Food Safety Issues in the Oltrepò Pavese Area: A SERS Sensing Perspective
Abstract
1. Introduction
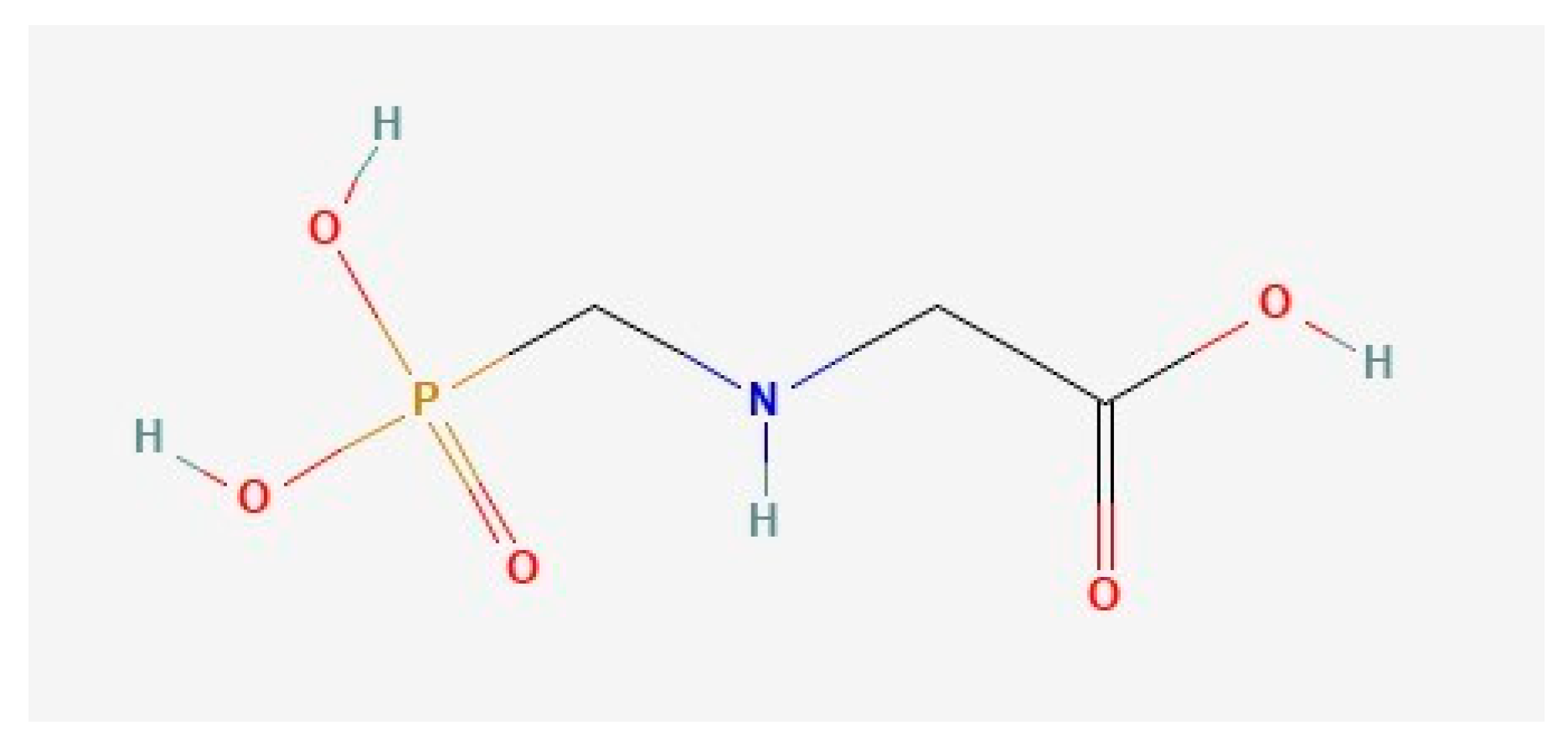
- glyphosate, a pesticide widely used in rice cultivations
- tetracycline in cultivation soils after sludge dispersion
- histamine in wine
- Sudan in saffron and other spices.
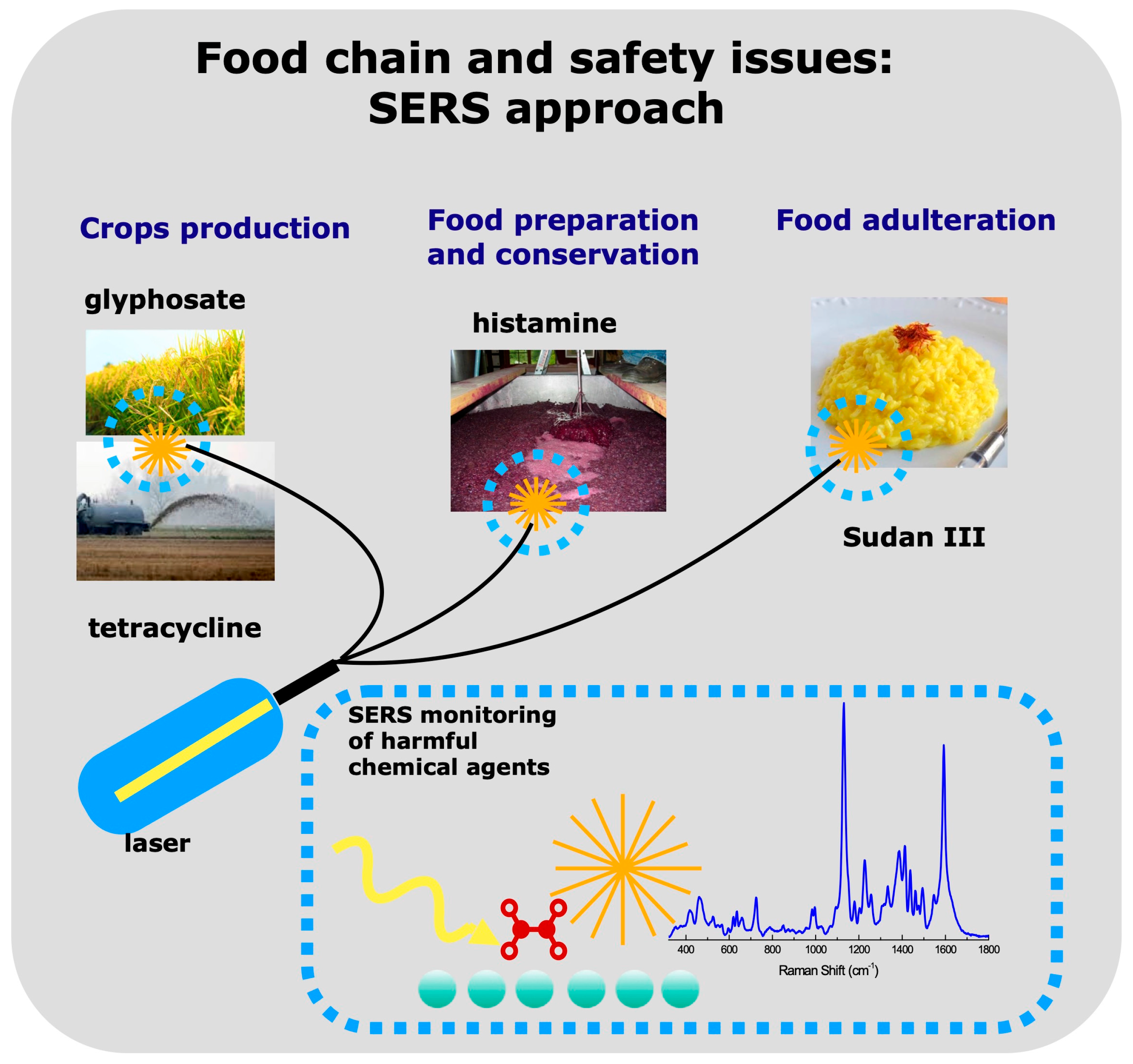
2. Raman Scattering and SERS
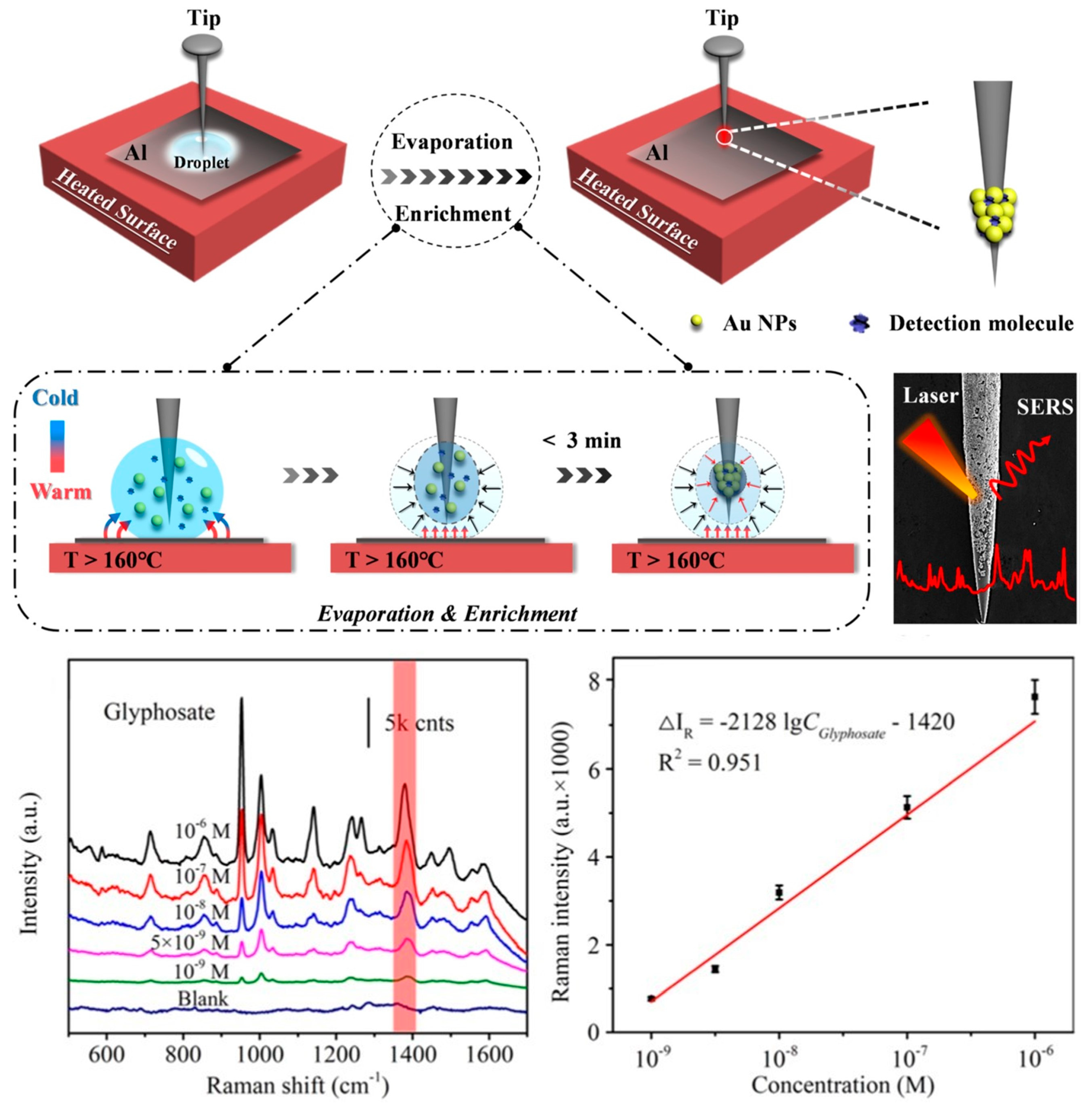
3. SERS Detection of Glyphosate
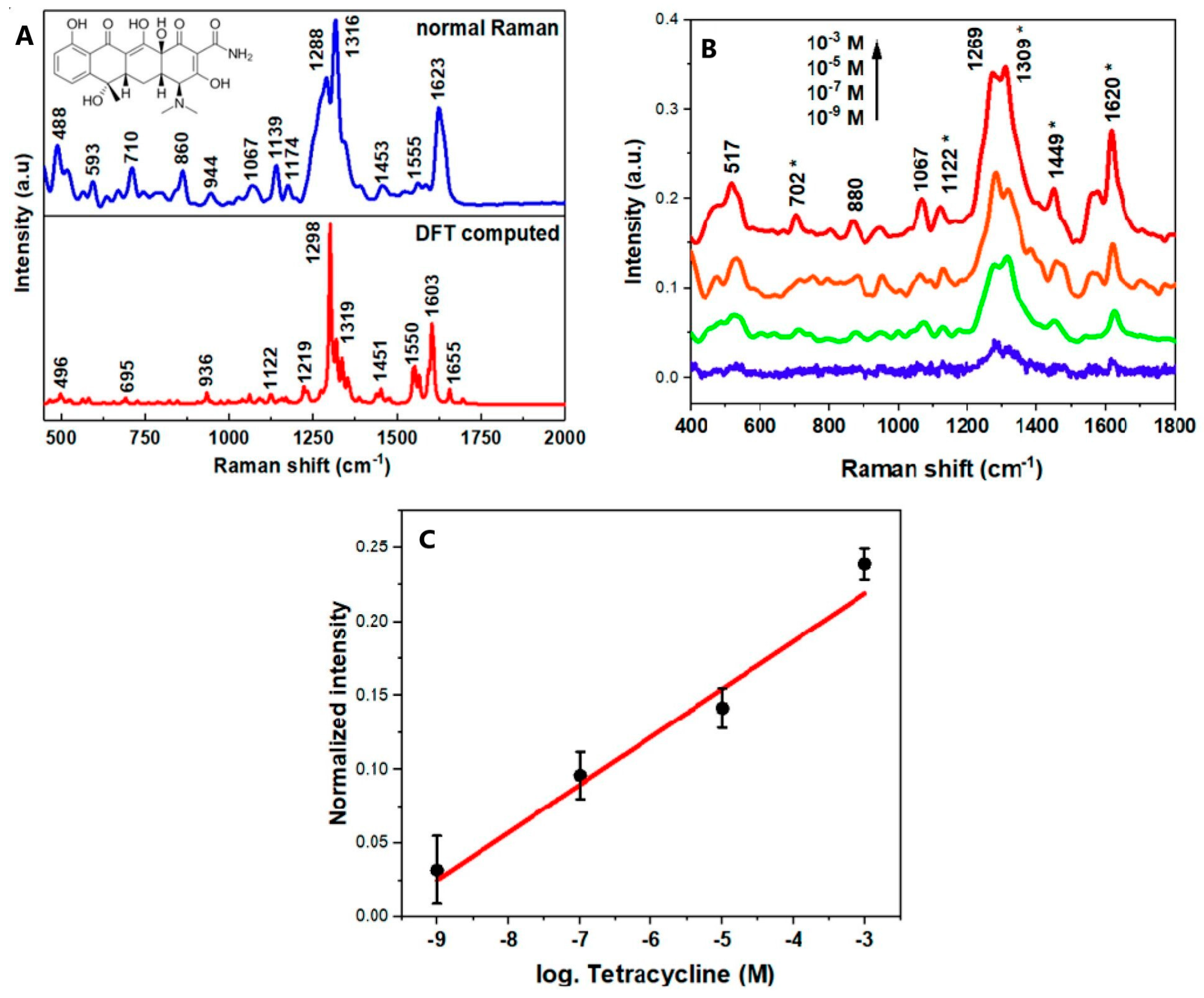
4. SERS Detection of Tetracycline
5. SERS Detection of Histamine
6. SERS Detection of Sudan in Saffron and Other Spices
7. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machado Nardi, V.A.; Auler, D.P.; Teixeira, R. Food Safety in Global Supply Chains: A Literature Review. J. Food Sci. 2020, 85, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Focker, M.; van Asselt, E.D.; Berendsen, B.J.A.; van de Schans, M.G.M.; van Leeuwen, S.P.J.; Visser, S.M.; van der Fels-Klerx, H.J. Review of Food Safety Hazards in Circular Food Systems in Europe. Food Res. Int. 2022, 158, 111505. [Google Scholar] [CrossRef] [PubMed]
- Sarno, E.; Pezzutto, D.; Rossi, M.; Liebana, E.; Rizzi, V. A Review of Significant European Foodborne Outbreaks in the Last Decade. J. Food Prot. 2021, 84, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. 21–Environmental Pollution: Causes, Effects, and the Remedies. In Microorganisms for Sustainable Environment and Health; Chowdhary, P., Raj, A., Verma, D., Akhter, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. ISBN 978-0-12-819001-2. [Google Scholar]
- European Parliament, Council of the European Union Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the Hygiene of Foodstuffs. Available online: https://eur-lex.europa.eu/EN/legal-content/summary/food-safety-from-farm-to-fork.html (accessed on 28 September 2023).
- Motarjemi, Y.; Warren, B.R. Chapter 36–Hazard Analysis and Critical Control Point System (HACCP). In Food Safety Management, 2nd ed.; Andersen, V., Lelieveld, H., Motarjemi, Y., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 799–818. ISBN 978-0-12-820013-1. [Google Scholar]
- Sapozhnikova, Y. Liquid and Gas Chromatography–Mass Spectrometry Methods in Food and Environmental Safety. In Mass Spectrometry in Food and Environmental Chemistry; Picó, Y., Campo, J., Eds.; The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2022; Volume 119, pp. 127–147. ISBN 978-3-031-19092-6. [Google Scholar]
- Kharbach, M.; Alaoui Mansouri, M.; Taabouz, M.; Yu, H. Current Application of Advancing Spectroscopy Techniques in Food Analysis: Data Handling with Chemometric Approaches. Foods 2023, 12, 2753. [Google Scholar] [CrossRef]
- Robert, B. Resonance Raman Spectroscopy. Photosynth. Res. 2009, 101, 147–155. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez De Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Mosca, S.; Conti, C.; Stone, N.; Matousek, P. Spatially Offset Raman Spectroscopy. Nat. Rev. Methods Primers 2021, 1, 21. [Google Scholar] [CrossRef]
- Xu, M.-L.; Gao, Y.; Han, X.-X.; Zhao, B. Innovative Application of SERS in Food Quality and Safety: A Brief Review of Recent Trends. Foods 2022, 11, 2097. [Google Scholar] [CrossRef]
- Jiang, L.; Hassan, M.M.; Ali, S.; Li, H.; Sheng, R.; Chen, Q. Evolving Trends in SERS-Based Techniques for Food Quality and Safety: A Review. Trends Food Sci. Technol. 2021, 112, 225–240. [Google Scholar] [CrossRef]
- Albini, B.; Parmigiani, M.; Pellegrini, G.; Taglietti, A.; Galinetto, P. Glass Supported SERS Chips for Emerging Pollutant Analyses. J. Mater. Sci. Mater. Electron. 2023, 34, 1619. [Google Scholar] [CrossRef]
- Parmigiani, M.; Albini, B.; Pellegrini, G.; Genovesi, M.; De Vita, L.; Pallavicini, P.; Dacarro, G.; Galinetto, P.; Taglietti, A. Surface-Enhanced Raman Spectroscopy Chips Based on Silver Coated Gold Nanostars. Nanomaterials 2022, 12, 3609. [Google Scholar] [CrossRef] [PubMed]
- Bassi, B.; Albini, B.; D’Agostino, A.; Dacarro, G.; Pallavicini, P.; Galinetto, P.; Taglietti, A. Robust, Reproducible, Recyclable SERS Substrates: Monolayers of Gold Nanostars Grafted on Glass and Coated with a Thin Silica Layer. Nanotechnology 2019, 30, 025302. [Google Scholar] [CrossRef]
- Bassi, B.; Taglietti, A.; Galinetto, P.; Marchesi, N.; Pascale, A.; Cabrini, E.; Pallavicini, P.; Dacarro, G. Tunable Coating of Gold Nanostars: Tailoring Robust SERS Labels for Cell Imaging. Nanotechnology 2016, 27, 265302. [Google Scholar] [CrossRef] [PubMed]
- Rovati, D.; Albini, B.; Galinetto, P.; Grisoli, P.; Bassi, B.; Pallavicini, P.; Dacarro, G.; Taglietti, A. High Stability Thiol-Coated Gold Nanostars Monolayers with Photo-Thermal Antibacterial Activity and Wettability Control. Nanomaterials 2019, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
- Zani, D. Comparto Vitivinicolo e Valorizzazione del Made in Italy All’Estero. Available online: https://www.assolombarda.it/le-imprese/filiere/filiera-agroalimentare/appuntamenti/comparto-vitivinicolo-e-valorizzazione-del-made-in-italy-all2019estero (accessed on 21 October 2023).
- Osservatorio Economico Di Zafferano Italiano. Available online: https://www.zafferanoitaliano.it/lo-zafferano-in-italia/osservatorio-economico.html (accessed on 12 September 2023).
- Smith, E.; Dent, G. Modern Raman Spectroscopy: A Practical Approach, 1st ed.; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-119-44055-0. [Google Scholar]
- Edwards, H.G.M.; Vandenabeele, P.; Colomban, P. Historical Overview of Raman Spectroscopy. In Raman Spectroscopy in Cultural Heritage Preservation. Cultural Heritage Science; Springer International Publishing: Cham, Switzerland, 2023; pp. 7–18. ISBN 978-3-031-14378-6. [Google Scholar]
- Esmonde-White, K.A.; Cuellar, M.; Uerpmann, C.; Lenain, B.; Lewis, I.R. Raman Spectroscopy as a Process Analytical Technology for Pharmaceutical Manufacturing and Bioprocessing. Anal. Bioanal. Chem. 2017, 409, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Jehlička, J.; Culka, A. Critical Evaluation of Portable Raman Spectrometers: From Rock Outcrops and Planetary Analogs to Cultural Heritage–A Review. Anal. Chim. Acta 2022, 1209, 339027. [Google Scholar] [CrossRef]
- Gu, X.; Trujillo, M.J.; Olson, J.E.; Camden, J.P. SERS Sensors: Recent Developments and a Generalized Classification Scheme Based on the Signal Origin. Annu. Rev. Anal. Chem. 2018, 11, 147–169. [Google Scholar] [CrossRef]
- PubChem Glyphosate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3496 (accessed on 12 September 2023).
- Glyphosate|EFSA. Available online: https://www.efsa.europa.eu/en/topics/topic/glyphosate (accessed on 12 September 2023).
- È vero che il glifosato è cancerogeno? Available online: https://www.airc.it/cancro/informazioni-tumori/corretta-informazione/vero-glifosato-un-erbicida-diffuso-mondo-cancerogeno (accessed on 12 September 2023).
- IARC Monograph on Glyphosate–IARC. Available online: https://www.iarc.who.int/featured-news/media-centre-iarc-news-glyphosate/ (accessed on 21 October 2023).
- Ezio Bosso Glifosato Nelle Risaie Italiane. Available online: https://www.risoitaliano.eu/glifosato-nelle-risaie-italiane/ (accessed on 12 September 2023).
- Ministero della Salute DECRETO 9 Agosto 2016–Revoca Di Autorizzazioni All’immissione in Commercio e Modifica Delle Condizioni d’impiego Di Prodotti Fitosanitari Contenenti La Sostanza Attiva «glifosate», in Attuazione Del Regolamento Di Esecuzione (UE) 2016/1313 Della Commissione Del 1° Agosto 2016. Available online: https://www.gazzettaufficiale.it/eli/id/2016/08/19/16A06170/sg (accessed on 12 September 2023).
- Cera, E. Glifosato, in Provincia di Pavia Scatta L’allerta Per L’alta Contaminazione|il Salvagente. Available online: https://ilsalvagente.it/2022/01/10/glifosato-in-provincia-di-pavia-scatta-lallerta-per-lalta-contaminazione/ (accessed on 12 September 2023).
- Simeone, L. Allerta Fitofarmaci in Provincia Glifosato, Alta Concentrazione. Available online: https://laprovinciapavese.gelocal.it/pavia/cronaca/2022/01/08/news/allerta-fitofarmaci-in-provincia-glifosato-alta-concentrazione-1.41106852 (accessed on 23 October 2023).
- European Commission; Directorate-General for Health and Food Safety Discussion at the Standing Committee on Plants, Animals, Food and Feed (PAFF Committee), Section Phytopharmaceuticals–Legislation on Agenda Item A.04.05–Exchange of Views on EFSA’s Conclusions on the Peer Review of the Risk Assessment for Glyphosate. Available online: https://food.ec.europa.eu/system/files/2023-08/sc_phyto_20230711_ppl_sum-glyphosate.pdf (accessed on 21 October 2023).
- European Food Safety Authority (EFSA); Álvarez, F.; Arena, M.; Auteri, D.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Crivellente, F.; Egsmose, M.; Fait, G.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Glyphosate. EFSA J. 2023, 21, 1–52. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Review of the Existing Maximum Residue Levels for Glyphosate According to Article 12 of Regulation (EC) No 396/2005–Revised Version to Take into Account Omitted Data. EFSA J. 2019, 17, 5862. [Google Scholar] [CrossRef]
- Mikac, L.; Rigó, I.; Škrabić, M.; Ivanda, M.; Veres, M. Comparison of Glyphosate Detection by Surface-Enhanced Raman Spectroscopy Using Gold and Silver Nanoparticles at Different Laser Excitations. Molecules 2022, 27, 5767. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.S.; Ando, R.A.; Sant’Ana, A.C.; Corio, P. Surface-Enhanced Raman Spectroscopy Studies of Organophosphorous Model Molecules and Pesticides. Phys. Chem. Chem. Phys. 2012, 14, 15645. [Google Scholar] [CrossRef]
- Ascolani Yael, J.; Fuhr, J.D.; Bocan, G.A.; Daza Millone, A.; Tognalli, N.; Dos Santos Afonso, M.; Martiarena, M.L. Abiotic Degradation of Glyphosate into Aminomethylphosphonic Acid in the Presence of Metals. J. Agric. Food Chem. 2014, 62, 9651–9656. [Google Scholar] [CrossRef] [PubMed]
- Feis, A.; Gellini, C.; Ricci, M.; Tognaccini, L.; Becucci, M.; Smulevich, G. Surface-Enhanced Raman Scattering of Glyphosate on Dispersed Silver Nanoparticles: A Reinterpretation Based on Model Molecules. Vib. Spectrosc. 2020, 108, 103061. [Google Scholar] [CrossRef]
- Emonds-Alt, G.; Mignolet, B.; Malherbe, C.; Monbaliu, J.-C.M.; Remacle, F.; Eppe, G. Understanding Chemical Interaction between Phosphonate-Derivative Molecules and a Silver Surface Cluster in SERS: A Combined Experimental and Computational Approach. Phys. Chem. Chem. Phys. 2019, 21, 22180–22187. [Google Scholar] [CrossRef]
- Li, J.; Shi, J.; Liang, A.; Jiang, Z. Highly Catalysis Amplification of MOF Nd -Loaded Nanogold Combined with Specific Aptamer SERS/RRS Assay of Trace Glyphosate. Analyst 2022, 147, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- De Góes, R.; Muller, M.; Fabris, J. Spectroscopic Detection of Glyphosate in Water Assisted by Laser-Ablated Silver Nanoparticles. Sensors 2017, 17, 954. [Google Scholar] [CrossRef]
- De Goes, R.E.; Possetti, G.R.C.; Muller, M.; Fabris, J.L. Tuning of Citrate-Stabilized Laser Ablated Silver Nanoparticles for Glyphosate Detection. IEEE Sens. J. 2020, 20, 1843–1850. [Google Scholar] [CrossRef]
- López-Castaños, K.A.; Ortiz-Frade, L.A.; Méndez, E.; Quiroga-González, E.; González-Fuentes, M.A.; Méndez-Albores, A. Indirect Quantification of Glyphosate by SERS Using an Incubation Process with Hemin as the Reporter Molecule: A Contribution to Signal Amplification Mechanism. Front. Chem. 2020, 8, 612076. [Google Scholar] [CrossRef]
- Emonds-Alt, G.; Malherbe, C.; Kasemiire, A.; Avohou, H.T.; Hubert, P.; Ziemons, E.; Monbaliu, J.-C.M.; Eppe, G. Development and Validation of an Integrated Microfluidic Device with an In-Line Surface Enhanced Raman Spectroscopy (SERS) Detection of Glyphosate in Drinking Water. Talanta 2022, 249, 123640. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Q.; Zhang, C.; Li, C.; Jiang, Z.; Liang, A. Aptamer Trimode Biosensor for Trace Glyphosate Based on FeMOF Catalytic Oxidation of Tetramethylbenzidine. Biosensors 2022, 12, 920. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Seixas Costa, L.; Schiefer, E.M.; Fabris, J.L.; Muller, M. Detection of Glyphosate in Water with Photonic-Tailored Silver Nanoparticles. In Proceedings of the 2022 SBFoton International Optics and Photonics Conference (SBFoton IOPC), Recife, Brazil, 13 October 2022; pp. 1–5. [Google Scholar]
- Xu, R.; Dai, S.; Dou, M.; Yang, J.; Wang, X.; Liu, X.; Wei, C.; Li, Q.; Li, J. Simultaneous, Label-Free and High-Throughput SERS Detection of Multiple Pesticides on Ag@Three-Dimensional Silica Photonic Microsphere Array. J. Agric. Food Chem. 2023, 71, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lai, Y.; Cai, J.; Jia, S.; Lin, L.; Feng, Z.; Zheng, Z.; Xie, R.; Li, J. A Photo-Responsive p-Si/TiO2/Ag Heterostructure with Charge Transfer for Recyclable Surface-Enhanced Raman Scattering Substrates. CrystEngComm 2022, 24, 1078–1084. [Google Scholar] [CrossRef]
- Ma, J.; Feng, G.; Ying, Y.; Shao, Y.; She, Y.; Zheng, L.; Abd EI-Aty, A.M.; Wang, J. Sensitive SERS Assay for Glyphosate Based on the Prevention of L-Cysteine Inhibition of a Au–Pt Nanozyme. Analyst 2021, 146, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yu, L.; You, H.; Hao, R.; Fang, J. Tip Enrichment Surface-Enhanced Raman Scattering Based on the Partial Leidenfrost Phenomenon for the Ultrasensitive Nanosensors. Sens. Actuators B Chem. 2022, 355, 131250. [Google Scholar] [CrossRef]
- Barreiro, A.; Cela-Dablanca, R.; Nebot, C.; Rodríguez-López, L.; Santás-Miguel, V.; Arias-Estévez, M.; Fernández-Sanjurjo, M.; Núñez-Delgado, A.; Álvarez-Rodríguez, E. Occurrence of Nine Antibiotics in Different Kinds of Sewage Sludge, Soils, Corn and Grapes After Sludge Spreading. Span. J. Soil Sci. 2022, 12, 10741. [Google Scholar] [CrossRef]
- Salute, M. Della Antibiotico-Resistenza nel Settore Ambientale. Available online: https://www.salute.gov.it/portale/antibioticoresistenza/dettaglioContenutiAntibioticoResistenza.jsp?lingua=italiano&id=5435&area=antibiotico-resistenza&menu=vuoto (accessed on 12 September 2023).
- Nelle acque di Pavia i Super Batteri Resistenti ai Farmaci. Available online: https://laprovinciapavese.gelocal.it/pavia/cronaca/2019/09/05/news/nelle-acque-di-pavia-i-super-batteri-resistenti-ai-farmaci-1.37419016 (accessed on 28 September 2023).
- AbuAlshaar, A.; Piazza, A.; Mercato, A.; Marchesini, F.; Mattioni Marchetti, V.; Bitar, I.; Hrabak, J.; Spalla, M.; Pilla, G.; Sconfietti, R.; et al. OXA-244-Producing ST131 Escherichia Coli From Surface and Groundwaters of Pavia Urban Area (Po Plain, Northern Italy). Front. Microbiol. 2022, 13, 920319. [Google Scholar] [CrossRef]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:015:0001:0072:en:PDF (accessed on 28 September 2023).
- Tetracycline|C22H24N2O8|CID 54675776–PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tetracycline#section=Structures (accessed on 28 September 2023).
- Muhammad, M.; Yan, B.; Yao, G.; Chao, K.; Zhu, C.; Huang, Q. Surface-Enhanced Raman Spectroscopy for Trace Detection of Tetracycline and Dicyandiamide in Milk Using Transparent Substrate of Ag Nanoparticle Arrays. ACS Appl. Nano Mater. 2020, 3, 7066–7075. [Google Scholar] [CrossRef]
- Leypold, C.F.; Reiher, M.; Brehm, G.; Schmitt, M.O.; Schneider, S.; Matousek, P.; Towrie, M. Tetracycline and Derivatives—Assignment of IR and Raman Spectra via DFT Calculations. Phys. Chem. Chem. Phys. 2003, 5, 1149–1157. [Google Scholar] [CrossRef]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Veigas, B.; Araújo, A.; Pagará, B.; Baptista, P.V.; Águas, H.; Martins, R.; Fortunato, E. Paper-Based SERS Platform for One-Step Screening of Tetracycline in Milk. Sci. Rep. 2019, 9, 17922. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Chao, K.; Huang, Q.; Kim, M.; Schmidt, W.; Qin, J.; Broadhurst, C. A Simple Surface-Enhanced Raman Spectroscopic Method for on-Site Screening of Tetracycline Residue in Whole Milk. Sensors 2018, 18, 424. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ma, C.; Gu, J.; Wu, Y.; Zhu, C.; Li, L.; Gao, H.; Zhang, Y.; Shang, Y.; Wang, C.; et al. A Sensitive Surface-Enhanced Raman Spectroscopy Method for Detecting Tetracycline in Milk. Appl. Spectrosc. 2021, 75, 589–595. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal Component Analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Pagano, R.; Ottolini, M.; Valli, L.; Bettini, S.; Giancane, G. Ag Nanodisks Decorated Filter Paper as a SERS Platform for Nanomolar Tetracycline Detection. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126787. [Google Scholar] [CrossRef]
- Bush, R.K.; Taylor, S.L. HISTAMINE. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdan, The Netherlands, 2003; pp. 3108–3111. ISBN 978-0-12-227055-0. [Google Scholar]
- PubChem Histamine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/774 (accessed on 20 September 2023).
- Kovacova-Hanuskova, E.; Buday, T.; Gavliakova, S.; Plevkova, J. Histamine, Histamine Intoxication and Intolerance. Allergol. Et Immunopathol. 2015, 43, 498–506. [Google Scholar] [CrossRef]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef]
- Histamine In Wine: What Are the Signs and Symptoms of Intolerance? Available online: https://omre.co/blogs/news/histamine-in-wine (accessed on 25 September 2023).
- Wantke, F.; Götz, M.; Jarisch, R. The Red Wine Provocation Test: Intolerance to Histamine as a Model for Food Intolerance. Allergy Asthma Proc. 1994, 15, 27–32. [Google Scholar] [CrossRef][Green Version]
- Konakovsky, V.; Focke, M.; Hoffmann-Sommergruber, K.; Schmid, R.; Scheiner, O.; Moser, P.; Jarisch, R.; Hemmer, W. Levels of Histamine and Other Biogenic Amines in High-Quality Red Wines. Food Addit. Contam. Part A 2011, 28, 408–416. [Google Scholar] [CrossRef]
- Wantke, F.; Moritz, K.; Sesztakgreinecker, G.; Gotz, M.; Hemmer, W.; Moser, P.; Jarisch, R. Histamine Content in Red and Sparkling Wine and Relationship with Wine Quality. J. Allergy Clin. Immunol. 2008, 121, S194. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Cano-Mozo, E.; Albors, C.; Santos, A.; Navascués, E. Autochthonous Oenococcus Oeni Strain to Avoid Histamine Formation in Red Wines: A Study in Real Winemaking Conditions. Am. J. Enol. Vitic. 2021, 72, 170–180. [Google Scholar] [CrossRef]
- Collado, J.A.; Ramírez, F.J. Vibrational Spectra and Assignments of Histamine Dication in the Solid State and in Solution. J. Raman Spectrosc. 2000, 31, 925–931. [Google Scholar] [CrossRef]
- Ramírez, F.J.; Tuñón, I.; Collado, J.A.; Silla, E. Structural and Vibrational Study of the Tautomerism of Histamine Free-Base in Solution. J. Am. Chem. Soc. 2003, 125, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Pal, S.; Sawalha, S.; Licciulli, A.; Valli, L.; Giancane, G.; Pagano, R. Cellulose-Based Substrate for SERS-Promoted Histamine Picomolar Detection in Beverages. ChemistrySelect 2019, 4, 2968–2975. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, E.; Jiao, A.; Jin, Z.; Irudayaraj, J. Bimodal Counterpropagating-Responsive Sensing Material for the Detection of Histamine. RSC Adv. 2017, 7, 44933–44944. [Google Scholar] [CrossRef]
- Yang, D.; Li, H.; Li, Q.; Li, K.; Xiao, F.; Yang, Y. Highly Selective Histamine Assay via SERS: Based on the Signal Enhancement of Carbon Dots and the Fluorescence Quenching of Gold Nanoparticles. Sens. Actuators B Chem. 2022, 350, 130866. [Google Scholar] [CrossRef]
- Kolosovas-Machuca, E.; Cuadrado, A.; Ojeda-Galván, H.; Ortiz-Dosal, L.; Hernández-Arteaga, A.; Rodríguez-Aranda, M.; Navarro-Contreras, H.; Alda, J.; González, F. Detection of Histamine Dihydrochloride at Low Concentrations Using Raman Spectroscopy Enhanced by Gold Nanostars Colloids. Nanomaterials 2019, 9, 211. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, S.; Guo, Z.; Yan, K.; Shen, J.; Zhang, Z.; Chen, J.; Guo, Y.; Liu, L.; Wu, X. Strong Histamine Torsion Raman Spectrum Enables Direct, Rapid, and Ultrasensitive Detection of Allergic Diseases. iScience 2021, 24, 103384. [Google Scholar] [CrossRef]
- Huynh, K.-H.; Pham, X.-H.; Hahm, E.; An, J.; Kim, H.-M.; Jo, A.; Seong, B.; Kim, Y.-H.; Son, B.S.; Kim, J.; et al. Facile Histamine Detection by Surface-Enhanced Raman Scattering Using SiO2@Au@Ag Alloy Nanoparticles. IJMS 2020, 21, 4048. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Wang, X.; Qiao, X.; Waterhouse, G.I.N.; Xu, Z. A Core-Satellite Self-Assembled SERS Aptasensor Containing a “Biological-Silent Region” Raman Tag for the Accurate and Ultrasensitive Detection of Histamine. Food Sci. Hum. Wellness 2023, 13, 1029–1039. [Google Scholar] [CrossRef]
- Li, P.; Zhou, B.; Ge, M.; Jing, X.; Yang, L. Metal Coordination Induced SERS Nanoprobe for Sensitive and Selective Detection of Histamine in Serum. Talanta 2022, 237, 122913. [Google Scholar] [CrossRef] [PubMed]
- Kumari, L.; Jaiswal, P.; Tripathy, S.S. Various Techniques Useful for Determination of Adulterants in Valuable Saffron: A Review. Trends Food Sci. Technol. 2021, 111, 301–321. [Google Scholar] [CrossRef]
- Commission Extends Sudan Dye Measures and Reminds Food Operators of Their Responsibilities. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_05_385 (accessed on 12 September 2023).
- Federal Institute for Risk Assessment (BfR) Dyes Sudan I to IV in Food. 2003. Available online: https://www.semanticscholar.org/paper/Federal-Institute-for-Risk-Assessment-(-BfR-)-Dyes/5803c5d5c42e692fc2f1042859f752b78ed15202 (accessed on 12 September 2023).
- Di Anibal, C.V.; Marsal, L.F.; Callao, M.P.; Ruisánchez, I. Surface Enhanced Raman Spectroscopy (SERS) and Multivariate Analysis as a Screening Tool for Detecting Sudan I Dye in Culinary Spices. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 87, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Rebane, R.; Leito, I.; Yurchenko, S.; Herodes, K. A Review of Analytical Techniques for Determination of Sudan I–IV Dyes in Food Matrixes. J. Chromatogr. A 2010, 1217, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Alomar, T.S.; AlMasoud, N.; Xu, Y.; Lima, C.; Akbali, B.; Maher, S.; Goodacre, R. Simultaneous Multiplexed Quantification of Banned Sudan Dyes Using Surface Enhanced Raman Scattering and Chemometrics. Sensors 2022, 22, 7832. [Google Scholar] [CrossRef]
- Ou, Y.; Pei, L.; Lai, K.; Huang, Y.; Rasco, B.A.; Wang, X.; Fan, Y. Rapid Analysis of Multiple Sudan Dyes in Chili Flakes Using Surface-Enhanced Raman Spectroscopy Coupled with Au–Ag Core-Shell Nanospheres. Food Anal. Methods 2017, 10, 565–574. [Google Scholar] [CrossRef]
- Jahn, M.; Patze, S.; Bocklitz, T.; Weber, K.; Cialla-May, D.; Popp, J. Towards SERS Based Applications in Food Analytics: Lipophilic Sensor Layers for the Detection of Sudan III in Food Matrices. Anal. Chim. Acta 2015, 860, 43–50. [Google Scholar] [CrossRef]
- März, A.; Henkel, T.; Cialla, D.; Schmitt, M.; Popp, J. Droplet Formation via Flow-through Microdevices in Raman and Surface Enhanced Raman Spectroscopy—Concepts and Applications. Lab Chip 2011, 11, 3584. [Google Scholar] [CrossRef]






| Analyte | Instrument | Substrate | Mechanism | Sensitivity (LOD) | References |
|---|---|---|---|---|---|
| Glyphosate | b 1, 532 nm | modified Ag NPs 3 | direct | 170 ppm | [38] |
| p 2, 785 nm | Au NPs | direct | 17 ppm | [38] | |
| b, 633 nm | Ag NPs | direct | not available | [39,41,42] | |
| b, not available | modified Au NPs | indirect | 0.0034 ppb | [43] | |
| b, 633 nm | Ag NPs | direct | 3.2 ppm | [44] | |
| b, 633 nm | Ag NPs | direct | 1.3 ppm | [45] | |
| b, 780 nm | Ag NPs | direct | 0.0016 ppb | [46] | |
| b, 647 nm | Ag NPs | direct | 40 ppb | [47] | |
| b, 633 nm | Ag NPs | direct | 0.0085 ppb | [48] | |
| b, 638 nm | Ag NPs | direct | 110 ppb | [49] | |
| b, 785 nm | Ag NPs | direct | 3.14 ppb | [50] | |
| b, 532 nm | TiO2/Ag heterostr. | direct | 0.1 ppb | [51] | |
| p, not available | Au-Ag nanochains | indirect | 5 ppb | [52] | |
| p, 785 nm | Au NPs | direct | 1.3 ppb | [53] | |
| Tetracycline | b, 633 nm | Ag NPs | direct | 0.1 ppm | [64] |
| p, 785 nm | Ag NPs | direct | 0.01 ppm | [65] | |
| b, 785 nm | Ag NPs | direct | 0.44 ppb | [61] | |
| b, 532 nm | Ag NPs | direct | 0.28 ppb | [66] | |
| b, 532 nm | Ag NPs | direct | 0.44 ppb | [68] | |
| Histamine | b, 532 nm | modified Ag NPs | direct | 10−4 ppb | [80] |
| b 785 nm | MIPs 4-Ag NPs | direct | 0.04 ppm | [81] | |
| b, 785 nm | carbon dots/Au NPs | direct | 1 ppm | [82] | |
| b, 785 nm | Au NPs | direct | 10 ppb | [83] | |
| b, 514.5 nm | Au NPs | direct | 0.01 ppb | [84] | |
| b, 532 nm | SiO2@Au@Ag NPs | direct | 3.7 ppm | [85] | |
| b, 532 nm | modified Ag NPs | indirect | 0.65 10−3 ppb | [86] | |
| b, 633 nm | Au NPs | indirect | 0.1 ppm | [87] | |
| Sudan I, II, III, IV | b, 785 nm | Au NPs | direct | 0.4 ppm | [93] |
| Sudan I, II, III, IV | b, 633 nm | Au@Ag NPs | direct | 0.08 to 0.2 ppm | [94] |
| Sudan III | b, 488 nm | modified Ag NPs | direct | 3 × 103 ppm | [95] |
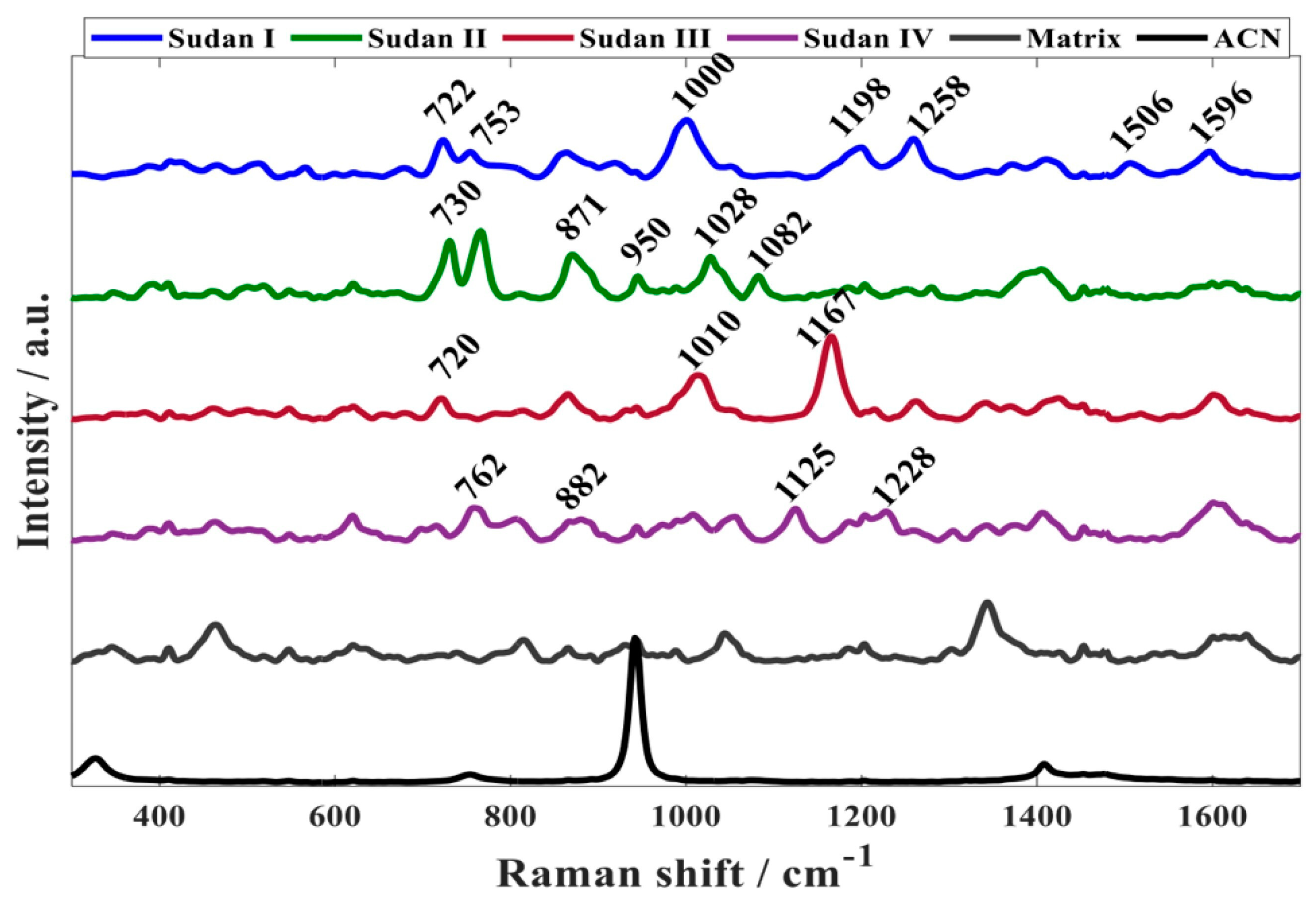
| Sudan III | b, 638 nm | Ag NPs | direct | LOD < 0.2 ppb | original results |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albini, B.; Galinetto, P.; Schiavi, S.; Giulotto, E. Food Safety Issues in the Oltrepò Pavese Area: A SERS Sensing Perspective. Sensors 2023, 23, 9015. https://doi.org/10.3390/s23229015
Albini B, Galinetto P, Schiavi S, Giulotto E. Food Safety Issues in the Oltrepò Pavese Area: A SERS Sensing Perspective. Sensors. 2023; 23(22):9015. https://doi.org/10.3390/s23229015
Chicago/Turabian StyleAlbini, Benedetta, Pietro Galinetto, Serena Schiavi, and Enrico Giulotto. 2023. "Food Safety Issues in the Oltrepò Pavese Area: A SERS Sensing Perspective" Sensors 23, no. 22: 9015. https://doi.org/10.3390/s23229015
APA StyleAlbini, B., Galinetto, P., Schiavi, S., & Giulotto, E. (2023). Food Safety Issues in the Oltrepò Pavese Area: A SERS Sensing Perspective. Sensors, 23(22), 9015. https://doi.org/10.3390/s23229015








