Sex Determination of Human Nails Based on Attenuated Total Reflection Fourier Transform Infrared Spectroscopy in Forensic Context
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Spectra Acquisition
2.3. Data Pre-Processing and Statistical Analysis
2.4. Partial Least-Square Discriminant Analysis (PLS-DA)
2.5. Artificial Neural Network (ANN)
3. Results
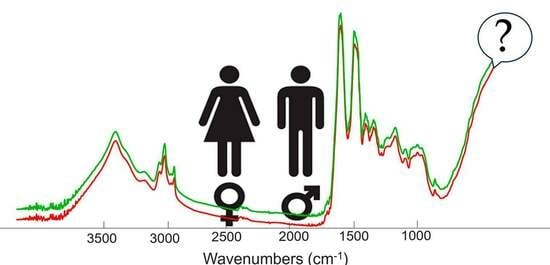
3.1. Average Mean Raw Spectra of Nails
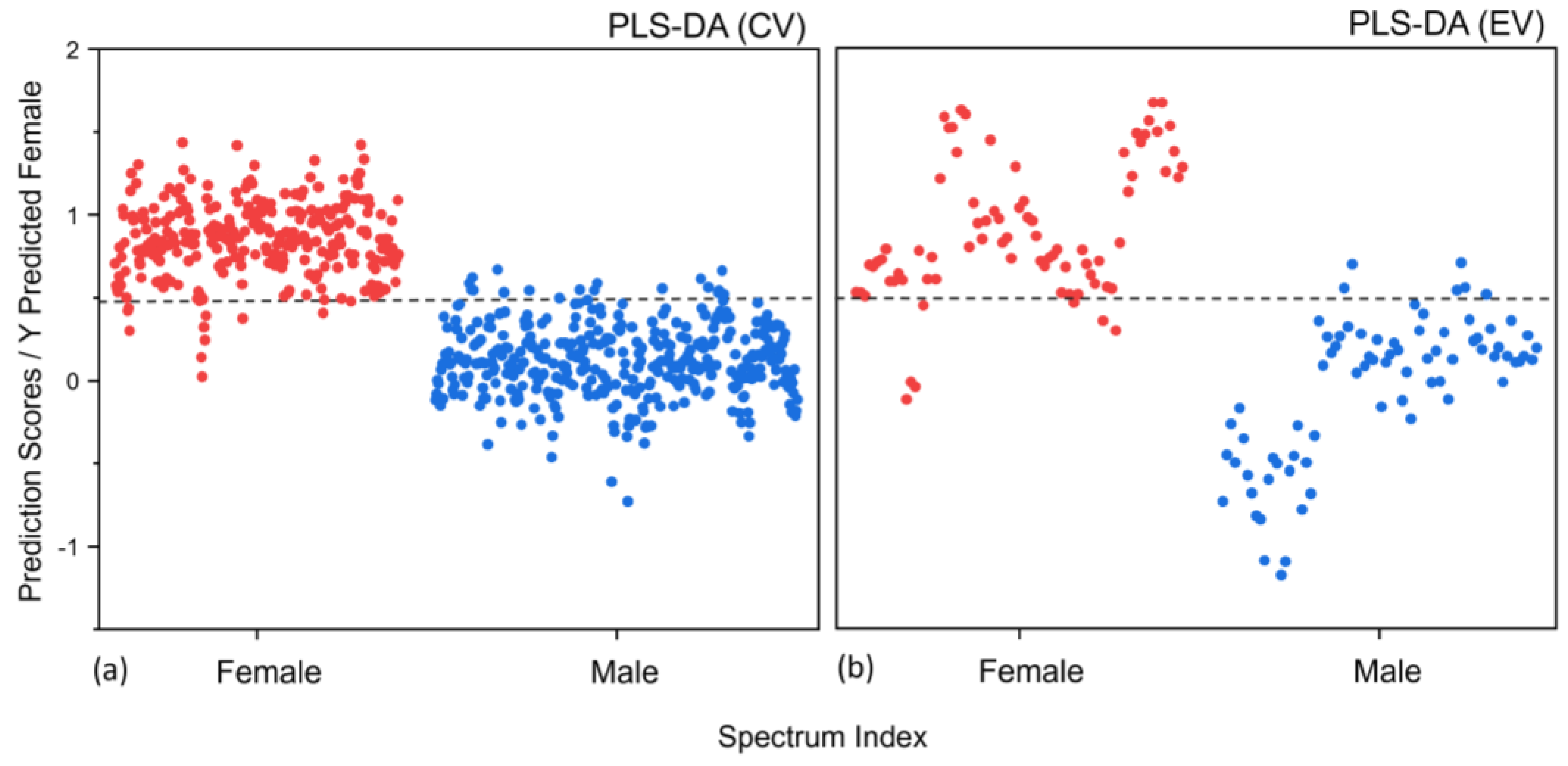
3.2. Partial Least-Square Classification (PLS-DA)
3.3. Artificial Neural Network (ANN)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmar, P.; Rathod, G.B. Forensic Onychology: An essential entity against crime. J. Indian Acad. Forensic Med. 2012, 34, 355–357. [Google Scholar]
- Parabon Snapshot, D. Phenotyping Service—Powered by Parabon NanoLabs; Parabon NanoLabs: Reston, VA, USA, 2017. [Google Scholar]
- Suzuki, O.; Hattori, H.; Asano, M. Nails as useful materials for detection of methamphetamine or amphetamine abuse. Forensic Sci. Int. 1984, 24, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lander, H.; Hodge, P.R.; Crisp, C.S. Arsenis in the Hair and Nails: Its Significance in Acute Arsenical Poisoning. J. Forensic Med. 1965, 12, 52–67. [Google Scholar] [PubMed]
- Mitu, B.; Cerda, M.; Hrib, R.; Trojan, V.; Halámková, L. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy for Forensic Screening of Long-Term Alcohol Consumption from Human Nails. ACS Omega 2023, 8, 22203–22210. [Google Scholar] [CrossRef] [PubMed]
- Toprak, S.; Kahriman, F.; Dogan, Z.; Ersoy, G.; Can, E.Y.; Akpolat, M.; Can, M. The potential of Raman and FT-IR spectroscopic methods for the detection of chlorine in human nail samples. Forensic Sci. Med. Pathol. 2020, 16, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.; Bansal, S. The nail as an investigative tool in medicine: What a dermatologist ought to know. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Verma, R.; Kumar, R.; Chauhan, R.; Sharma, V. Chemometric analysis of ATR-FTIR spectra of fingernail clippings for classification and prediction of sex in forensic context. Microchem. J. 2020, 159, 105504. [Google Scholar] [CrossRef]
- Daniel, C.R., III; Piraccini, B.M.; Tosti, A. The nail and hair in forensic science. J. Am. Acad. Dermatol. 2004, 50, 258–261. [Google Scholar] [CrossRef]
- Widjaja, E.; Lim, G.H.; An, A. A novel method for human gender classification using Raman spectroscopy of fingernail clippings. Analyst 2008, 133, 493–498. [Google Scholar] [CrossRef]
- Liu, G.-L.; Kazarian, S.G. Recent advances and applications to cultural heritage using ATR-FTIR spectroscopy and ATR-FTIR spectroscopic imaging. Analyst 2022, 147, 1777–1797. [Google Scholar] [CrossRef]
- Dittmar, M.; Dindorf, W.; Banerjee, A. Organic elemental composition in fingernail plates varies between sexes and changes with increasing age in healthy humans. Gerontology 2008, 54, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, A. Human nails as a biomarker of element exposure. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Nigg, H.N., Doerge, D.R., Eds.; Springer: New York, NY, USA, 2005; Volume 185, pp. 141–177. [Google Scholar]
- Baswan, S.; Kasting, G.B.; Li, S.K.; Wickett, R.; Adams, B.; Eurich, S.; Schamper, R. Understanding the formidable nail barrier: A review of the nail microstructure, composition and diseases. Mycoses 2017, 60, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Kwon, K.H. A Comparative Analysis of Major Mineral Contents of Nails by Gender in Adolescents. Korean J. Aesthet. Cosmetol. 2014, 12, 837–843. [Google Scholar]
- Rodushkin, I.; Axelsson, M.D. Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part I. Analytical methodology. Sci. Total Environ. 2000, 250, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Benzeval, I.; Bowen, C.R.; Guy, R.H.; Delgado-Charro, M.B. Effects of iontophoresis, hydration, and permeation enhancers on human nail plate: Infrared and impedance spectroscopy assessment. Pharm. Res. 2013, 30, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Muddasani, S.; Lin, G.; Hooper, J.; Sloan, S.B. Nutrition and nail disease. Clin. Dermatol. 2021, 39, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Brzózka, P.; Kolodziejski, W. Sex-related chemical differences in keratin from fingernail plates: A solid-state carbon-13 NMR study. Rsc Adv. 2017, 7, 28213–28223. [Google Scholar] [CrossRef]
- He, K. Trace elements in nails as biomarkers in clinical research. Eur. J. Clin. Investig. 2011, 41, 98–102. [Google Scholar] [CrossRef]
- Coopman, R.; Van de Vyver, T.; Kishabongo, A.S.; Katchunga, P.; Van Aken, E.H.; Cikomola, J.; Monteyne, T.; Speeckaert, M.M.; Delanghe, J.R. Glycation in human fingernail clippings using ATR-FTIR spectrometry, a new marker for the diagnosis and monitoring of diabetes mellitus. Clin. Biochem. 2017, 50, 62–67. [Google Scholar] [CrossRef]
- Jurgeleviciene, I.; Stanislovaitiene, D.; Tatarunas, V.; Jurgelevicius, M.; Zaliuniene, D. Assessment of absorption of glycated nail proteins in patients with diabetes mellitus and diabetic retinopathy. Medicina 2020, 56, 658. [Google Scholar] [CrossRef]
- Farhan, K.M.; Sastry, T.P.; Mandal, A.B. Comparative study on secondary structural changes in diabetic and non-diabetic human finger nail specimen by using FTIR spectra. Clin. Chim. Acta 2011, 412, 386–389. [Google Scholar] [CrossRef]
- Sakudo, A.; Kuratsune, H.; Kato, Y.H.; Ikuta, K. Secondary structural changes of proteins in fingernails of chronic fatigue syndrome patients from Fourier-transform infrared spectra. Clin. Chim. Acta 2009, 402, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Al-Jorani, K.; Rüther, A.; Martin, M.; Haputhanthri, R.; Deacon, G.B.; Li, H.L.; Wood, B.R. The application of ATR-FTIR spectroscopy and the reversible DNA conformation as a sensor to test the effectiveness of platinum (II) anticancer drugs. Sensors 2018, 18, 4297. [Google Scholar] [CrossRef] [PubMed]
- Vigano, C.; Ruysschaert, J.-M.; Goormaghtigh, E. Sensor applications of attenuated total reflection infrared spectroscopy. Talanta 2005, 65, 1132–1142. [Google Scholar] [CrossRef]
- Mizaikoff, B. Peer reviewed: Mid-IR fiber-optic sensors. Anal. Chem. 2003, 75, 258A–267A. [Google Scholar] [CrossRef]
- Rigler, P.; Ulrich, W.P.; Hoffmann, P.; Mayer, M.; Vogel, H. Reversible immobilization of peptides: Surface modification and in situ detection by attenuated total reflection FTIR spectroscopy. ChemPhysChem 2003, 4, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Lucena, R.; Cárdenas, S.; Gallego, M.; Valcárcel, M. ATR-FTIR membrane-based sensor for the simultaneous determination of surfactant and oil total indices in industrial degreasing baths. Analyst 2006, 131, 415–421. [Google Scholar] [CrossRef]
- Srivastava, R.; Mallick, P.K.; Rautaray, S.S.; Pandey, M. Computational Intelligence for Machine Learning and Healthcare Informatics; Walter de Gruyter GmbH & Co KG: Berlin, Germany; Boston, MA, USA, 2020; Volume 1. [Google Scholar]
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaría, J.; Fadhel, M.A.; Al-Amidie, M.; Farhan, L. Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions. J. Big Data 2021, 8, 53. [Google Scholar] [CrossRef]
- Günther, F.; Fritsch, S. neuralnet: Training of Neural Networks. R J. 2010, 2, 30–38. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Baker, M.J.; Hussain, S.R.; Lovergne, L.; Untereiner, V.; Hughes, C.; Lukaszewski, R.A.; Thiéfin, G.; Sockalingum, G.D. Developing and understanding biofluid vibrational spectroscopy: A critical review. Chem. Soc. Rev. 2016, 45, 1803–1818. [Google Scholar] [CrossRef]
- Balki, I.; Amirabadi, A.; Levman, J.; Martel, A.L.; Emersic, Z.; Meden, B.; Garcia-Pedrero, A.; Ramirez, S.C.; Kong, D.; Moody, A.R.; et al. Sample-Size Determination Methodologies for Machine Learning in Medical Imaging Research: A Systematic Review. Can. Assoc. Radiol. J. 2019, 7, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, D.N.; Roger, J.-M.; Lesnoff, M. Different Methods for Determining the Dimensionality of Multivariate Models. Front. Anal. Sci. 2021, 1, 754447. [Google Scholar] [CrossRef]
- Mendez, K.M.; Reinke, S.N.; Broadhurst, D.I. A comparative evaluation of the generalised predictive ability of eight machine learning algorithms across ten clinical metabolomics data sets for binary classification. Metabolomics 2019, 15, 150. [Google Scholar] [CrossRef]
- Riedmiller, M. Advanced supervised learning in multi-layer perceptrons—From backpropagation to adaptive learning algorithms. Comput. Stand. Interfaces 1994, 16, 265–278. [Google Scholar] [CrossRef]
- Diago, M.P.; Fernández-Novales, J.; Gutiérrez, S.; Marañón, M.; Tardaguila, J. Development and Validation of a New Methodology to Assess the Vineyard Water Status by On-the-Go Near Infrared Spectroscopy. Front. Plant Sci. 2018, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: Berlin/Heidelberg, Germany, 2009; p. 764. [Google Scholar]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef]
- Barton, P.M. A Forensic Investigation of Single Human Hair Fibres Using FTIR-ATR Spectroscopy and Chemometrics. Ph.D. Thesis, Queensland University of Technology, Brisbane City, Australia, 2011. [Google Scholar]
- Al-Hetlani, E.; Halámková, L.; Amin, M.O.; Lednev, I.K. Differentiating smokers and nonsmokers based on Raman spectroscopy of oral fluid and advanced statistics for forensic applications. J. Biophotonics 2020, 13, e201960123. [Google Scholar] [CrossRef]
- Golik, P.; Doetsch, P.; Ney, H. Cross-Entropy vs. Squared Error Training: A Theoretical and Experimental Comparison; Interspeech: Dublin, Ireland, 2013. [Google Scholar]
- Biancolillo, A.; Marini, F.; Ruckebusch, C.; Vitale, R. Chemometric strategies for spectroscopy-based food authentication. Appl. Sci. 2020, 10, 6544. [Google Scholar] [CrossRef]
- Takamura, A.; Halámková, L.; Ozawa, T.; Lednev, I.K. Phenotype Profiling for Forensic Purposes: Determining Donor Sex Based on Fourier Transform Infrared Spectroscopy of Urine Traces. Anal. Chem. 2019, 91, 6288–6295. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Alonso-Salces, R.M.; Héberger, K. Supervised pattern recognition in food analysis. J. Chromatogr. A 2007, 1158, 196–214. [Google Scholar] [CrossRef] [PubMed]
- Montesinos López, O.A.; Montesinos López, A.; Crossa, J. Fundamentals of Artificial Neural Networks and Deep Learning. In Multivariate Statistical Machine Learning Methods for Genomic Prediction; Montesinos López, O.A., Montesinos López, A., Crossa, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 379–425. [Google Scholar]
- Song, K.; Li, L.; Li, S.; Tedesco, L.P.; Duan, H.; Li, Z.; Shi, K.; Du, J.; Zhao, Y.; Shao, T. Using Partial Least Squares-Artificial Neural Network for Inversion of Inland Water Chlorophyll-a. Geosci. Remote Sens. IEEE Trans. 2014, 52, 1502–1517. [Google Scholar] [CrossRef]
- Lavine, B.K.; Workman, J., Jr. Chemometrics: Past, Present, and Future; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2005. [Google Scholar]
- Lavine, B.; Workman, J. Chemometrics. Anal. Chem. 2008, 80, 4519–4531. [Google Scholar] [CrossRef] [PubMed]
- Ralbovsky, N.M.; Halámková, L.; Wall, K.; Anderson-Hanley, C.; Lednev, I.K. Screening for Alzheimer’s Disease Using Saliva: A New Approach Based on Machine Learning and Raman Hyperspectroscopy. J. Alzheimers Dis. 2019, 71, 1351–1359. [Google Scholar] [CrossRef]
- Krogh, A. What are artificial neural networks? Nat. Biotechnol. 2008, 26, 195–197. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitu, B.; Trojan, V.; Halámková, L. Sex Determination of Human Nails Based on Attenuated Total Reflection Fourier Transform Infrared Spectroscopy in Forensic Context. Sensors 2023, 23, 9412. https://doi.org/10.3390/s23239412
Mitu B, Trojan V, Halámková L. Sex Determination of Human Nails Based on Attenuated Total Reflection Fourier Transform Infrared Spectroscopy in Forensic Context. Sensors. 2023; 23(23):9412. https://doi.org/10.3390/s23239412
Chicago/Turabian StyleMitu, Bilkis, Václav Trojan, and Lenka Halámková. 2023. "Sex Determination of Human Nails Based on Attenuated Total Reflection Fourier Transform Infrared Spectroscopy in Forensic Context" Sensors 23, no. 23: 9412. https://doi.org/10.3390/s23239412
APA StyleMitu, B., Trojan, V., & Halámková, L. (2023). Sex Determination of Human Nails Based on Attenuated Total Reflection Fourier Transform Infrared Spectroscopy in Forensic Context. Sensors, 23(23), 9412. https://doi.org/10.3390/s23239412