The State of the Art on Graphene-Based Sensors for Human Health Monitoring through Breath Biomarkers
Abstract
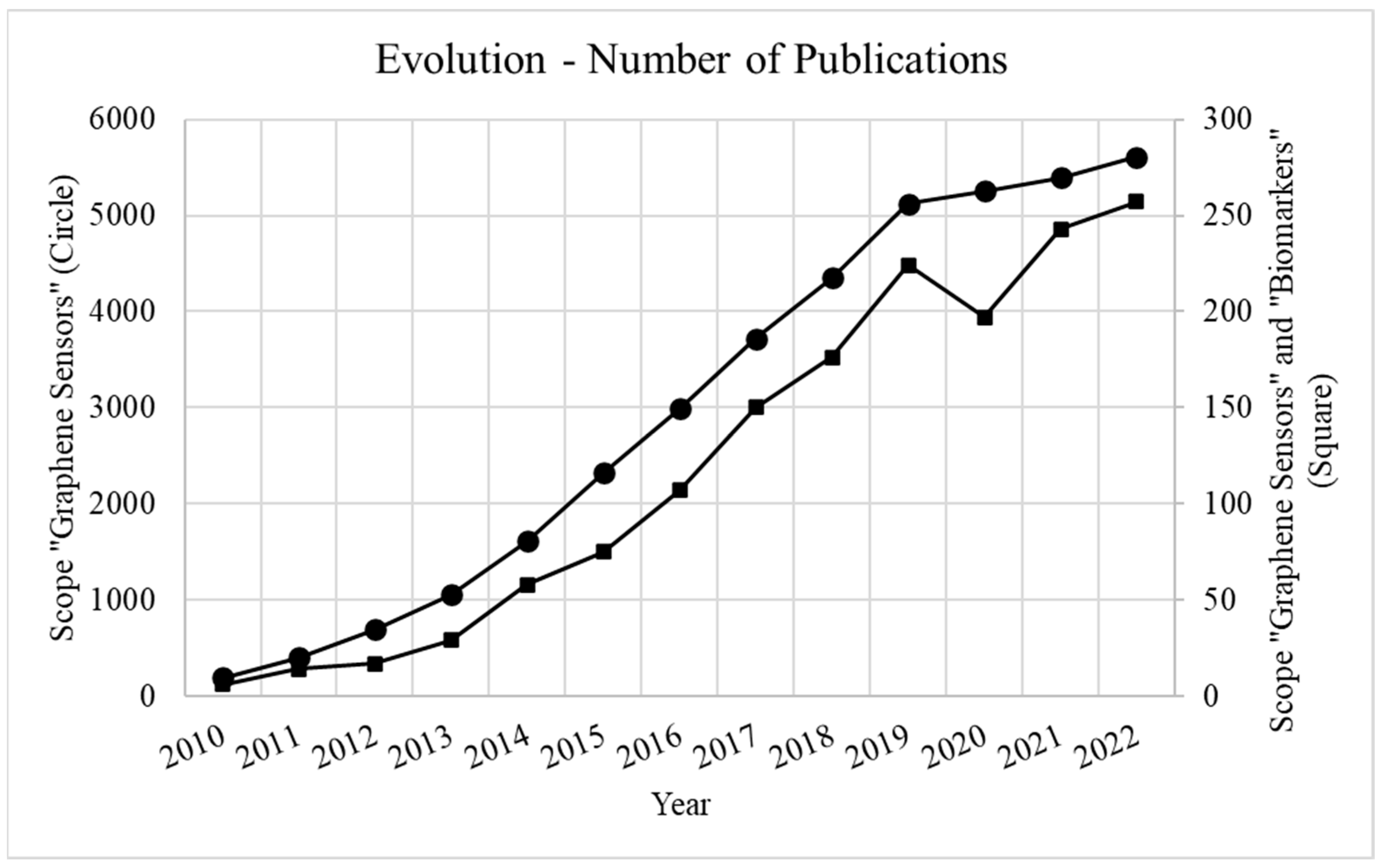
1. Introduction
1.1. Biomarkers
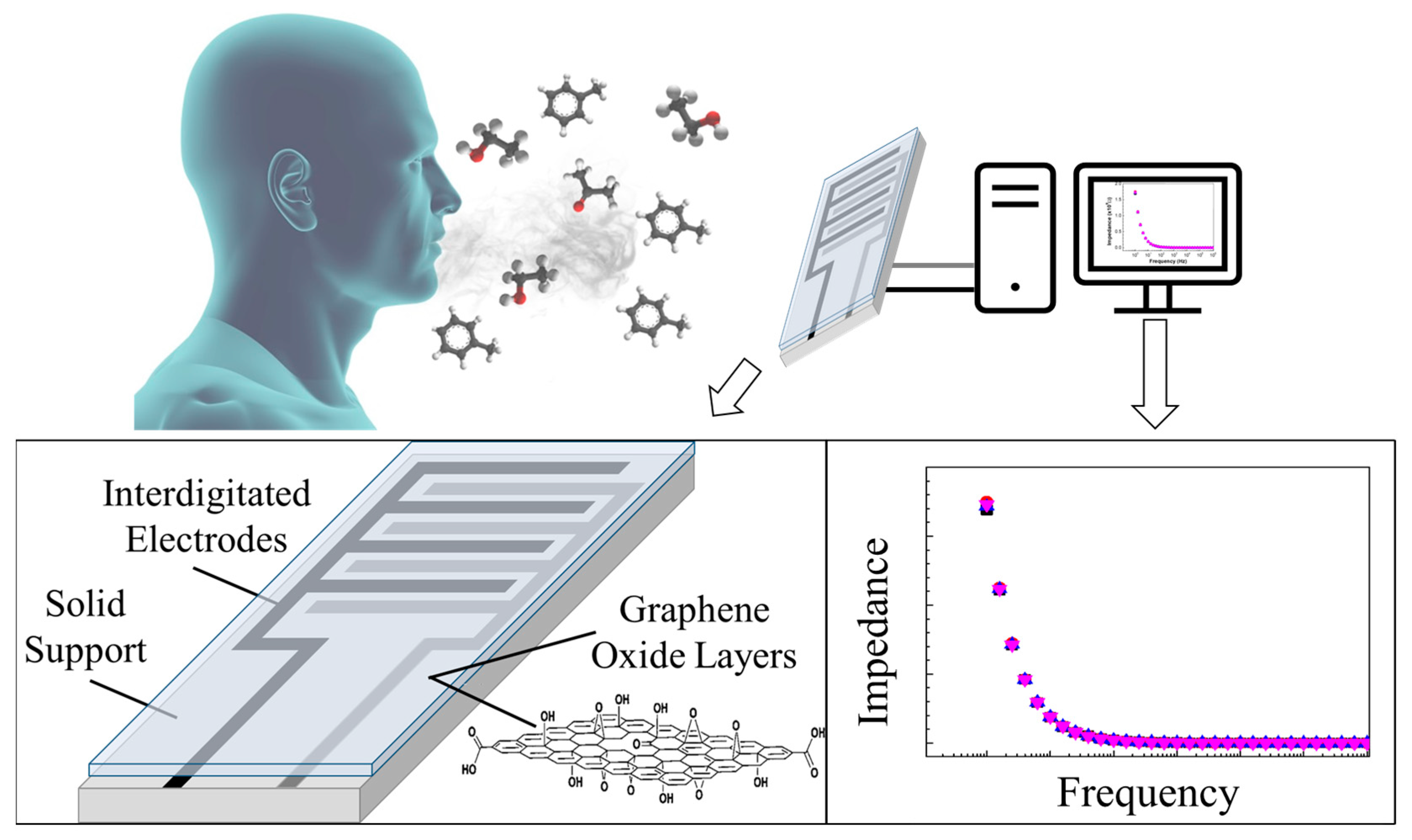
1.2. Graphene-Based Sensors
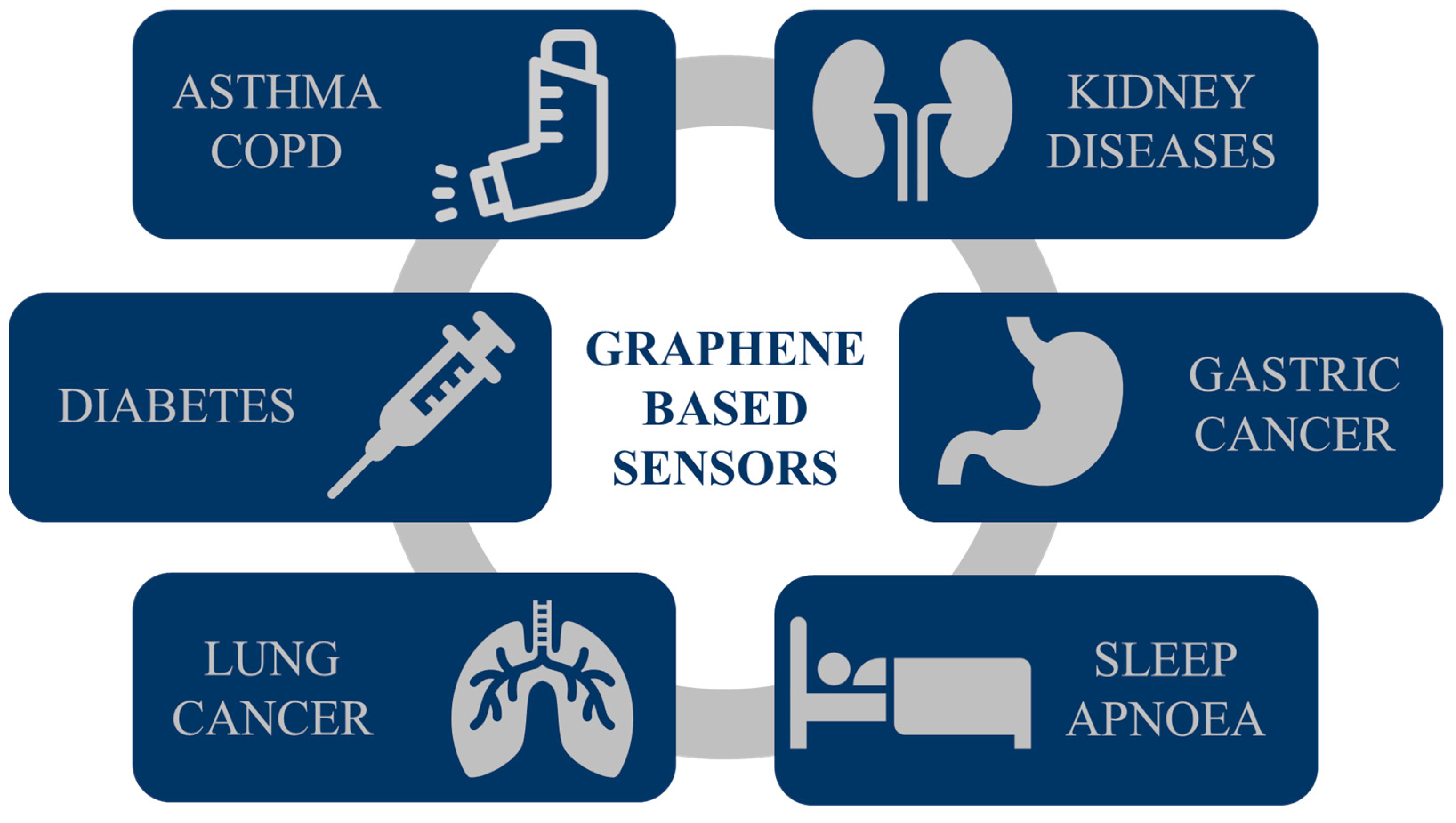
2. Graphene Sensors for Biomarker Detection
2.1. Asthma and COPD
2.2. Chronic Kidney Diseases
2.3. Diabetes
2.4. Gastric Cancer
2.5. Lung Cancer
2.6. Sleep Apnoea
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Etzioni, R.; Urban, N.; Ramsey, S.; McIntosh, M.; Schwartz, S.; Reid, B.; Radich, J.; Anderson, G.; Hartwell, L. The case for early detection. Nat. Rev. Cancer 2003, 3, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, Z.A.; Kumar, U.; Chen, D.D.Y. Review of recent developments in determining volatile organic compounds in exhaled breath as biomarkers for lung cancer diagnosis. Anal. Chim. Acta 2017, 996, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haworth, J.J.; Pitcher, C.K.; Ferrandino, G.; Hobson, A.R.; Pappan, K.L.; Lawson, J.L.D. Breathing new life into clinical testing and diagnostics: Perspectives on volatile biomarkers from breath. Crit. Rev. Clin. Lab. Sci. 2022, 59, 353–372. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M. Biomarker definitions and their application. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Sawyers, C.L. The cancer biomarker problem. Nature 2008, 452, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.C.; Raposo, M.; Vassilenko, V. Breath Volatile Organic Compounds (VOCs) as Biomarkers for the Diagnosis of Pathological Conditions: A Review. Biomed. J. 2023, 46, 100623. [Google Scholar] [CrossRef]
- Amann, A.; Smith, D. Volatile Biomarkers: Non-Invasive Diagnosis in Physiology and Medicine, 1st ed.; Elsevier: Waltham, MA, USA, 2013. [Google Scholar]
- Daneshkhah, A.; Siegel, A.P.; Agarwal, M. Volatile organic compounds: Potential biomarkers for improved diagnosis and monitoring of diabetic wounds. In Wound Healing, Tissue Repair, and Regeneration Diabetes, 1st ed.; Bagchi, D., Das, A., Sashwati, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 491–512. [Google Scholar]
- Amann, A.; Costello, B.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ractiffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Cauchi, M.; Weber, C.M.; Bolt, B.J.; Spratt, P.B.; Bessant, C.; Turner, D.C.; Willis, C.M.; Britton, L.E.; Turner, C.; Morgan, G. Evaluation of gas chromatography mass spectrometry and pattern recognition for the identification of bladder cancer from urine headspace. Anal. Methods 2016, 8, 4037. [Google Scholar] [CrossRef]
- Bond, A.; Greenwood, R.; Lewis, S.; Corfe, B.; Sarkar, S.; O’Toole, P.; Rooney, P.; Burkitt, M.; Hold, G.; Probert, C. Volatile organic compounds emitted from faeces as a biomarker for colorectal cancer. Aliment. Pharmacol. Ther. 2019, 49, 1005–1012. [Google Scholar] [CrossRef]
- Aoki, T.; Nagaoka, T.; Kobayashi, N.; Kurahashi, M.; Tsuji, C.; Takiguchi, H.; Tomomatsu, K.; Oguma, T.; Kobayashi, N.; Magatani, K.; et al. Prospective Analyses of Volatile Organic Compounds in Obstructive Sleep Apnea Patients. Toxicol. Sci. 2017, 156, 362–374. [Google Scholar] [CrossRef][Green Version]
- Berna, A.Z.; McCarthy, J.S.; Wang, R.X.; Saliba, K.J.; Bravo, F.G.; Cassells, J.; Padovan, B.; Trowell, S. Analysis of Breath Specimens for Biomarkers of Plasmodium falciparum Infection. J. Infect. Dis. 2015, 212, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Castro, M.; Feller, J.F. An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J. Mater. Chem. B 2013, 1, 4563. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Che, X.; Su, H.; Mai, Z.; Huang, Z.; Huang, W.; Chen, W.; Liu, S.; Gao, W.; Zhou, Z.; et al. Exhaled breath analysis using on-line preconcentration mass spectrometry for gastric cancer diagnosis. J. Mass Spectrom. 2021, 56, e4588. [Google Scholar] [CrossRef] [PubMed]
- Halbritter, S.; Fedrigo, M.; Höllriegl, V.; Szymczak, W.; Maier, J.M.; Ziegler, A.G.; Hummel, M. Human Breath Gas Analysis in the Screening of Gestational Diabetes Mellitus. Diabetes Technol. Ther. 2012, 14, 917–925. [Google Scholar] [CrossRef]
- Neerincx, A.H.; Geurts, B.P.; Loon, J.; Tiemes, V.; Jansen, J.J.; Harren, F.J.M.; Kluijtmans, L.A.J.; Merkus, P.J.F.M.; Cristescu, S.M.; Buydens, L.M.C.; et al. Detection of Staphylococcus aureus in cystic fibrosis patients using breath VOC profiles. J. Breath Res. 2016, 10, 046014. [Google Scholar] [CrossRef]
- Chen, H.; Qi, X.; Zhang, L.; Li, X.; Ma, J.; Zhang, C.; Feng, H.; Yao, M. COVID-19 screening using breath-borne volatile organic compounds. J. Breath Res. 2021, 15, 047104. [Google Scholar] [CrossRef]
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath testing as potential colorectal cancer screening tool. Int. J. Cancer 2016, 138, 229–236. [Google Scholar] [CrossRef]
- Hanouneh, I.A.; Zein, N.N.; Cikach, F.; Dababneh, L.; Grove, D.; Alkhouri, N.; Lopez, R.; Dweik, R.A. The Breathprints in Patients with Liver Disease Identify Novel Breath Biomarkers in Alcoholic Hepatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 516–523. [Google Scholar] [CrossRef]
- Grabowska-Polanowska, B.; Skowron, M.; Miarka, P.; Pietrzycka, A.; Sliwka, I. The application of chromatographic breath analysis in the search of volatile biomarkers of chronic kidney disease and coexisting type 2 diabetes mellitus. J. Chromatogr. B 2017, 1060, 103–110. [Google Scholar] [CrossRef]
- Smolinska, A.; Klaassen, E.M.M.; Dallinga, J.W.; Kant, K.D.G.; Jobsis, Q.; Moonen, E.J.C.; Schayck, O.C.P.; Dompeling, E.; Schooten, F.J. Profiling of Volatile Organic Compounds in Exhaled Breath as a Strategy to Find Early Predictive Signatures of Asthma in Children. PLoS ONE 2014, 9, e95668. [Google Scholar] [CrossRef]
- Phillips, C.; Parthaláin, N.M.; Syed, Y.; Deganello, D.; Claypole, T.; Lewis, K. Short-Term Intra-Subject Variation in Exhaled Volatile Organic Compounds (VOCs) in COPD Patients and Healthy Controls and Its Effect on Disease Classification. Metabolites 2014, 4, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Marom, O.; Nakhoul, F.; Tisch, U.; Shiban, A.; Abassi, Z.; Haick, H. Gold nanoparticle sensors for detecting chronic kidney disease and disease progression. Nanomedicine 2012, 7, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Dadamio, J.; Velde, S.; Laleman, W.; Hee, P.; Coucke, W.; Nevens, F.; Quirynen, M. Breath biomarkers of liver cirrhosis. J. Chromatogr. B 2012, 905, 17–22. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Saunders, C.; Hope, P.; Schmitt, P.; Wai, J. Volatile biomarkers in the breath of women with breast cancer. J. Breath Res. 2010, 4, 026003. [Google Scholar] [CrossRef] [PubMed]
- Dryahina, K.; Sovová, K.; Nemec, A.; Spanel, P. Differentiation of pulmonary bacterial pathogens in cystic fibrosis by volatile metabolites emitted by their in vitro cultures: Pseudomonas aeruginosa, Staphylococcus aureus, Stenotrophomonas maltophilia and the Burkholderia cepacia complex. J. Breath Res. 2016, 10, 037102. [Google Scholar] [CrossRef]
- Ruszkiewicz, D.M.; Sanders, D.; O’Brien, R.; Hempel, F.; Reed, M.J.; Riepe, A.C.; Bailie, K.; Brodrick, E.; Darnley, K.; Ellerkmann, R.; et al. Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry—A feasibility study. EClinicalMedicine 2020, 29, 100609. [Google Scholar] [CrossRef]
- Xu, Z.; Broza, Y.Y.; Ionsecu, R.; Tisch, U.; Ding, L.; Liu, H.; Song, Q.; Pan, Y.; Xiong, F.; Gu, K.; et al. A nanomaterial-based breath test for distinguishing gastric cancer from benign gastric conditions. Br. J. Cancer 2013, 108, 941–950. [Google Scholar] [CrossRef]
- Neupane, S.; Peverall, R.; Richmond, G.; Blaikie, T.P.J.; Taylor, D.; Hancock, G.; Evans, M.L. Exhaled Breath Isoprene Rises During Hypoglycemia in Type 1 Diabetes. Diabetes Care 2016, 39, e97–e98. [Google Scholar] [CrossRef]
- Schaber, C.L.; Katta, N.; Bollinger, L.B.; Mwale, M.; Mlotha-Mitole, R.; Trehan, I.; Raman, B.; John, A.R.O. Breathprinting Reveals Malaria-Associated Biomarkers and Mosquito Attractants. J. Infect. Diss 2018, 217, 1553–1560. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Chen, J.; Wang, H.; Cheng, T.; Chen, K.; Xu, S. Analysis of human breath samples of lung cancer patients and healthy controls with solid-phase microextraction (SPME) and flow-modulated comprehensive two-dimensional gas chromatography (GC-GC). Anal. Methods 2014, 6, 6841–6849. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; Ramírez-García, S.; Ilizaliturri-Hernández, C.; Gómez-Gómez, A.; Van-Brussel, E.; Díaz-Barriga, F.; Medellín-Garibay, S.; Flores-Ramírez, R. Ultrafast gas chromatography coupled to electronic nose to identify biomarkers in exhaled breath from chronic obstructive pulmonary disease patients: A pilot study. Biomed. Chromatogr. 2019, 33, e4684. [Google Scholar] [CrossRef]
- Mastrigt, E.; Reyes-Reyes, A.; Brand, K.; Bhattacharya, N.; Urbach, H.P.; Stubbs, A.P.; Jongste, J.C.; Pijnenburg, M.W. Exhaled breath profiling using broadband quantum cascade laser-based spectroscopy in healthy children and children with asthma and cystic fibrosis. J. Breath Res. 2016, 10, 026003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Huang, C.; Zou, X.; Lu, Y.; Shen, C.; Ding, X.; Wang, H.; Jiang, H.; Chu, Y. Exhaled breath online measurement for cervical cancer patients and healthy subjects by proton transfer reaction mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 5603–5612. [Google Scholar] [CrossRef]
- Phillips, M.; Basa-Dalay, V.; Bothamley, G.; Cataneo, R.N.; Lam, P.K.; Natividad, M.P.R.; Schmitt, P.; Wai, J. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis 2010, 90, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.S.; Fill, E.; Strittmatter, F.; Volz, Y.; Sroka, R.; Apolonski, A. Towards reliable diagnostics of prostate cancer via breath. Sci. Rep. 2021, 11, 18381. [Google Scholar] [CrossRef]
- Moura, P.C.; Vassilenko, V.; Ribeiro, P.A. Ion Mobility Spectrometry Towards Environmental Volatile Organic Compounds Identification and Quantification: A Comparative Overview over Infrared Spectroscopy. Emiss. Control Sci. Technol. 2023, 9, 25–46. [Google Scholar] [CrossRef]
- Boeker, P. On ‘Electronic Nose’ methodology. Sens. Actuators B Chem. 2014, 204, 2–17. [Google Scholar] [CrossRef]
- Dragonieri, S.; Pennazza, G.; Carratu, P.; Resta, O. Electronic Nose Technology in Respiratory Diseases. Lung 2017, 195, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.C.; Vassilenko, V. Contemporary ion mobility spectrometry applications and future trends towards environmental, health and food research: A review. Int. J. Mass Spectrom. 2023, 486, 117012. [Google Scholar] [CrossRef]
- Ludwig, K.R.; Hummon, A.B. Mass spectrometry for the discovery of biomarkers of sepsis. Mol. Biosyst. 2017, 13, 648. [Google Scholar] [CrossRef]
- Kouremenos, K.A.; Johansson, M.; Marriott, P.J. Advances in Gas Chromatographic Methods for the Identification of Biomarkers in Cancer. J. Cancer 2012, 3, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Binson, V.A.; Subramoniam, M.; Mathew, L. Discrimination of COPD and lung cancer from controls through breath analysis using a self-developed e-nose. J. Breath Res. 2021, 15, 046003. [Google Scholar] [CrossRef]
- Al-Hamry, A.; Panzardi, E.; Mugnaini, M.; Kanoun, O. Human Breathing Monitoring by Graphene Oxide Based Sensors. In Advanced Sensors for Biomedical Applications, 1st ed.; Kanoun, O., Derbel, N., Eds.; Springer: Cham, Switzerland, 2021; pp. 97–105. [Google Scholar]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, S.S.; Kaur, P.; Kim, Y.H.; Sekhon, S.S. 2D graphene oxide–aptamer conjugate materials for cancer diagnosis. NPJ 2D Mater. Appl. 2021, 5, 21. [Google Scholar] [CrossRef]
- Shahdeo, D.; Roberts, A.; Abbineni, N.; Gandhi, S. Graphene based sensors. Compr. Anal. Chem. 2020, 91, 175–199. [Google Scholar]
- Zagalo, P.M.; Sério, S.; Ribeiro, P.A.; Raposo, M. Graphene-based biosensors. In Recent Advances in Graphene-Based Technologies, 1st ed.; Chandran, A., Unnikrishan, N.V., Jayaraj, M.K., George, J., Eds.; IOP Publishing: Bristol, UK, 2023; pp. 1–20. [Google Scholar]
- Moura, P.C.; Pivetta, T.P.; Vassilenko, V.; Ribeiro, P.A.; Raposo, M. Graphene Oxide Thin Films for Detection and Quantification of Industrially Relevant Alcohols and Acetic Acid. Sensors 2023, 23, 462. [Google Scholar] [CrossRef]
- Magro, C.; Gonçalves, O.C.; Morais, M.; Ribeiro, P.A.; Sério, S.; Vieira, P.; Raposo, M. Volatile Organic Compound Monitoring during Extreme Wildfires: Assessing the Potential of Sensors Based on LbL and Sputtering Films. Sensors 2022, 22, 6677. [Google Scholar] [CrossRef]
- Magro, C.; Mateus, E.P.; Raposo, M.; Ribeiro, A.B. Overview of electronic tongue sensing in environmental aqueous matrices: Potential for monitoring emerging organic contaminants. Environ. Rev. 2019, 27, 202–214. [Google Scholar] [CrossRef]
- Magro, C.; Zagalo, P.; Pereira-da-Silva, J.; Mateus, E.P.; Ribeiro, A.B.; Ribeiro, P.; Raposo, M. Polyelectrolyte Based Sensors as Key to Achieve Quantitative Electronic Tongues: Detection of Triclosan on Aqueous Environmental Matrices. Nanomaterials 2020, 10, 640. [Google Scholar] [CrossRef]
- Pienutsa, N.; Roongruangsree, P.; Seedokbuab, V.; Yannawibut, K.; Phatoomvijitwong, C.; Srinives, S. SnO2-graphene composite gas sensor for a room temperature detection of ethanol. Nanotechnology 2020, 32, 115502. [Google Scholar] [CrossRef]
- Phasuksom, K.; Prissanaroon-Ouajai, W.; Sirivat, A. A highly responsive methanol sensor based on graphene oxide/polyindole composites. RSC Adv. 2020, 10, 15206–15220. [Google Scholar] [CrossRef] [PubMed]
- Minta, D.; González, Z.; Wiench, P.; Gryglewicz, S.; Gryglewicz, G. N-Doped Reduced Graphene Oxide/Gold Nanoparticles Composite as an Improved Sensing Platform for Simultaneous Detection of Dopamine, Ascorbic Acid, and Uric Acid. Sensors 2020, 20, 4427. [Google Scholar] [CrossRef] [PubMed]
- Vitoria, I.; Gallego, E.E.; Melendi-Espina, S.; Hernaez, M.; Zamarreño, C.R.; Matías, I.R. Gas Sensor Based on Lossy Mode Resonances by Means of Thin Graphene Oxide Films Fabricated onto Planar Coverslips. Sensors 2023, 23, 1459. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, M.; Zamarreño, C.R.; Melendi-Espina, S.; Bird, L.R.; Mayes, A.G.; Arregui, F.J. Optical Fibre Sensors Using Graphene-Based Materials: A Review. Sensors 2017, 17, 155. [Google Scholar] [CrossRef]
- Li, D.; Shao, Y.; Zhang, Q.; Qu, M.; Ping, J.; Fu, Y.; Xie, J. A flexible virtual sensor array based on laserinduced graphene and MXene for detecting volatile organic compounds in human breath. Analyst 2021, 146, 5704. [Google Scholar] [CrossRef]
- Chronic Obstructive PULMONARY Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 21 October 2023).
- Asthma. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 21 October 2023).
- Hargreave, F.E.; Nair, P. The definition and diagnosis of asthma. Clin. Exp. Allergy 2009, 38, 1652–1658. [Google Scholar] [CrossRef]
- Mannino, D.M. COPD: Epidemiology, Prevalence, Morbidity and Mortality, and Disease Heterogeneity. Chest 2002, 121, 121S–126S. [Google Scholar] [CrossRef]
- Beach, J.; Russell, K.; Blitz, S.; Hooton, N.; Spooner, C.; Lemiere, C.; Tarlo, S.; Rowe, B. A Systematic Review of the Diagnosis of Occupational Asthma. Chest 2007, 131, 569–578. [Google Scholar] [CrossRef]
- Rennard, S.I.; Drummond, M.B. Early chronic obstructive pulmonary disease: Definition, assessment, and prevention. Lancet 2015, 385, 1778–1788. [Google Scholar] [CrossRef]
- Caldeira, M.; Perestrelo, R.; Barros, A.S.; Bilelo, M.J.; Morête, A.; Câmara, J.S.; Rocha, S.M. Allergic asthma exhaled breath metabolome: A challenge for comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2012, 1254, 87–97. [Google Scholar] [CrossRef]
- Schleich, F.N.; Zanella, D.; Stefanuto, P.H.; Bessonov, K.; Smolinska, A.; Dallinga, J.W.; Henket, M.; Paulus, V.; Guissard, F.; Graff, S.; et al. Exhaled Volatile Organic Compounds Are Able to Discriminate between Neutrophilic and Eosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2019, 15, 444–453. [Google Scholar] [CrossRef]
- Gahleitner, F.; Guallar-Hoyas, C.; Beardsmore, C.S.; Pandya, H.C.; Thomas, C.P. Metabolomics pilot study to identify volatile organic compound markers of childhood asthma in exhaled breath. Bioanalysis 2013, 5, 2239–2247. [Google Scholar] [CrossRef]
- Cazzola, M.; Segreti, A.; Capuano, R.; Bergamini, A.; Martinelli, E.; Calzetta, L.; Rogliani, P.; Ciaprini, C.; Ora, J.; Paolesse, R.; et al. Analysis of exhaled breath fingerprints and volatile organic compounds in COPD. COPD Res. Pract. 2015, 1, 7. [Google Scholar] [CrossRef]
- Jareño-Esteban, J.J.; Muñoz-Lucas, M.A.; Gómez-Martín, O.; Utrilla-Trigo, S.; Gutiérrez-Ortega, C.; Aguilar-Ros, A.; Collado-Yurrita, L.; Callol-Sánchez, L.M. Study of 5 Volatile Organic Compounds in Exhaled Breath in Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2017, 53, 251–256. [Google Scholar] [CrossRef]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2017, 89, 152–166. [Google Scholar] [CrossRef]
- Ghoizadeh, A.; Voiry, D.; Weisel, C.; Gow, A.; Laumbach, R.; Kipen, H.; Chhwalla, M. Toward point-of-care management of chronic respiratory conditions: Electrochemical sensing of nitrite content in exhaled breath condensate using reduced graphene oxide. Microsys. Nanoeng. 2017, 3, 17022. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, A.; Black, K.; Kipen, H.; Laumbach, R.; Gow, A.; Weisel, C.; Javanmard, M. Detection of respiratory inflammation biomarkers in non-processed exhaled breath condensate samples using reduced graphene oxide. RSC Adv. 2022, 12, 35627. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ghosh, R. Selective determination of ammonia, ethanol and acetone by reduced graphene oxide based gas sensors at room temperature. Sens. Bio-Sens. Res. 2022, 28, 100336. [Google Scholar] [CrossRef]
- Samadi, S.; Nouroozshad, M.; Zakaria, S.A. ZnO@SiO2/rGO core/shell nanocomposite: A superior sensitive, selective and reproducible performance for 1-propanol gas sensor at room temperature. Mater. Chem. Phys. 2021, 271, 124884. [Google Scholar] [CrossRef]
- Hussein, H.T.; Nayef, U.M.; Hussien, A.M.A. Synthesis of graphene on porous silicon for vapor organic sensor by using photoluminescence. Optik 2019, 180, 61–70. [Google Scholar] [CrossRef]
- Murashima, Y.; Karim, M.R.; Furue, R.; Matsui, T.; Takehira, H.; Wakata, K.; Toda, K.; Ohtani, R.; Nakamura, M.; Hayami, S. Reduced graphene oxide–transition metal hybrids as p-type semiconductors for acetaldehyde sensing. Inorg. Chem. Front. 2016, 3, 842–848. [Google Scholar] [CrossRef]
- Khan, A.A.P.; Khan, A.; Rahman, M.M.; Asiri, A.M. Conventional surfactant-doped poly (o-anisidine)/GO nanocomposites for benzaldehyde chemical sensor development. J. Solgel Sci. Technol. 2016, 77, 361–370. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, D.; Lin, L.; Wu, J. Bridging interdigitated electrodes by electrochemical-assisted deposition of graphene oxide for constructing flexible gas sensor. Sens. Actuators B Chem. 2019, 286, 591–599. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Kam, K.W.L.; Cheung, W.F.; Zhao, N.; Zheng, B. Functionalized graphene-based chemiresistive electronic nose for discrimination of disease-related volatile organic compounds. Biosens. Bioelectron. X 2019, 1, 100016. [Google Scholar] [CrossRef]
- Turner, J.M.; Bauer, C.; Abramowitz, M.K.; Melamed, M.L.; Hostetter, T.H. Treatment of chronic kidney disease. Kidney Int. 2012, 81, 351–362. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Ordoñez, J.D.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Go, A.S. Thre risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008, 74, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Hutchison, A. Cardiovascular disease in patients with chronic kidney disease. Vasc. Health Risk Manag. 2009, 5, 713–722. [Google Scholar]
- Taniselass, S.; Arshad, M.K.; Gopinath, S.C.B. Graphene-based electrochemical biosensors for monitoring noncommunicable disease biomarkers. Biosens. Bioelectron. 2019, 130, 276–292. [Google Scholar] [CrossRef]
- Bayrakli, I.; Turkmen, A.; Akman, H.; Sezer, M.T.; Kutluhan, S. Applications of external cavity diode laser-based technique to noninvasive clinical diagnosis using expired breath ammonia analysis: Chronic kidney disease, epilepsy. J. Biomed. Opt. 2016, 21, 087004. [Google Scholar] [CrossRef]
- Grabowska-Polanowska, B.; Faber, J.; Skowron, M.; Miarka, P.; Pietrzycka, A.; Sliwka, I.; Amann, A. Detection of potential chronic kidney disease markers in breath using gas chromatography with mass-spectral detection coupled with thermal desorption method. J. Chromatogr. A 2013, 1301, 179–189. [Google Scholar] [CrossRef]
- Obermeier, J.; Trefz, P.; Happ, J.; Schubbert, J.K.; Staude, H.; Fischer, D.C.; Miekisch, W. Exhaled volatile substances mirror clinical conditions in pediatric chronic kidney disease. PLoS ONE 2017, 12, e0178745. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Eetemadi, A.; Tagkopoulos, I. Reduced Graphene Oxide-Metalloporphyrin Sensors for Human Breath Screening. Appl. Sci. 2021, 11, 11290. [Google Scholar] [CrossRef]
- Majidi, R.; Nadafan, M. Application of nitrogenated holey graphene for detection of volatile organic biomarkers in exhaled breath of humans with chronic kidney disease: A density functional theory study. J. Comput. Electron. 2021, 20, 1930–1937. [Google Scholar] [CrossRef]
- Tung, T.T.; Tran, M.T.; Feller, J.F.; Castro, M.; Ngo, T.V.; Hassan, K.; Nine, J.; Losic, D. Graphene and metal organic frameworks (MOFs) hybridization for tunable chemoresistive sensors for detection of volatile organic compounds (VOCs) biomarkers. Carbon 2020, 159, 333–344. [Google Scholar] [CrossRef]
- Choi, S.J.; Fuchs, F.; Demadrille, R.; Grévin, B.; Jang, B.H.; Lee, S.J.; Lee, J.H.; Tuller, H.; Kim, I.D. Fast Responding Exhaled-Breath Sensors Using WO3 Hemitubes Functionalized by Graphene-Based Electronic Sensitizers for Diagnosis of Diseases. Appl. Mater. Interfaces 2014, 6, 9061–9070. [Google Scholar] [CrossRef] [PubMed]
- Brannelly, N.T.; Hamilton-Shield, J.P.; Killard, A.J. The Measurement of Ammonia in Human Breath and its Potential in Clinical Diagnostics. Crit. Rev. Anal. Chem. 2016, 46, 490–501. [Google Scholar] [CrossRef]
- Ogimoto, Y.; Selyanchyn, R.; Takahara, N.; Wakamatsu, S.; Lee, S. Detection of ammonia in human breath using quartz crystal microbalance sensors with functionalized mesoporous SiO2 nanoparticle films. Sens. Actuators B Chem. 2015, 215, 428–436. [Google Scholar] [CrossRef]
- Narasimhan, L.R.; Goodman, W.; Patel, C.K. Correlation of breath ammonia with blood urea nitrogen and creatinine during hemodialysis. Proc. Natl. Acad. Sci. USA 2001, 98, 4617–4621. [Google Scholar] [CrossRef]
- Shahmoradi, A.; Hosseini, A.; Akbarinejad, A.; Alizadeh, N. Noninvasive Detection of Ammonia in the Breath of Hemodialysis Patients Using a Highly Sensitive Ammonia Sensor Based on a Polypyrrole/Sulfonated Graphene Nanocomposite. Anal. Chem. 2021, 93, 6706–6714. [Google Scholar] [CrossRef]
- Yabaş, E.; Biçer, E.; Altındal, A. Novel reduced graphene oxide/zinc phthalocyanine and reduced graphene oxide/cobalt phthalocyanine hybrids as high sensitivity room temperature volatile organic compound gas sensors. J. Mol. Struct. 2023, 1271, 134076. [Google Scholar] [CrossRef]
- Deshpande, A.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Iglay, K.; Hannachi, H.; Howie, P.J.; Xu, J.; Li, X.; Engel, S.S.; Moore, L.M.; Rajpathak, S. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr. Med. Res. Opin. 2016, 32, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E. Diagnosis of Diabetes. N. Engl. J. Med. 2012, 367, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Dixit, K.; Fardindoost, S.; Ravishankara, A.; Tasnim, N.; Hoorfar, M. Exhaled Breath Analysis for Diabetes Diagnosis and Monitoring: Relevance, Challenges and Possibilities. Biosensors 2021, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Liu, Y.; Cheng, S.; Duan, Y. Exhaled isopropanol: New potential biomarker in diabetic breathomics and its metabolic correlations with acetone. RSC Adv. 2017, 7, 17480. [Google Scholar] [CrossRef]
- Righettoni, M.; Schmid, A.; Amann, A.; Pratsinis, S.E. Correlations between blood glucose and breath components from portable gas sensors and PTR-TOF-MS. J. Breath Res. 2013, 7, 037110. [Google Scholar] [CrossRef]
- Yan, H.; Zhou, Y.G. Electrical sensing of volatile organic compounds in exhaled breath for disease diagnosis. Curr. Opin. Electrochem. 2022, 33, 100922. [Google Scholar] [CrossRef]
- Kalidoss, R.; Umapathy, S.; Sivalingam, Y. An investigation of GO-SnO2-TiO2 ternary nanocomposite for the detection of acetone in diabetes mellitus patient’s breath. Appl. Surf. Sci. 2018, 449, 677–684. [Google Scholar] [CrossRef]
- Kalidoss, R.; Umapathy, S. A comparison of online and offline measurement of exhaled breath for diabetes pre-screening by graphene-based sensors; from powder processing to clinical monitoring prototype. J. Breath Res. 2019, 13, 036008. [Google Scholar] [CrossRef] [PubMed]
- Kalidoss, R.; Umapathy, S.; Kothalam, R.; Sakthivelu, U. Adsorption kinetics feature extraction from breathprint obtained by graphene based sensors for diabetes diagnosis. J. Breath Res. 2021, 15, 016005. [Google Scholar] [CrossRef] [PubMed]
- Thakur, U.N.; Bhardwaj, R.; Hazra, A. A Multivariative Computational Approach with Hybrid Graphene Oxide Sensor Array for Partial Fulfillment of Breath Acetone Sensing. IEEE Sens. J. 2022, 22, 20207–20216. [Google Scholar] [CrossRef]
- Choi, S.J.; Jang, B.; Lee, S.; Min, B.K.; Rothschild, A.; Kim, I. Selective Detection of Acetone and Hydrogen Sulfide for the Diagnosis of Diabetes and Halitosis Using SnO2 Nanofibers Functionalized with Reduced Graphene Oxide Nanosheets. Appl. Mater. Interfaces 2014, 6, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Maity, S.; Kundu, S. Reduced graphene oxide (rGO) decorated NiO-SnO2 nanocomposite based sensor towards room temperature diabetic biomarker detection. J. Alloys Compd. 2023, 966, 171553. [Google Scholar] [CrossRef]
- Yempally, S.; Hegazy, S.M.; Aly, A.; Kannan, K.; Sadasivuni, K.K. Non-Invasive Diabetic Sensor Based on Cellulose Acetate/Graphene Nanocomposite. Macromol. Symp. 2020, 392, 2000024. [Google Scholar] [CrossRef]
- Rakkesh, R.A.; Durgalakshmi, D.; Balakumar, S. Scalable approach to fabricate paper-based biomass reduced graphene sensor for the detection of exhaled diabetic breath. Nanotechnology 2022, 33, 495703. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Labianca, R.; Beretta, G.D.; Gatta, G.; Braud, F.; Cutsem, E. Gastric Cancer. Crit. Rev. Oncol. Hematol. 2009, 71, 127–164. [Google Scholar] [CrossRef]
- Blair, V.; Martin, I.; Shaw, D.; Winship, I.; Kerr, D.; Arnold, J.; Harawira, P.; McLeod, M.; Parry, S.; Charlton, A.; et al. Hereditary Diffuse Gastric Cancer: Diagnosis and Management. Clin. Gastroenterol. Hepatol. 2006, 4, 262–275. [Google Scholar] [CrossRef]
- Xiang, L.; Wu, S.; Hua, Q.; Bao, C.; Liu, H. Volatile Organic Compounds in Human Exhaled Breath to Diagnose Gastrointestinal Cancer: A Metal-Analysis. Front. Oncol. 2021, 11, 606915. [Google Scholar] [CrossRef]
- Jung, Y.J.; Seo, H.S.; Kim, J.H.; Song, K.Y.; Park, C.H.; Lee, H.H. Advanced Diagnostic Technology of Volatile Organic Compounds Real Tiem Analysis from Exhaled Breath of Gastric Cancer Patients Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry. Front. Oncol. 2021, 11, 560591. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Pan, F.; Liu, J.; Wang, K.; Zhang, C.; Cheng, S.; Lu, L.; Zhang, W.; Zhang, Z.; et al. Breath Analysis Based on Surface-Enhanced Raman Scattering Sensors Distinguishes Early and Advanced Gastric Cancer Patients from Healthy Persons. ACS Nano 2016, 10, 8169–8179. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, G.; Liu, H.; Fu, H.; Fan, J.; Wang, K.; Chen, Y.; Li, B.; Zhang, C.; Zhi, X.; et al. Identification of Volatile Biomarkers of Gastric Cancer Cells and Ultrasensitive Electrochemical Detection based on Sensing Interface of Au-Ag Alloy coated MWCNTs. Theranostics 2014, 4, 154–162. [Google Scholar] [CrossRef]
- Amal, H.; Leja, M.; Funka, K.; Skarpars, R.; Sivins, A.; Ancans, G.; Liepniece-Karele, I.; Kikuste, I.; Lasina, I.; Haick, H. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut 2016, 65, 400–407. [Google Scholar] [CrossRef]
- Tombel, N.S.M.; Badaruddin, S.A.M.; Yakin, F.S.; Zaki, H.F.M.; Syono, M.I. Detection of low PPM of volatile organic compounds using nanomaterial functionalized reduced graphene oxide sensor. AIP Conf. Proc. 2021, 2368, 020004. [Google Scholar]
- Velumani, M.; Prasanth, A.; Narasimman, S.; Chandrasekhar, A.; Sampson, A.; Meher, S.R.; Rajalingam, S.; Rufus, E.; Alex, Z.C. Nanomaterial-Based Sensors for Exhaled Breath Analysis: A Review. Coatings 2022, 12, 1989. [Google Scholar] [CrossRef]
- Jia, X.; Yu, S.; Cheng, C.; Yang, J.; Li, Y.; Wang, S.; Song, H. Ag nanoparticles modified Fe3O4/reduced graphene oxide and their acetone sensing properties. Mater. Chem. Phys. 2022, 276, 125378. [Google Scholar] [CrossRef]
- Pastorino, U. Lung cancer screening. Br. J. Cancer 2010, 102, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Lung Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/lung-cancer-statistics/ (accessed on 21 October 2023).
- Thriumani, R.; Zakaria, A.; Hashim, Y.; Jeffree, A.I.; Helmy, K.M.; Kamarudin, L.M.; Omar, M.I.; Shakaff, A.Y.; Adom, A.H.; Persaud, K.C. A study on volatile organic compounds emitted by in-vitro lung cancer cultured cells using gas sensor array and SPME-GCMS. BMC Cancer 2018, 18, 362. [Google Scholar] [CrossRef]
- Kischkel, S.; Miekisch, W.; Sawacki, A.; Straker, E.M.; Trefz, P.; Amann, A.; Schubert, J.K. Breath biomarkers for lung cancer detection and assessment of smoking related effects—Confounding variables, influence of normalization and statistical algorithms. Clin. Chim. Acta 2010, 411, 1637–1644. [Google Scholar] [CrossRef]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J. Chromatogr. B 2010, 878, 2643–2651. [Google Scholar] [CrossRef]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Mieksich, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.A.; Li, M.; Knipp, R.J.; Nantz, M.H.; Bousamra, M. Noninvasive detection of lung cancer using exhaled breath. Cancer Med. 2014, 3, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef]
- Saalberg, Y.; Bruhns, H.; Wolff, M. Photoacoustic Spectroscopy for the Determination of Lung Cancer Biomarkers—A Preliminary Investigation. Sensors 2017, 17, 210. [Google Scholar] [CrossRef]
- Buszewski, B.; Ulanowska, A.; Kowalkowski, T.; Cieslinski, K. Investigation of lung cancer biomarkers by hyphenated separation techniques and chemometrics. Clin. Chem. Lab. Med. 2011, 50, 573–581. [Google Scholar] [CrossRef]
- Kushch, I.; Schwarz, K.; Schwentner, L.; Baumann, B.; Dzien, A.; Schmid, A.; Unterkofler, K.; Gastl, G.; Španěl, P.; Smith, D.; et al. Compounds enhanced in a mass spectrometric profile of smokers’ exhaled breath versus non-smokers as determined in a pilot study using PTR-MS. J. Breath Res. 2008, 2, 026002. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, Z.; Chen, X.; Huang, C.; Bai, W.; Lu, Z.; Wang, T. Nanomaterial-based gas sensors used for breath diagnosis. J. Mater. Chem. B 2020, 8, 3231. [Google Scholar] [CrossRef] [PubMed]
- Emam, S.; Nasrollahpour, M.; Allen, J.P.; He, Y.; Hussein, H.; Shah, H.S.; Tavangarian, F.; Sun, N.X. A handheld electronic device with the potential to detect lung cancer biomarkers from exhaled breath. Biomed. Microdevices 2022, 24, 41. [Google Scholar] [CrossRef]
- Shanmugasundaram, A.; Lee, D.W. SnO2/rGO nanocomposite for the detection of biomarkers of lung cancer. Micro Nano Syst. Lett. 2022, 10, 13. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; He, Y.; Liu, Y.; Li, S.; Fang, J.; Zhang, X.; Peng, G. Sniffing lung cancer related biomarkers using an oxidized graphene SAW sensor. Front. Phys. 2016, 11, 116801. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Z.; Liu, D.; He, Z.; Wu, J. Constructing an E Nose Using Metal-Ion-Induced Assembly of Graphene Oxide for Diagnosis of Lung Cancer via Exhaled Breath. App. Mater. Interfaces 2020, 12, 17713–17724. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vicente, C.; Santos, J.P.; Lozano, J.; Sayago, I.; Sanjurjo, J.L.; Azabal, A.; Ruiz-Valdepeñas, S. Graphene-Doped Tin Oxide Nanofibers and Nanoribbons as Gas Sensors to Detect Biomarkers of Different Diseases through the Breath. Sensors 2020, 20, 7223. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, A.; Manorama, S.V.; Kim, D.S.; Jeong, Y.J.; Lee, D.W. Toward Point-of-Care chronic disease Management: Biomarker detection in exhaled breath using an E-Nose sensor based on rGO/SnO2 superstructures. Chem. Eng. J. 2022, 448, 137736. [Google Scholar] [CrossRef]
- Sleep Apnea Statistics. Available online: https://www.ncoa.org/adviser/sleep/sleep-apnea-statistics/ (accessed on 21 October 2023).
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Dragonieri, S.; Porcelli, F.; Longobardi, F.; Carratù, P.; Aliani, M.; Ventura, V.A.; Tutino, M.; Quaranta, V.N.; Resta, O.; Gennaro, G. An electric nose in the discrimination of obese patients with and without obstructive sleep apnoea. J. Breath Res. 2015, 9, 026005. [Google Scholar] [CrossRef]
- Casanova-Chafer, J.; Garcia-Aboal, R.; Atienzar, P.; Bittencourt, C.; Lobet, E. Perovskite@Graphene Nanohybrids for Breath Analysis: A Proof-of-Concept. Chemosensors 2021, 9, 215. [Google Scholar] [CrossRef]
- Casanova-Chafer, J.; Garcia-Aboal, R.; Atienzar, P.; Llobet, E. The role of anions and cations in the gas sensing mechanisms of graphene decorated with lead halide perovskite nanocrystals. Chem. Comm. 2020, 56, 8956–8959. [Google Scholar] [CrossRef]
- Sen, S.; Kundu, S. Reduced graphene oxide (rGO) decorated ZnO-SnO2: A ternary nanocomposite towards improved low concentration VOC sensing performance. J. Alloys Compd. 2021, 881, 160406. [Google Scholar] [CrossRef]
- Caccami, M.C.; Miozzi, C.; Mulla, M.; Natale, C.; Marrocco, G. An epidermal graphene oxide-based RFID sensor for the wireless analysis of human breath. In Proceedings of the IEEE International Conference on RFID Technology & Application (RFID-TA), Warsaw, Poland, 20–22 September 2017. [Google Scholar]
- Caccami, M.C.; Mulla, M.; Occhiuzzi, C.; Natale, C.; Marrocco, G. Design and Experimentation of a Batteryless On-Skin RFID Graphene-Oxide Sensor for the Monitoring and Discrimination of Breath Anomalies. IEEE Sens. J. 2018, 18, 8893–8900. [Google Scholar] [CrossRef]
- Ray, B.; Parmar, S.; Vijayan, V.; Vishwakarma, S.; Datar, S. Detection of trace volatile organic compounds in spiked breath samples: A leap towards breathomics. Nanotechnology 2022, 33, 205505. [Google Scholar] [CrossRef] [PubMed]
| Target Biomarker | Population | Sensors | References | |||||
|---|---|---|---|---|---|---|---|---|
| Name | HMDB | Target | Notes | Sensitivity | Detection Limits | Response/Recovery Time | Accuracy | |
| Nitrite | HMDB0002786 | Human subjects | Exhaled air condensate | – | – | – | 100% | [72] |
| Nitrite | HMDB0002786 | Human subjects | Exhaled air condensate | – | – | – | 100% | [73] |
| Acetone | HMDB0001659 | Standard solutions | Two known concentrations | – | 1000–2000 ppmv | 100/–s | – | [74] |
| Propanol | HMDB0000820 | Standard solutions | Known concentrations (150–450 ppmv) | 300 ppmv | 4 ppmv | 156.85 | 95% | [75] |
| Hexane | HMDB0029600 | Standard solutions | Solutions of three compounds | – | – | – | – | [76] |
| Acetaldehyde | HMDB0000990 | Standard solutions | Gaseous samples | 1.012–1.043 | – | 30–70/45–85 s | – | [77] |
| Benzaldehyde | HMDB0006115 | Standard solutions | Gaseous samples | 1.2277 | 0.03 nM | 10/–s | – | [78] |
| Isoprene | HMDB0253673 | Standard solutions | Gaseous samples (5–160 ppmv) | – | 237 ppbv | – | – | [79] |
| Nonanal | HMDB0059835 | Standard solutions | Binary mixture | – | 25 ppmv | 61–200/97–416 s | – | [80] |
| Target Biomarker | Population | Sensors | References | |||||
|---|---|---|---|---|---|---|---|---|
| Name | HMDB | Target | Notes | Sensitivity | Detection Limits | Response/Recovery Time | Accuracy | |
| Ammonia | HMDB0000051 | Standard solutions + Synthetic Breath | 24 Levels of concentration | – | – | –/– | 91.7% | [88] |
| Isoprene | HMDB0253673 | Standard solutions | Known concentrations | – | – | –/– | – | [89] |
| Pentanal | HMDB0031206 | |||||||
| Hexanal | HMDB0005994 | |||||||
| Heptanal | HMDB0031475 | |||||||
| Acetone | HMDB0001659 | Standard solutions | Gaseous samples | – | 2.82 ppbv | –/– | – | [90] |
| Ethanol | HMDB0000108 | |||||||
| Acetone | HMDB0001659 | Standard solutions | Solutions of 1 ppmv | 1.7 | 100 ppbv | 11.5–13.5/–s | – | [91] |
| Ammonia | HMDB0000051 | Standard solutions | Known concentrations | – | 0.2 ppbv–12 ppmv | 48/234 s | – | [95] |
| Acetone | HMDB0001659 | Standard solutions | Known concentrations (30–210 ppmv) | – | 82 ppbv | 190–250/–s | – | [96] |
| Ethanol | HMDB0000108 | |||||||
| Ammonia | HMDB0000051 | |||||||
| Target Biomarker | Population | Sensors | References | |||||
|---|---|---|---|---|---|---|---|---|
| Name | HMDB | Target | Notes | Sensitivity | Detection Limits | Response/Recovery Time | Accuracy | |
| Acetone | HMDB0001659 | Standard solutions | – | 6.28 | 0.25–30 ppmv | –/– | – | [104] |
| Acetone | HMDB0001659 | Human subjects | 30 Volunteers: 17 diabetic patients and 13 healthy individuals | 5.66 | – | 10/12 s | 60% | [105] |
| Acetone | HMDB0001659 | Human subjects | 60 Volunteers: 30 diabetic patients and 30 healthy individuals | – | 0–3 ppmv | –/– | 70% | [106] |
| Acetone | HMDB0001659 | Synthetic breath | Four distinct solutions | 0.5–3.5 | 400 ppbv–80 ppmv | –/– | 100% | [107] |
| Acetone | HMDB0001659 | Standard solutions | – | 10.0 | >100 ppbv | –/– | – | [108] |
| Acetone | HMDB0001659 | Human subjects | – | 7.8 | <1 ppmv | 10/30 s | – | [109] |
| Acetone | HMDB0001659 | Standard solutions | Mixture of known concentrations | – | 1–100 ppmv | –/– | – | [110] |
| Methanol | HMDB0001875 | |||||||
| Ethanol | HMDB0000108 | |||||||
| Acetone | HMDB0001659 | Standard solutions | – | – | 1–5 ppmv | 1.11/41.25 s | – | [111] |
| Target Biomarker | Population | Sensors | References | |||||
|---|---|---|---|---|---|---|---|---|
| Name | HMDB | Target | Notes | Sensitivity | Detection Limits | Response/Recovery Time | Accuracy | |
| Acetic acid | HMDB0000042 | Standard solutions | Three known concentrations | 30.3 | 0.04 ppmv | – | – | [50] |
| Acetone | HMDB0001659 | Standard solutions | Concentrations up to 6 ppmv | – | 1 ppmv | 15/75 | – | [119] |
| Isoprene | HMDB0253673 | |||||||
| Toluene | HMDB0034168 | |||||||
| 2-Methylhexane | HMDB0245230 | Synthetic breath | Mixture of 14 compounds at known concentrations | 83% | – | –/– | 92% | [116] |
| 2-Methylpentane | HMDB0061884 | |||||||
| 3-Methylpentane | HMDB0061885 | |||||||
| Dodecane | HMDB0031444 | |||||||
| Tetradecane | HMDB0059907 | |||||||
| Menthol | HMDB0003352 | |||||||
| Phenyl acetate | HMDB0040733 | |||||||
| Hexanol | HMDB0012971 | |||||||
| Pivalic acid | HMDB0041992 | |||||||
| 3-Methylhexane | HMDB0245932 | |||||||
| 2,3-Dimethylpentane | HMDB0245455 | |||||||
| Hexane | HMDB0029600 | |||||||
| Isoprene | HMDB0253673 | |||||||
| Acetone | HMDB0001659 | |||||||
| Acetone | HMDB0001659 | Standard solutions | Concentrations up to 800 ppmv | – | 35 ppmv | – | – | [121] |
| Target Biomarker | Population | Sensors | References | |||||
|---|---|---|---|---|---|---|---|---|
| Name | HMDB | Target | Notes | Sensitivity | Detection Limits | Response/Recovery Time | Accuracy | |
| Butyraldehyde | HMDB0003543 | Standard solutions | Known concentrations | – | 1–20 ppbv | –/– | – | [135] |
| Tetrahydrofuran | HMDB0303508 | |||||||
| Acetonitrile | HMDB0061869 | |||||||
| Heptane | HMDB0031447 | |||||||
| Hexanal | HMDB0005994 | |||||||
| Benzene | HMDB0001505 | |||||||
| Pentane | HMDB0029603 | |||||||
| 2-Butanone | HMDB0000474 | |||||||
| Furan | HMDB0013785 | |||||||
| Decane | HMDB0031450 | Standard solutions | Known concentrations | – | 1 ppmv | 15/90 | – | [136] |
| Heptane | HMDB0031447 | 19/48 | ||||||
| Decane | HMDB0031450 | Standard solutions | Known concentrations | – | 0.2 ppmv | 28/37 s | – | [137] |
| Acetone | HMDB0001659 | Human subjects | 108 Volunteers: 48 healthy individuals and 60 lung cancer patients | – | 0.05–10 ppmv | 60/180 s | 96% | [138] |
| Isoprene | HMDB0253673 | |||||||
| Ammonia | HMDB0000051 | |||||||
| Ethanol | Synthetic breath | Known concentrations | – | 0.5 ppmv | 50/60 s | – | [139] | |
| Acetone | HMDB0001659 | |||||||
| Formaldehyde | HMDB0001426 | Synthetic breath | Lung cancer samples with 83 ppbv and healthy samples with 49 ppbv | 100 ppbv | 10 ppbv | – | – | [140] |
| Target Biomarker | Population | Sensors | References | |||||
|---|---|---|---|---|---|---|---|---|
| Name | HMDB | Target | Notes | Sensitivity | Detection Limits | Response/Recovery Time | Accuracy | |
| Toluene | HMDB0034168 | Standard solutions | Concentrations of 2, 4, 6 and 8 ppmv | 0.5 | – | –/– | – | [146] |
| Acetone | HMDB0001659 | Standard solutions | Concentrations of 1–10 ppmv | – | 0.675 ppmv | 10/100 | 91% | [147] |
| Isopropanol | HMDB0000863 | Human subjects | Breath spiked with 0.5 ppmv of several compounds | – | 0.5 ppmv | - | 92.8–96% | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, P.C.; Ribeiro, P.A.; Raposo, M.; Vassilenko, V. The State of the Art on Graphene-Based Sensors for Human Health Monitoring through Breath Biomarkers. Sensors 2023, 23, 9271. https://doi.org/10.3390/s23229271
Moura PC, Ribeiro PA, Raposo M, Vassilenko V. The State of the Art on Graphene-Based Sensors for Human Health Monitoring through Breath Biomarkers. Sensors. 2023; 23(22):9271. https://doi.org/10.3390/s23229271
Chicago/Turabian StyleMoura, Pedro Catalão, Paulo António Ribeiro, Maria Raposo, and Valentina Vassilenko. 2023. "The State of the Art on Graphene-Based Sensors for Human Health Monitoring through Breath Biomarkers" Sensors 23, no. 22: 9271. https://doi.org/10.3390/s23229271
APA StyleMoura, P. C., Ribeiro, P. A., Raposo, M., & Vassilenko, V. (2023). The State of the Art on Graphene-Based Sensors for Human Health Monitoring through Breath Biomarkers. Sensors, 23(22), 9271. https://doi.org/10.3390/s23229271











