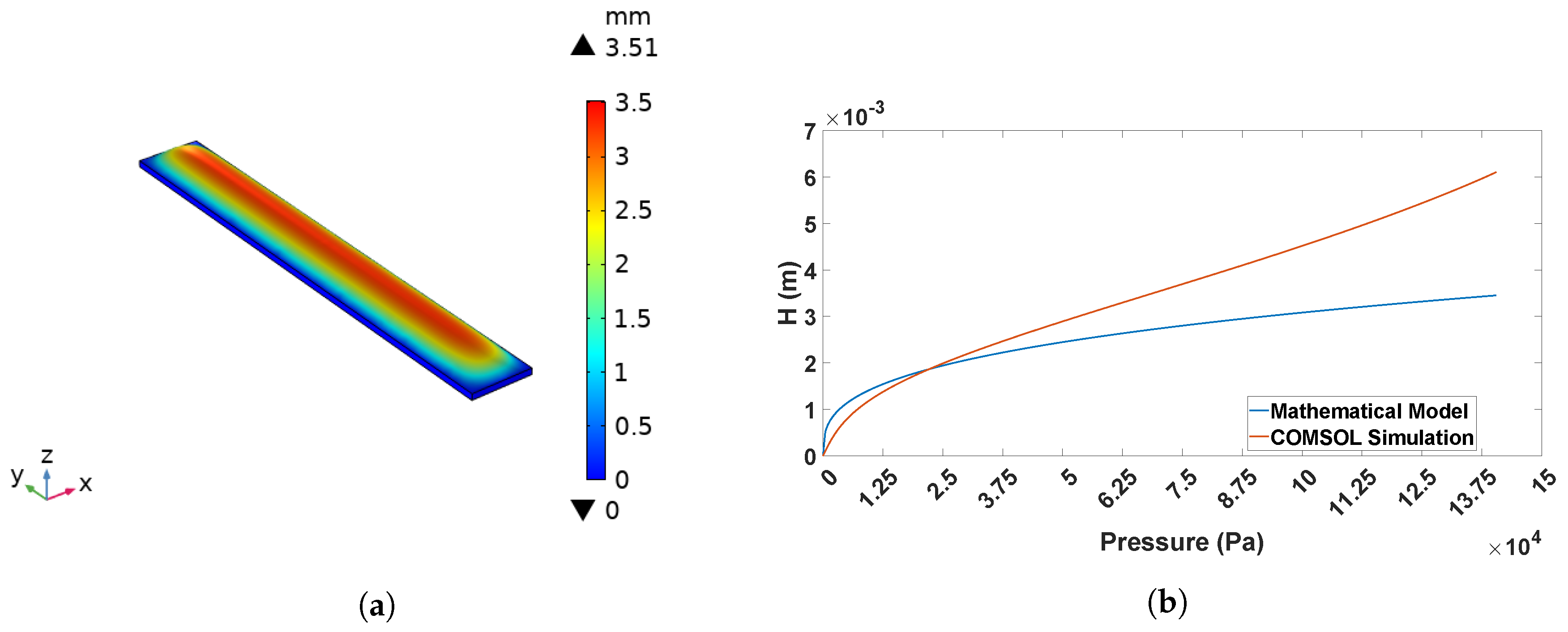
2.3. Mechanical Design Model and Validation
2.4. Stretch Monitoring Applied to Cardiac Research
- Animal models.
- In silico models: software simulations of the cardiac structure under the compound effect.
- In vitro models: 3D tissue slices or human-induced pluripotent stem-cell-derived cardiomyocytes (hiPSC-DM) that are cultured and kept under standard conditions.
- Continuous electrical stimulation.
- Mechanical loading.
- Oxygen and nutrients supply.
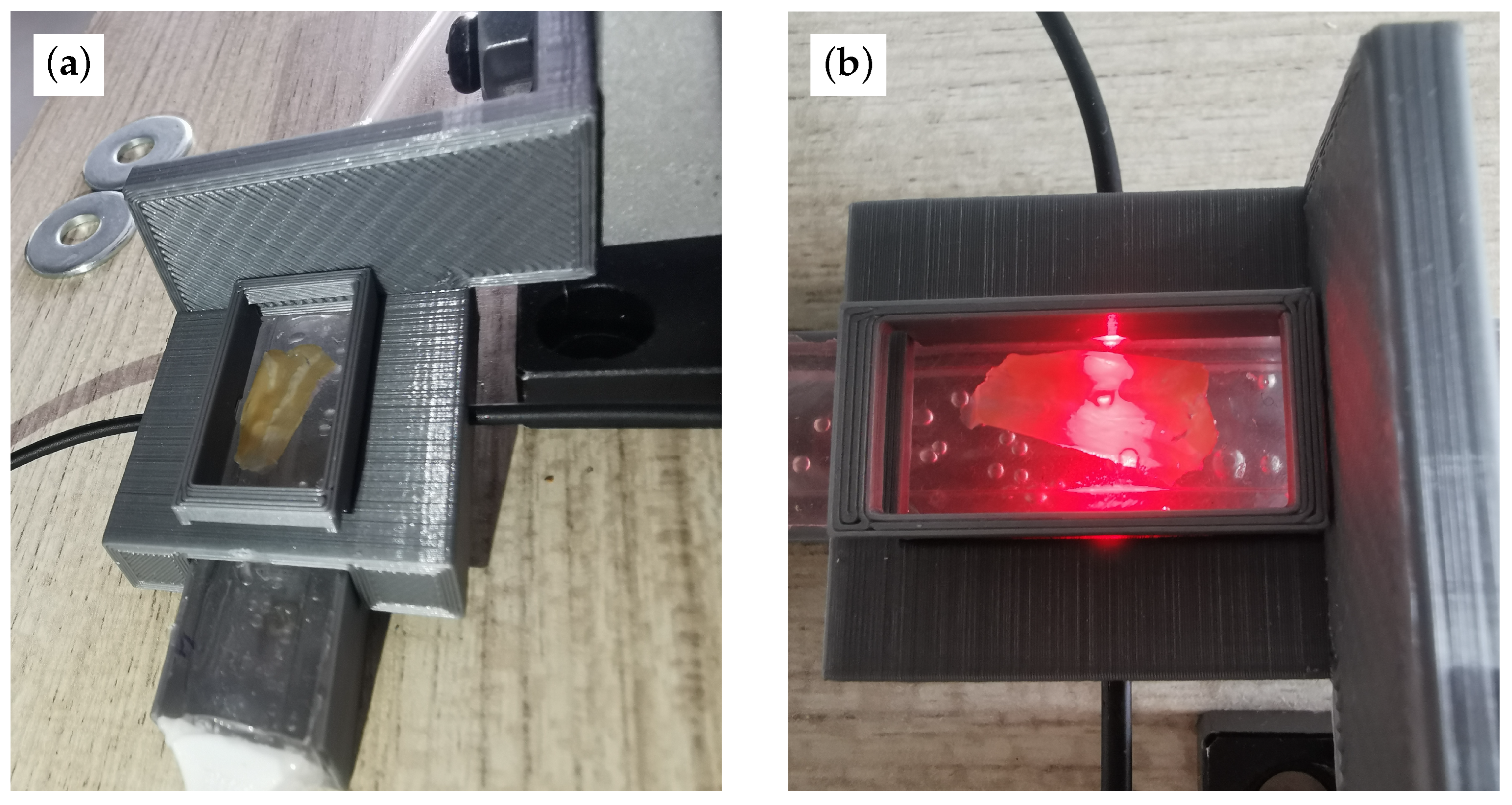
2.5. Mold and Sensor Fabrication
- Evacuation of the silicone encapsulant in a vacuum chamber for 3 h to remove bubbles and subsequent drying heat treatment at 70 C for 1 h.
- Centrifugation at 1200 rpm for 5 min of the container and the encapsulant to eliminate entrapped air and 3 h of 70 C heat-curing.
- Mold with encapsulant left at room temperature for 48 h.
- Prototype 1: Sensing layer of 1 mm and funnels glued with Loctite Super Glue-3®.
- Prototype 2: Sensing layer of 1 mm and funnels glued with Pattex Repair Extreme®.
- Prototype 3: Sensing layer of 1.2 mm and funnels glued with Pattex Repair Extreme®.
3. Measurements Setup
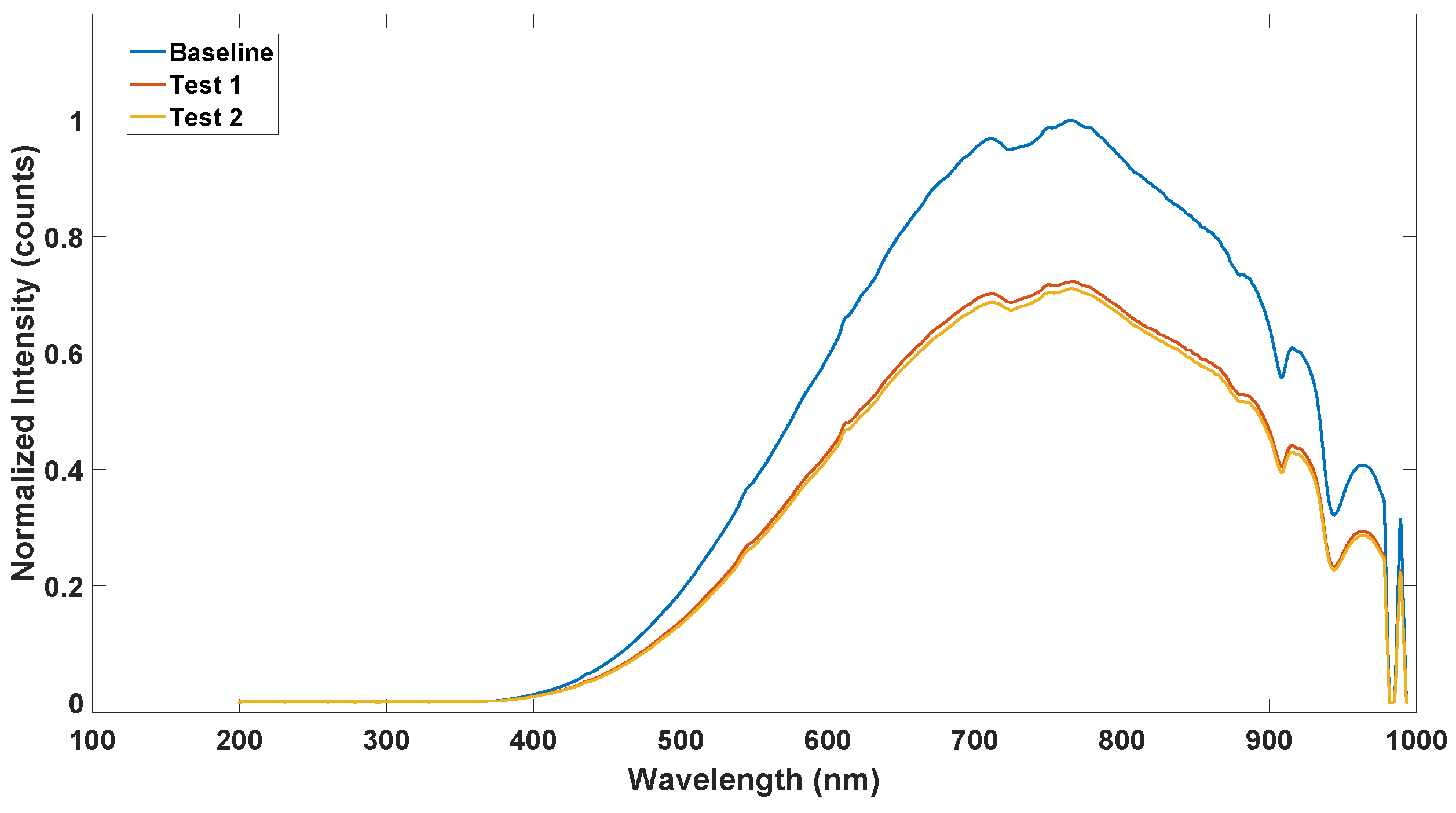
3.1. Optical Path Characterization
3.2. Mechanical Setup
- Pneumatic circuit.
- Sensor mechanical support.
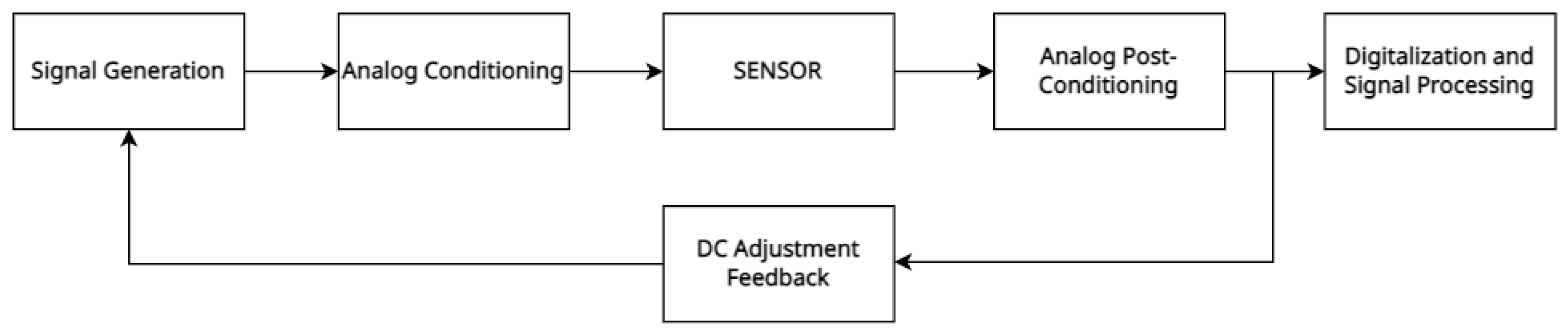
3.3. Electronic Signal Conditioning and Data Acquisition
4. Results
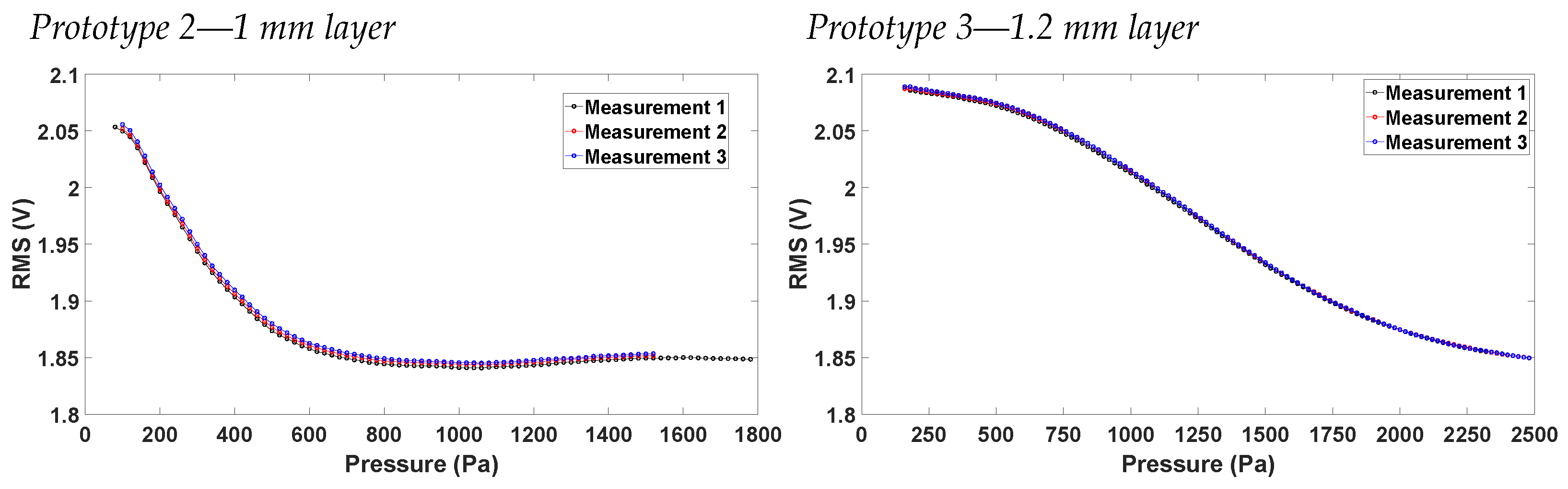
4.1. Voltage vs. Pressure Characterization
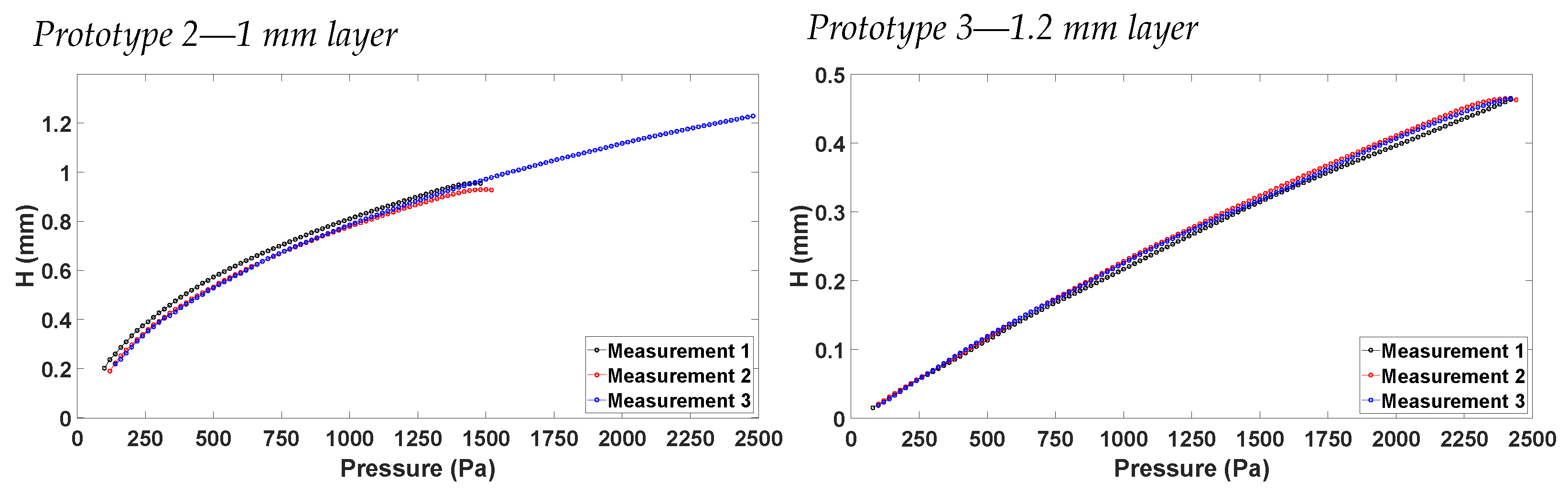
4.2. Vertical Displacement Characterization
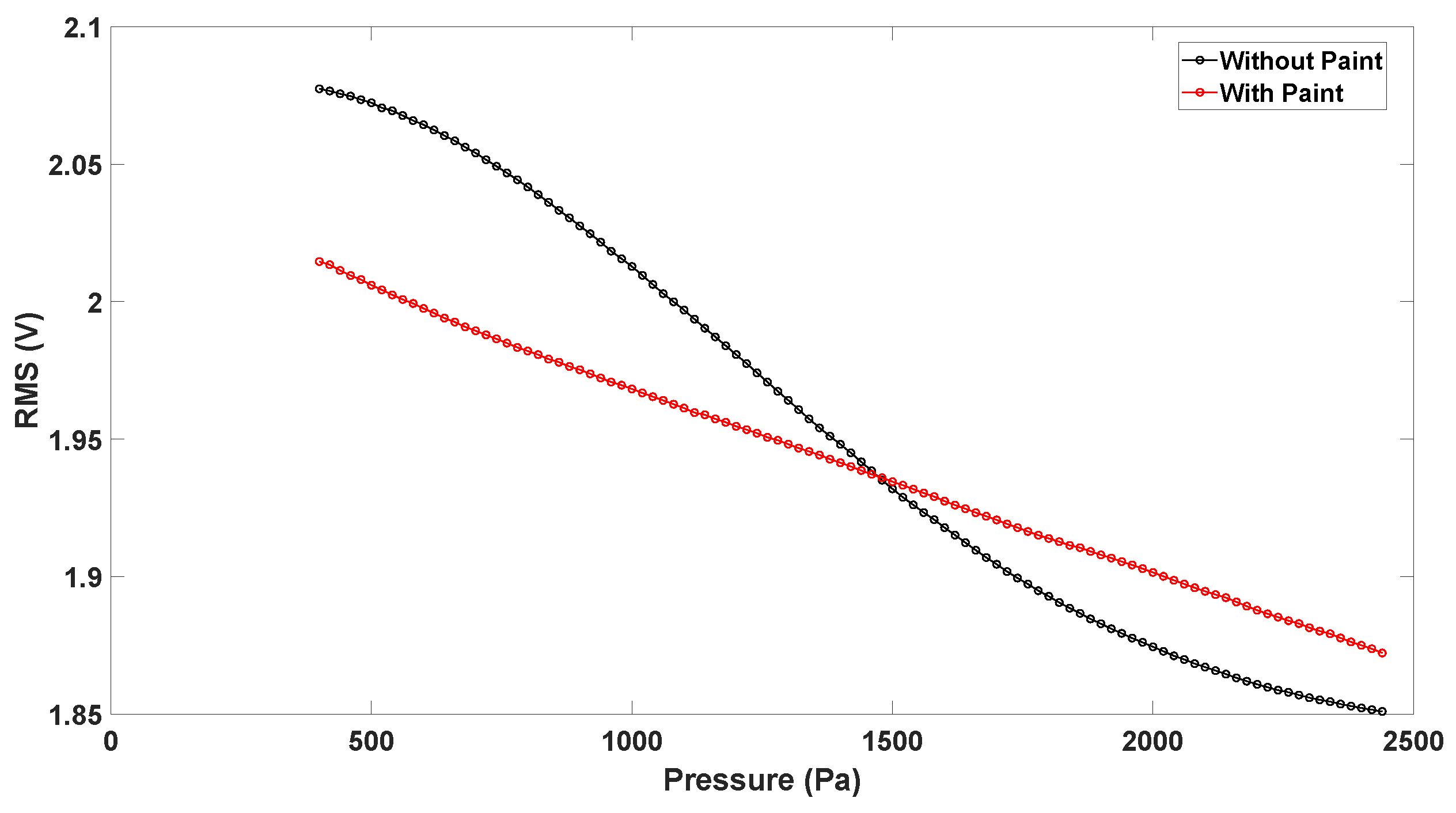
- The paint changes the way light is guided in the sensing layer. It appeared that light was better confined in the membrane, changing the Vrms vs. pressure behaviour.
- Measuring the vertical displacement without painting the top layer was not possible, since PDMS is highly transparent under red light, preventing the proper vertical measurements and changing the Vrms characteristics due to interference from the laser light and fiberoptic radiation.
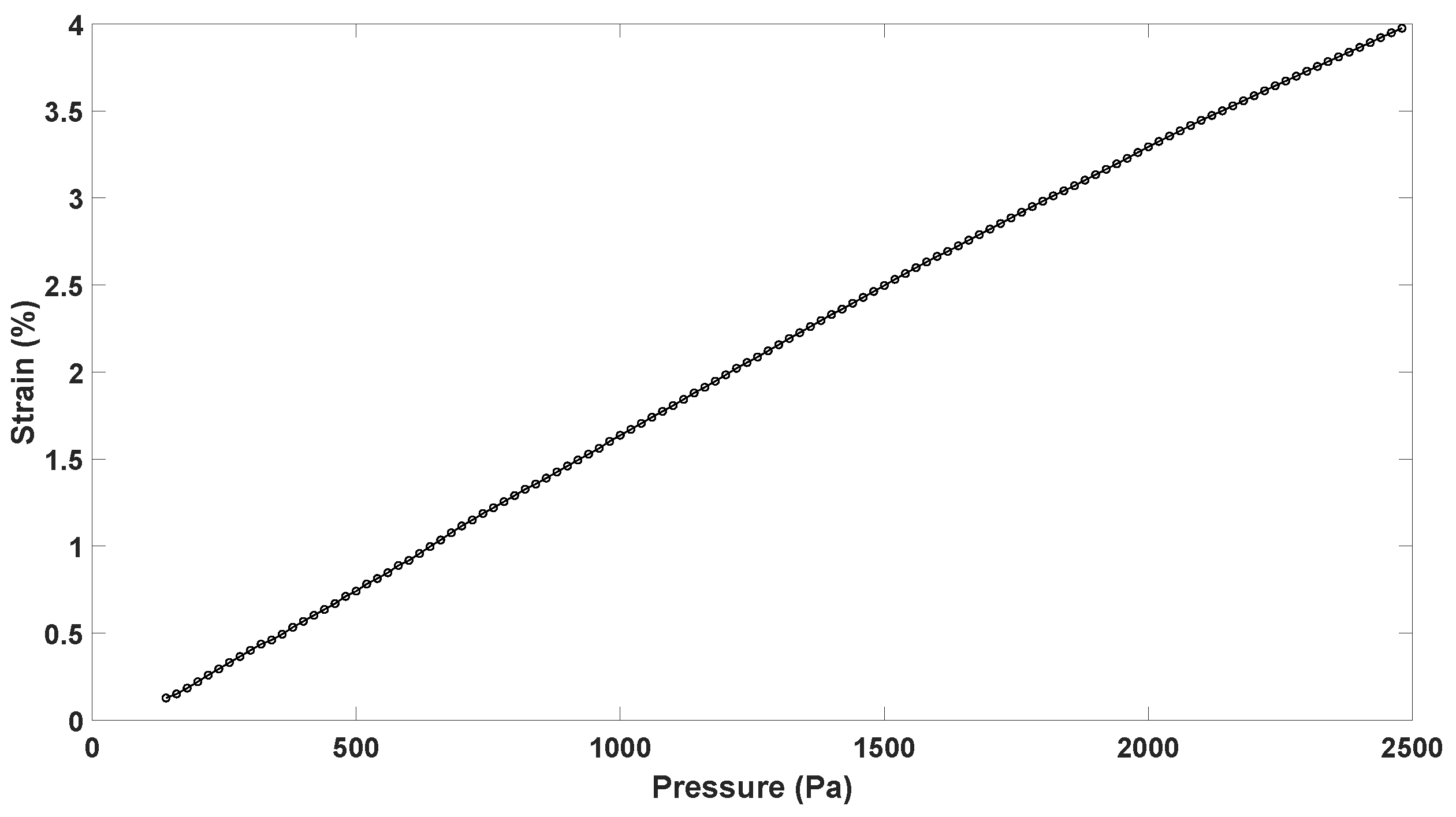
4.3. Stretch Calculation
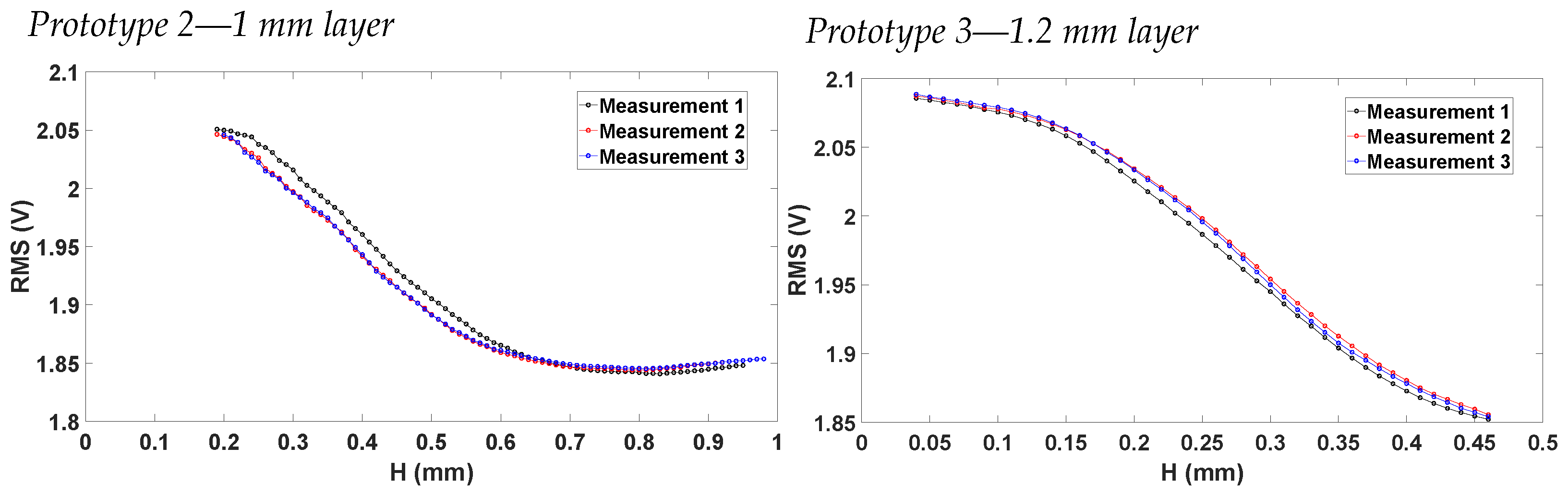
4.4. Vrms vs. Displacement Characteristics
4.5. Impact of Painting on the Vrms Characteristic
5. Discussion and Future Work
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalid, M.A.U.; Chang, S.H. Flexible strain sensors for wearable applications fabricated using novel functional nanocomposites: A review. Compos. Struct. 2022, 284, 115214. [Google Scholar] [CrossRef]
- Liman, M.L.R.; Islam, M.T.; Hossain, M.M. Mapping the Progress in Flexible Electrodes for Wearable Electronic Textiles: Materials, Durability, and Applications. Adv. Electron. Mater. 2022, 8, 2100578. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; He, H.; Zhang, L.; Li, C.; Ouyang, J. Wearable Stretchable Dry and Self-Adhesive Strain Sensors with Conformal Contact to Skin for High-Quality Motion Monitoring. Adv. Funct. Mater. 2021, 31, 2007495. [Google Scholar] [CrossRef]
- Gaio, N.; van Meer, B.; Solano, W.Q.; Bergers, L.; van de Stolpe, A.; Mummery, C.; Sarro, P.M.; Dekker, R. Cytostretch, an Organ-on-Chip platform. Micromachines 2016, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ota, H.; Kiriya, D.; Takei, K.; Javey, A. Flexible Electronics toward Wearable Sensing. Acc. Chem. Res. 2019, 52, 523–533. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Y.; Chen, J. Wearable Biosensors for Non-Invasive Sweat Diagnostics. Biosensors 2021, 11, 245. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Chang, X.; Pan, D.; Song, G.; Guo, Z.; Naik, N. Recent Progress in Essential Functions of Soft Electronic Skin. Adv. Funct. Mater. 2021, 31, 2104686. [Google Scholar] [CrossRef]
- Yuan, Z.; Han, S.T.; Gao, W.; Pan, C. Flexible and Stretchable Strategies for Electronic Skins: Materials, Structure, and Integration. ACS Appl. Electron. Mater. 2022, 4, 1–26. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, J.; Gao, Q.; Zhang, J.; Zhang, J.; Omisore, O.; Wang, L.; Li, H. Polydimethylsiloxane (PDMS)-Based Flexible Resistive Strain Sensors for Wearable Applications. Appl. Sci. 2018, 8, 345. [Google Scholar] [CrossRef]
- Tan, Y.J.; Susanto, G.J.; Anwar Ali, H.P.; Tee, B.C.K. Progress and Roadmap for Intelligent Self-Healing Materials in Autonomous Robotics. Adv. Mater. 2021, 33, 2002800. [Google Scholar] [CrossRef]
- Wang, H.; Ma, X.; Hao, Y. Electronic Devices for Human-Machine Interfaces. Adv. Mater. Interfaces 2017, 4, 1600709. [Google Scholar] [CrossRef]
- Roh, E.; Hwang, B.U.; Kim, D.; Kim, B.Y.; Lee, N.E. Stretchable, Transparent, Ultrasensitive, and Patchable Strain Sensor for Human–Machine Interfaces Comprising a Nanohybrid of Carbon Nanotubes and Conductive Elastomers. ACS Nano 2015, 9, 6252–6261. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Son, D.; Kim, J.; Lee, Y.B.; Song, J.K.; Choi, S.; Lee, D.J.; Kim, J.H.; Lee, M.; Hyeon, T.; et al. Transparent and Stretchable Interactive Human Machine Interface Based on Patterned Graphene Heterostructures. Adv. Funct. Mater. 2015, 25, 375–383. [Google Scholar] [CrossRef]
- Gaumet, A.V.; Ball, R.J.; Nogaret, A. Graphite-polydimethylsiloxane composite strain sensors for in-situ structural health monitoring. Sens. Actuators A Phys. 2021, 332, 113139. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.; Chen, H.; Zhu, Y.; Yang, D.; Hou, Y. Graphene-Based Flexible Strain Sensor Based on PDMS for Strain Detection of Steel Wire Core Conveyor Belt Joints. Sensors 2023, 23, 7473. [Google Scholar] [CrossRef]
- Quirós-Solano, W.F. Wafer-level fabrication of strain gauges on PDMS membranes for low-pressure sensing. In Proceedings of the 2015 IEEE Sensors, Busan, Republic of Korea, 1–4 November 2015. [Google Scholar]
- Souri, H.; Banerjee, H.; Jusufi, A.; Radacsi, N.; Stokes, A.A.; Park, I.; Sitti, M.; Amjadi, M. Wearable and Stretchable Strain Sensors: Materials, Sensing Mechanisms, and Applications. Adv. Intell. Syst. 2020, 2, 2000039. [Google Scholar] [CrossRef]
- Zou, Y.; Tan, P.; Shi, B.; Ouyang, H.; Jiang, D.; Liu, Z.; Li, H.; Yu, M.; Wang, C.; Qu, X.; et al. A bionic stretchable nanogenerator for underwater sensing and energy harvesting. Nat. Commun. 2019, 10, 2695. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Zhang, P.; Chang, C.; Xu, F.; Pu, X. Ultra-stretchable and healable hydrogel-based triboelectric nanogenerators for energy harvesting and self-powered sensing. RSC Adv. 2021, 11, 17437–17444. [Google Scholar] [CrossRef] [PubMed]
- Ra, Y.; Choi, J.H.; La, M.; Park, S.J.; Choi, D. Development of a highly transparent and flexible touch sensor based on triboelectric effect. Funct. Compos. Struct. 2019, 1, 045001. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, B.; Zong, R.; Pan, L.; Li, X.; Yu, X.; Yang, C.; Kong, L.; Dai, Q. Stretchable and Highly Sensitive Optical Strain Sensors for Human-Activity Monitoring and Healthcare. ACS Appl. Mater. Interfaces 2019, 11, 33589–33598. [Google Scholar] [CrossRef]
- Velarte, A.; Otin, A.; Giménez-Gómez, P.; Muñoz-Berbel, X.; Pueyo, E. Fiber-Optic-Based System for High-Resolution Monitoring of Stretch in Excised Tissues. Biosensors 2023, 13, 900. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, X.; Vlassak, J. Plane-strain Bulge Test for Thin Films. J. Mater. Res. 2005, 20, 2360–2370. [Google Scholar] [CrossRef]
- Khoshfetrat Pakazad, S. Stretchable Micro-Electrode Arrays for Electrophysiology. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2015. [Google Scholar]
- Wang, W.E.; Li, L.; Xia, X.; Fu, W.; Liao, Q.; Lan, C.; Yang, D.; Chen, H.; Yue, R.; Zeng, C.; et al. Dedifferentiation, Proliferation, and Redifferentiation of Adult Mammalian Cardiomyocytes After Ischemic Injury. Circulation 2017, 136, 834–848. [Google Scholar] [CrossRef]
- Louch, W.E.; Sheehan, K.A.; Wolska, B.M. Methods in cardiomyocyte isolation, culture, and gene transfer. J. Mol. Cell. Cardiol. 2011, 51, 288–298. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- Fischer, C.; Milting, H.; Fein, E.; Reiser, E.; Lu, K.; Seidel, T.; Schinner, C.; Schwarzmayr, T.; Schramm, R.; Tomasi, R.; et al. Long-term functional and structural preservation of precision-cut human myocardium under continuous electromechanical stimulation in vitro. Nat. Commun. 2019, 10, 117. [Google Scholar] [CrossRef]
- Watson, S.A.; Duff, J.; Bardi, I.; Zabielska, M.; Atanur, S.S.; Jabbour, R.J.; Simon, A.; Tomas, A.; Smolenski, R.T.; Harding, S.E.; et al. Biomimetic electromechanical stimulation to maintain adult myocardial slices in vitro. Nat. Commun. 2019, 10, 2168. [Google Scholar] [CrossRef]
- Carlos-Oliveira, M.; Lozano-Juan, F.; Occhetta, P.; Visone, R.; Rasponi, M. Current strategies of mechanical stimulation for maturation of cardiac microtissues. Biophys. Rev. 2021, 13, 717–727. [Google Scholar] [CrossRef]
- Khokhlova, A.; Iribe, G.; Katsnelson, L.; Naruse, K.; Solovyova, O. The effects of load on transmural differences in contraction of isolated mouse ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2018, 114, 276–287. [Google Scholar] [CrossRef]
- Miller, J.M.; Meki, M.H.; Elnakib, A.; Ou, Q.; Abouleisa, R.R.E.; Tang, X.L.; Salama, A.B.M.; Gebreil, A.; Lin, C.; Abdeltawab, H.; et al. Biomimetic cardiac tissue culture model (CTCM) to emulate cardiac physiology and pathophysiology ex vivo. Commun. Biol. 2022, 5, 934. [Google Scholar] [CrossRef]
- Ashesives Research. Product Information Sheet ARcare 8939; Adhesives Research: Glen Rock, PA, USA, 2014. [Google Scholar]
- Papanicolaou, G.; Zaoutsos, S. Viscoelastic constitutive modeling of creep and stress relaxation in polymers and polymer matrix composites. In Creep and Fatigue in Polymer Matrix Composites, 2nd ed.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Swatson, UK, 2019; pp. 3–59. [Google Scholar] [CrossRef]
- Abgrall, P.; Conedera, V.; Camon, H.; Gue, A.-M.; Nguyen, N.-T. SU-8 as a structural material for labs-on-chips and microelectromechanical systems. Electrophoresis 2007, 28, 4539–4551. [Google Scholar] [CrossRef]
- Parida, O.P.; Bhat, N. Characterization of Optical Properties of Su-8 and Fabrication of Optical Componenets. In Proceedings of the International Conference on Optics and Photonics, Chandigarh, India, 30 October–1 November 2009. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sannino, A.; Velarte, A.; Otín, A.; Artigas, J.I.; Oliván-Viguera, A. A Flexible PDMS-Based Optical Biosensor for Stretch Monitoring in Cardiac Tissue Samples. Sensors 2023, 23, 9454. https://doi.org/10.3390/s23239454
Sannino A, Velarte A, Otín A, Artigas JI, Oliván-Viguera A. A Flexible PDMS-Based Optical Biosensor for Stretch Monitoring in Cardiac Tissue Samples. Sensors. 2023; 23(23):9454. https://doi.org/10.3390/s23239454
Chicago/Turabian StyleSannino, Andrea, Antonio Velarte, Aránzazu Otín, José Ignacio Artigas, and Aida Oliván-Viguera. 2023. "A Flexible PDMS-Based Optical Biosensor for Stretch Monitoring in Cardiac Tissue Samples" Sensors 23, no. 23: 9454. https://doi.org/10.3390/s23239454
APA StyleSannino, A., Velarte, A., Otín, A., Artigas, J. I., & Oliván-Viguera, A. (2023). A Flexible PDMS-Based Optical Biosensor for Stretch Monitoring in Cardiac Tissue Samples. Sensors, 23(23), 9454. https://doi.org/10.3390/s23239454





