The Application of Graphene Field-Effect Transistor Biosensors in COVID-19 Detection Technology: A Review
Abstract
:1. Introduction
2. GFET Biosensors
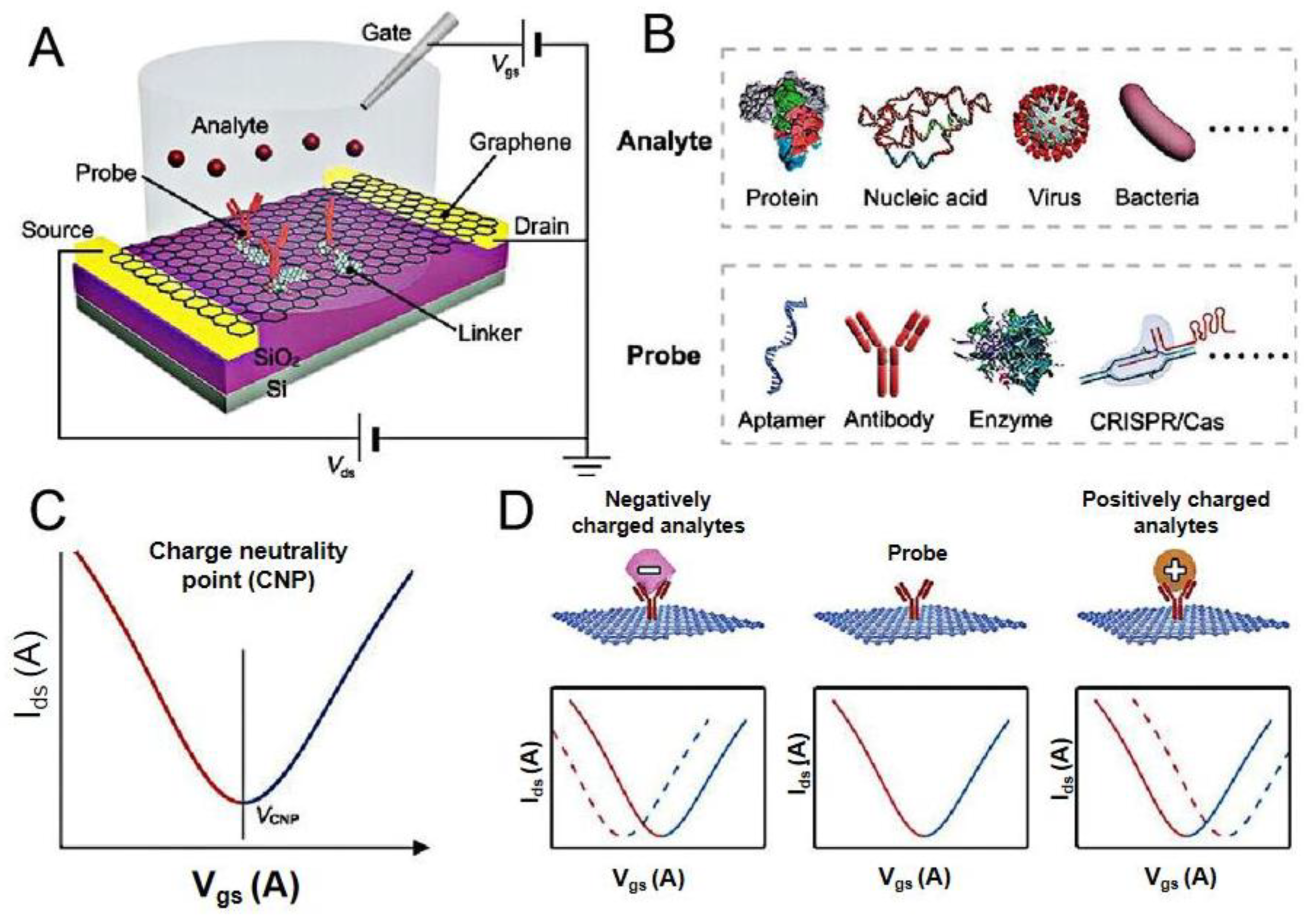
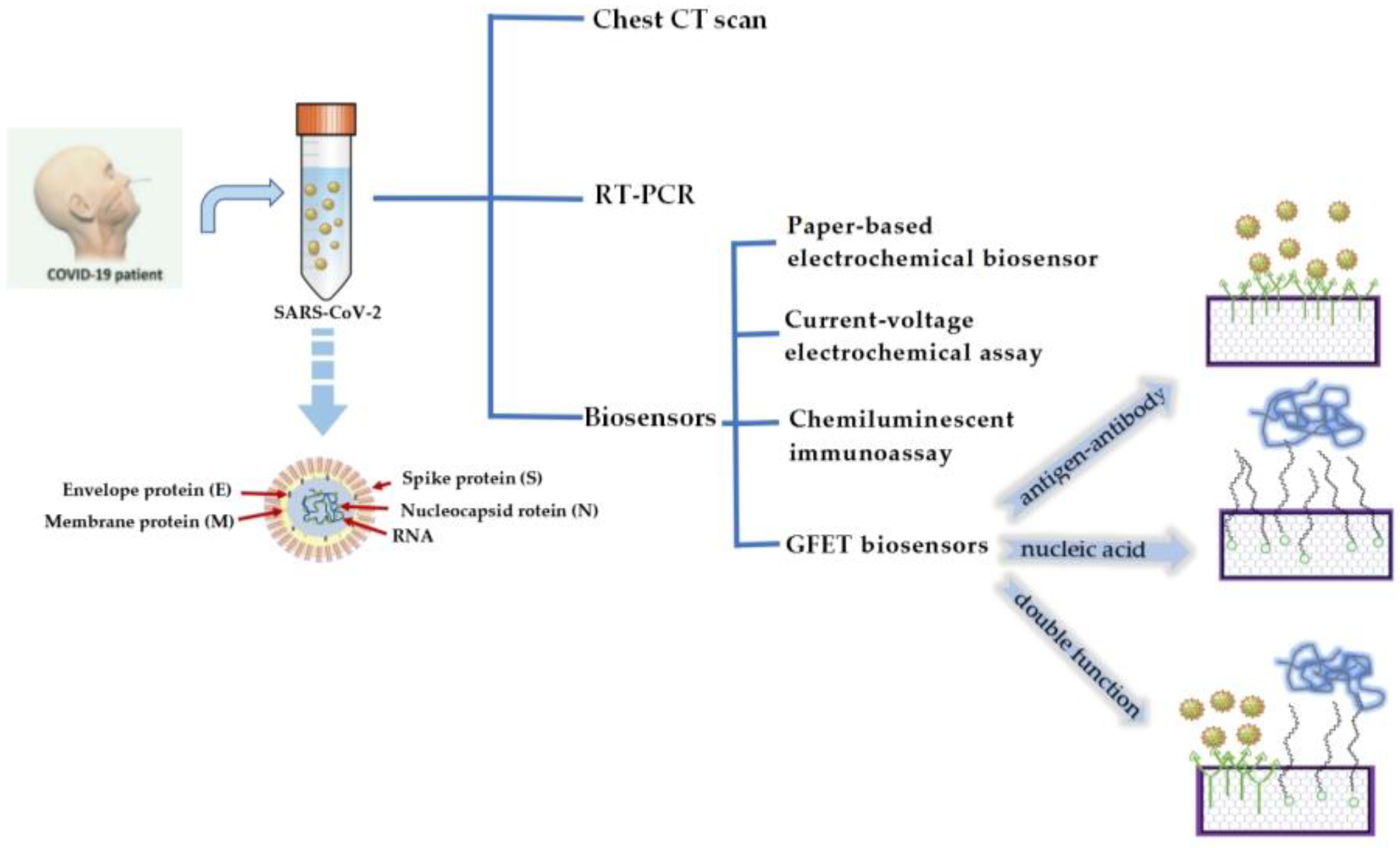
3. Application of GFET Biosensors in the Diagnosis of COVID-19
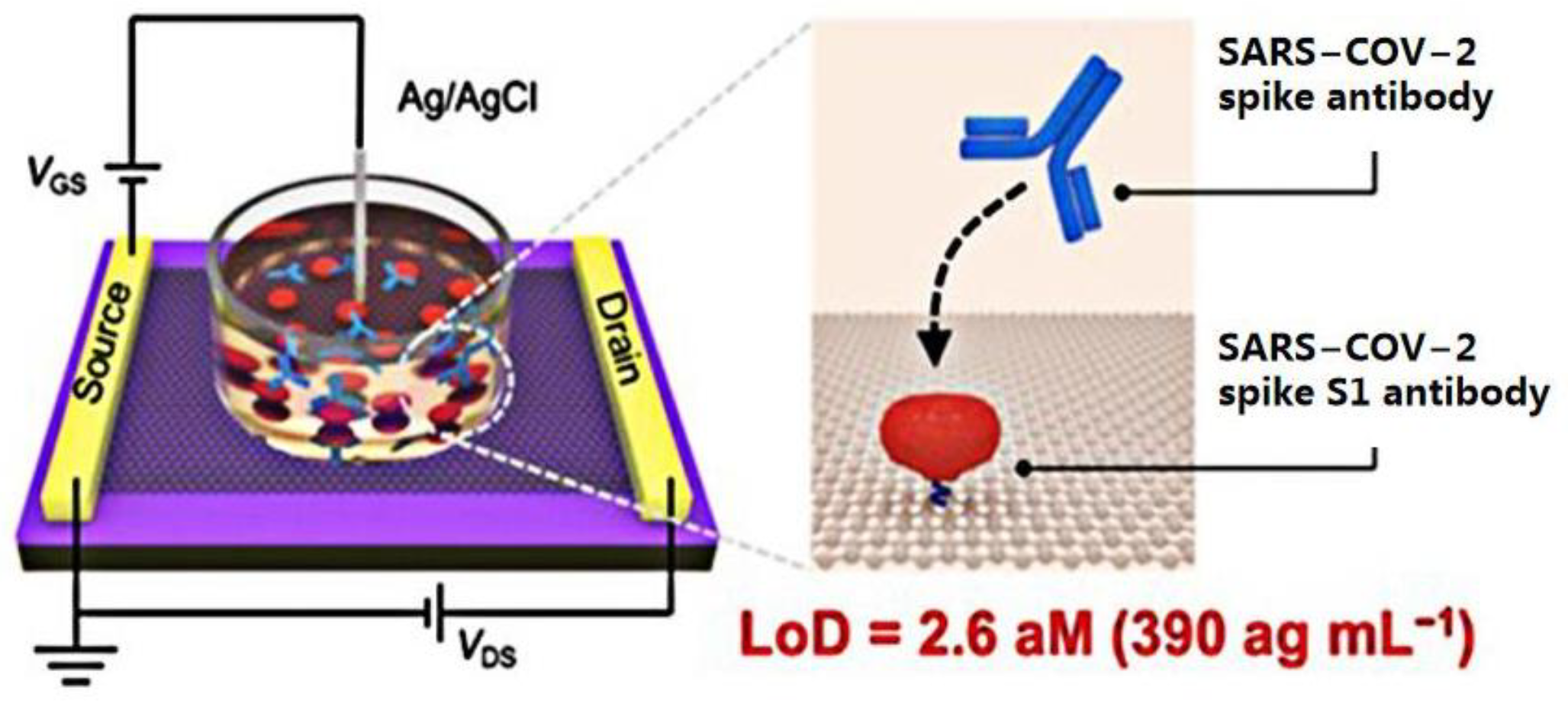
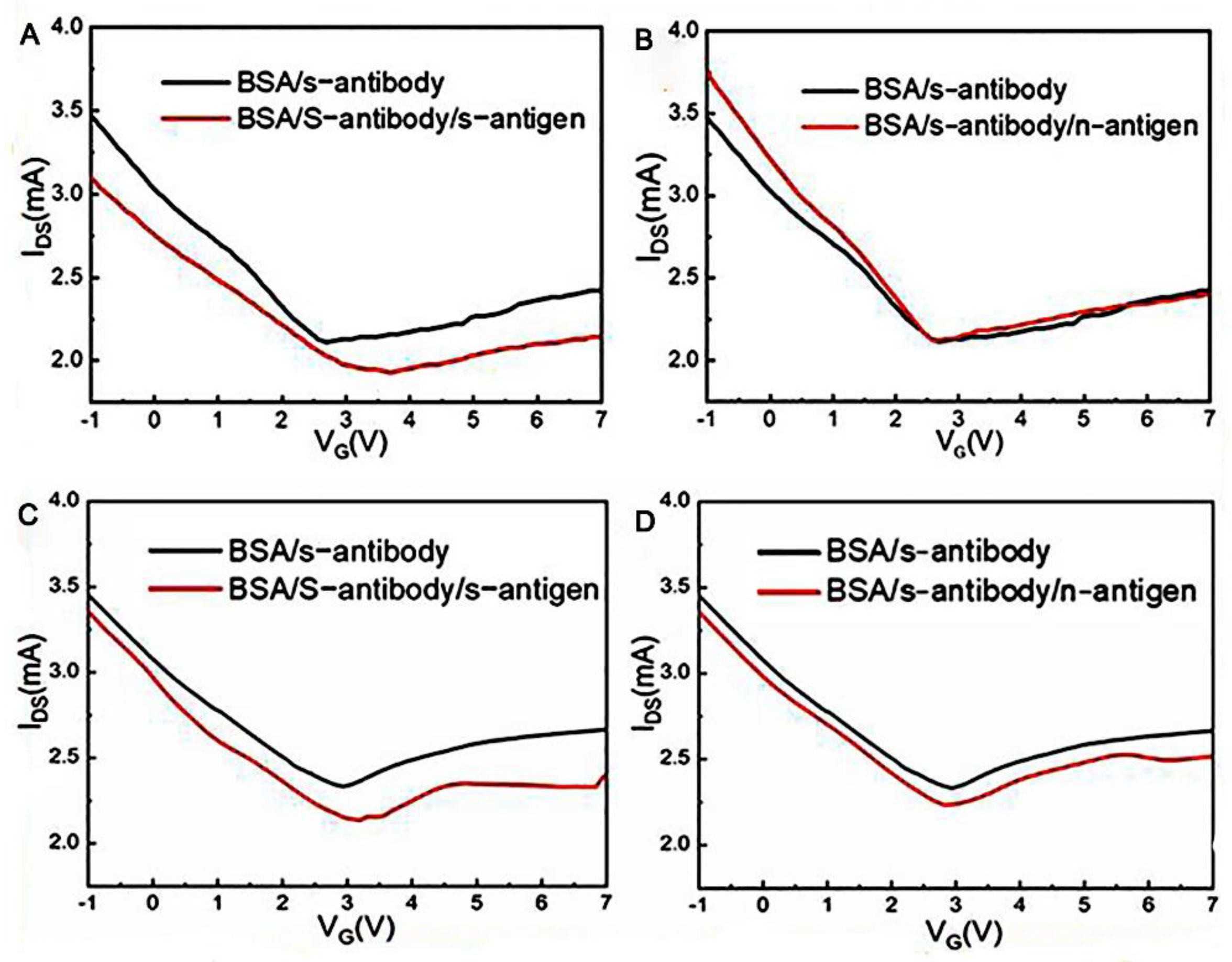
3.1. GFET Biosensors Detect SARS-CoV-2 Based on Specific Antigen–Antibody Binding
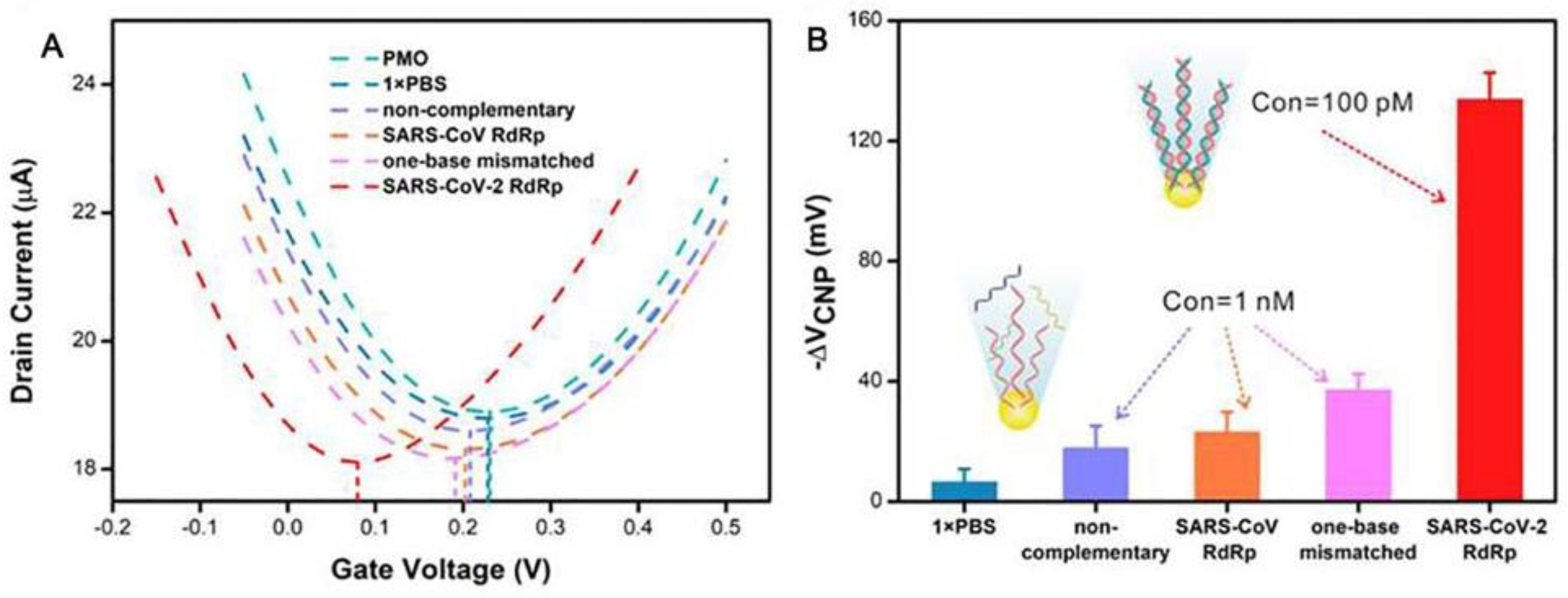
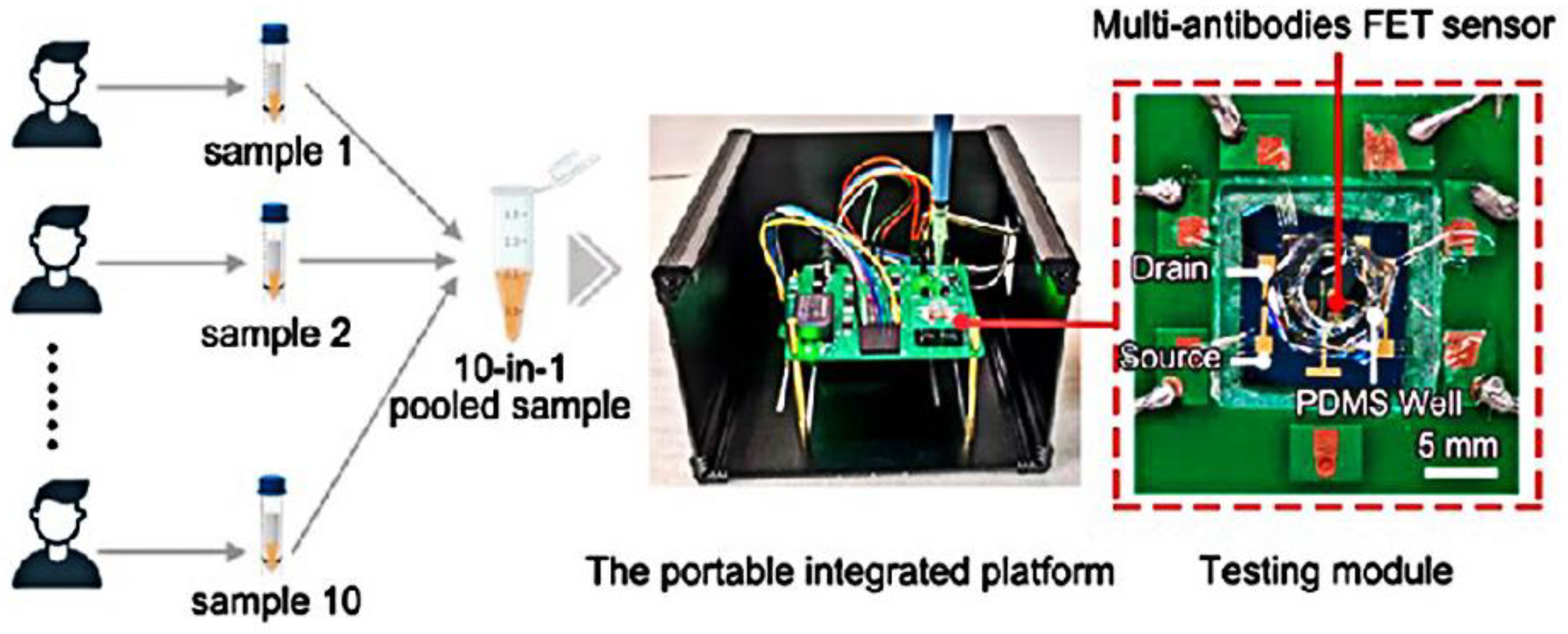
3.2. GFET Biosensors Based on Nucleic Acid Hybridization Detection of SARS-CoV-2
3.3. Double Function of GFET Biosensors in Response to the Detection of SARS-CoV-2
4. Other Types of Biosensors to Detect SARS-CoV-2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Leung, K.; Leung, G.M. Nowcasting and Forecasting the Potential Domestic and International Spread of the 2019-nCoV Outbreak Originating in Wuhan, China: A Modelling Study. Lancet 2020, 395, 689–697. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, H.S. Diagnostic Methods and Potential Portable Biosensors for Coronavirus Disease 2019. Biosens. Bioelectron. 2020, 165, 112349. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Tang, Y.-N.; Jiang, D.; Wang, X.; Liu, Y.; Wei, D. Recent Progress on Rapid Diagnosis of COVID-19 by Point-of-Care Testing Platforms. Chin. Chem. Lett. 2023, 108688. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 296, E32–E40. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.; Cortade, D.L.; Ng, E.; Wang, S.X. Diagnostics for SARS-CoV-2 Detection: A Comprehensive Review of the FDA-EUA COVID-19 Testing Landscape. Biosens. Bioelectron. 2020, 165, 112454. [Google Scholar] [CrossRef]
- Giovannini, G.; Haick, H.; Garoli, D. Detecting COVID-19 from Breath: A Game Changer for a Big Challenge. ACS Sens. 2021, 6, 1408–1417. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, H.; Xie, J.; Lin, M.; Ying, L.; Pang, P.; Ji, W. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020, 296, E115–E117. [Google Scholar] [CrossRef] [PubMed]
- Etienne, E.E.; Nunna, B.B.; Talukder, N.; Wang, Y.; Lee, E.S. COVID-19 Biomarkers and Advanced Sensing Technologies for Point-of-Care (POC) Diagnosis. Bioengineering 2021, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wen, D.; Wu, J.; Liu, L.; Wu, W.; Fang, X.; Kong, J. Microfluidic Immunoassays for Sensitive and Simultaneous Detection of IgG/IgM/Antigen of SARS-CoV-2 within 15 Min. Anal. Chem. 2020, 92, 9454–9458. [Google Scholar] [CrossRef]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-Based Electrochemical Biosensor for Diagnosing COVID-19: Detection of SARS-CoV-2 Antibodies and Antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef]
- Novodchuk, I.; Kayaharman, M.; Prassas, I.; Soosaipillai, A.; Karimi, R.; Goldthorpe, I.A.; Abdel-Rahman, E.; Sanderson, J.; Diamandis, E.P.; Bajcsy, M.; et al. Electronic Field Effect Detection of SARS-CoV-2 N-Protein before the Onset of Symptoms. Biosens. Bioelectron. 2022, 210, 114331. [Google Scholar] [CrossRef]
- Lim, W.Y.; Lan, B.L.; Ramakrishnan, N. Emerging Biosensors to Detect Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Review. Biosensors 2021, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Ajmal, M.; Ashraf, G.; Muhammad, N.; Aziz, A.; Iftikhar, T.; Wang, J.; Liu, H. The Role of Biosensors in Coronavirus Disease-2019 Outbreak. Curr. Opin. Electrochem. 2020, 23, 174–184. [Google Scholar] [CrossRef]
- Han, S.; Chen, C.; Chen, C.; Wu, L.; Wu, X.; Lu, C.; Zhang, X.; Chao, P.; Lv, X.; Jia, Z.; et al. Coupling Annealed Silver Nanoparticles with a Porous Silicon Bragg Mirror SERS Substrate and Machine Learning for Rapid Non-Invasive Disease Diagnosis. Anal. Chim. Acta 2023, 1254, 341116. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Yang, Y.; Xiong, H.; Wang, X.; Gou, J.; Li, P.; Wu, Y.; Chen, Y.; Kong, D.; Yang, Y.; et al. Accurately Detecting Trace-Level Infectious Agents by an Electro-Enhanced Graphene Transistor. Adv. Funct. Mater. 2023, 33, 2300151. [Google Scholar] [CrossRef]
- Fan, Z.; Geng, Z.; Fang, W.; Lv, X.; Su, Y.; Wang, S.; Chen, H. Smartphone Biosensor System with Multi-Testing Unit Based on Localized Surface Plasmon Resonance Integrated with Microfluidics Chip. Sensors 2020, 20, 446. [Google Scholar] [CrossRef]
- Sun, D.; Wu, Y.; Chang, S.-J.; Chen, C.-J.; Liu, J.-T. Investigation of the Recognition Interaction between Glycated Hemoglobin and Its Aptamer by Using Surface Plasmon Resonance. Talanta 2021, 222, 121466. [Google Scholar] [CrossRef] [PubMed]
- Chaibun, T.; Puenpa, J.; Ngamdee, T.; Boonapatcharoen, N.; Athamanolap, P.; O’Mullane, A.P.; Vongpunsawad, S.; Poovorawan, Y.; Lee, S.Y.; Lertanantawong, B. Rapid Electrochemical Detection of Coronavirus SARS-CoV-2. Nat. Commun. 2021, 12, 802. [Google Scholar] [CrossRef]
- Shahdeo, D.; Chauhan, N.; Majumdar, A.; Ghosh, A.; Gandhi, S. Graphene-Based Field-Effect Transistor for Ultrasensitive Immunosensing of SARS-CoV-2 Spike S1 Antigen. ACS Appl. Bio Mater. 2022, 5, 3563–3572. [Google Scholar] [CrossRef] [PubMed]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing Based on Field-Effect Transistors (FET): Recent Progress and Challenges. TrAC Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef]
- Fernandes, R.S.; De Oliveira Silva, J.; Gomes, K.B.; Azevedo, R.B.; Townsend, D.M.; De Paula Sabino, A.; Branco De Barros, A.L. Recent Advances in Point of Care Testing for COVID-19 Detection. Biomed. Pharmacother. 2022, 153, 113538. [Google Scholar] [CrossRef]
- Shabani, E.; Dowlatshahi, S.; Abdekhodaie, M.J. Laboratory Detection Methods for the Human Coronaviruses. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.A.; Park, S.; Khan, M.F.; Bhopal, M.F.; Nazir, G.; Kim, M.; Farooq, A.; Ha, J.; Rehman, S.; Jun, S.C.; et al. Development of Directly Grown-graphene–Silicon Schottky Barrier Solar Cell Using Co-doping Technique. Int. J. Energy Res. 2022, 46, 11510–11522. [Google Scholar] [CrossRef]
- Khan, M.F.; Elahi, E.; Hassan, N.U.; Rehman, M.A.; Khalil, H.M.W.; Khan, M.A.; Rehman, S.; Hao, A.; Noh, H.; Khan, K.; et al. Bipolar Photoresponse of a Graphene Field-Effect Transistor Induced by Photochemical Reactions. ACS Appl. Electron. Mater. 2023, 5, 5111–5119. [Google Scholar] [CrossRef]
- Yao, H.; Sun, Z.; Liang, L.; Yan, X.; Wang, Y.; Yang, M.; Hu, X.; Wang, Z.; Li, Z.; Wang, M.; et al. Hybrid Metasurface Using Graphene/Graphitic Carbon Nitride Heterojunctions for Ultrasensitive Terahertz Biosensors with Tunable Energy Band Structure. Photon. Res. 2023, 11, 858. [Google Scholar] [CrossRef]
- Varghese, N.; Mogera, U.; Govindaraj, A.; Das, A.; Maiti, P.K.; Sood, A.K.; Rao, C.N.R. Binding of DNA Nucleobases and Nucleosides with Graphene. ChemPhysChem 2009, 10, 206–210. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for Biosensing Applications: A Review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Song, B.; Li, D.; Qi, W.; Elstner, M.; Fan, C.; Fang, H. Graphene on Au (111): A Highly Conductive Material with Excellent Adsorption Properties for High-Resolution Bio/Nanodetection and Identification. ChemPhysChem 2010, 11, 585–589. [Google Scholar] [CrossRef]
- Roberts, A.; Chauhan, N.; Islam, S.; Mahari, S.; Ghawri, B.; Gandham, R.K.; Majumdar, S.S.; Ghosh, A.; Gandhi, S. Graphene Functionalized Field-Effect Transistors for Ultrasensitive Detection of Japanese Encephalitis and Avian Influenza Virus. Sci. Rep. 2020, 10, 14546. [Google Scholar] [CrossRef]
- Lu, C.-H.; Yang, H.-H.; Zhu, C.-L.; Chen, X.; Chen, G.-N. A Graphene Platform for Sensing Biomolecules. Angew. Chem. Int. Ed. 2009, 48, 4785–4787. [Google Scholar] [CrossRef] [PubMed]
- Panahi, A.; Sadighbayan, D.; Forouhi, S.; Ghafar-Zadeh, E. Recent Advances of Field-Effect Transistor Technology for Infectious Diseases. Biosensors 2021, 11, 103. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Sreejith, S.; Ajayan, J.; Radhika, J.M.; Sivasankari, B.; Tayal, S.; Saravanan, M. A Comprehensive Review on Graphene FET Bio-Sensors and Their Emerging Application in DNA/RNA Sensing & Rapid COVID-19 Detection. Measurement 2023, 206, 112202. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, D.; Qiu, L.; Wu, Y.; Dai, C.; Luo, S.; Huang, Z.; Lin, Q.; Chen, H.; Xie, S.; et al. Artificial Nucleotide Aptamer-Based Field-Effect Transistor for Ultrasensitive Detection of Hepatoma Exosomes. Anal. Chem. 2022, 95, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, C.; Chu, Y.; Han, Y.; Gao, Y.; Wang, Y.; Wang, C.; Liu, H.; Han, L.; Zhang, Y. Graphene Oxide-Graphene Van Der Waals Heterostructure Transistor Biosensor for SARS-CoV-2 Protein Detection. Talanta 2022, 240, 123197. [Google Scholar] [CrossRef]
- Thriveni, G.; Ghosh, K. Advancement and Challenges of Biosensing Using Field Effect Transistors. Biosensors 2022, 12, 647. [Google Scholar] [CrossRef]
- Dai, C.; Kong, D.; Chen, C.; Liu, Y.; Wei, D. Graphene Transistors for In Vitro Detection of Health Biomarkers. Adv. Funct. Mater. 2023, 33, 2301948. [Google Scholar] [CrossRef]
- Cai, J.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, S.; Muoth, M.; Seitsonen, A.P.; Saleh, M.; Feng, X.; et al. Atomically Precise Bottom-up Fabrication of Graphene Nanoribbons. Nature 2010, 466, 470–473. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A Review on Mechanical Exfoliation for the Scalable Production of Graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Minhas-Khan, A.; Ghafar-Zadeh, E. Laser-Induced Graphene-Functionalized Field-Effect Transistor-Based Biosensing: A Potent Candidate for COVID-19 Detection. IEEE Trans. Nanobiosci. 2022, 21, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wang, X. Use of In-Situ Polymerization in the Preparation of Graphene/Polymer Nanocomposites. New Carbon Mater. 2020, 35, 336–343. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Wagner, S.; Ruhkopf, J.; Gahoi, A.; Pandey, H.; Bornemann, R.; Vaziri, S.; Smith, A.D.; Ostling, M.; Lemme, M.C. Chemical Vapor Deposited Graphene: From Synthesis to Applications: Chemical Vapor Deposited Graphene. Phys. Status Solidi A 2014, 211, 2439–2449. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-Induced Porous Graphene Films from Commercial Polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Alafeef, M.; Dighe, K.; Moitra, P.; Pan, D. Rapid, Ultrasensitive, and Quantitative Detection of SARS-CoV-2 Using Antisense Oligonucleotides Directed Electrochemical Biosensor Chip. ACS Nano 2020, 14, 17028–17045. [Google Scholar] [CrossRef]
- Zambrano, G.; Nastri, F.; Pavone, V.; Lombardi, A.; Chino, M. Use of an Artificial Miniaturized Enzyme in Hydrogen Peroxide Detection by Chemiluminescence. Sensors 2020, 20, 3793. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Yip, C.C.-Y.; To, K.K.-W.; Tang, T.H.-C.; Wong, S.C.-Y.; Leung, K.-H.; Fung, A.Y.-F.; Ng, A.C.-K.; Zou, Z.; Tsoi, H.-W.; et al. Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. J. Clin. Microbiol. 2020, 58, e00310-20. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Kang, H.; Wang, X.; Guo, M.; Dai, C.; Chen, R.; Yang, L.; Wu, Y.; Ying, T.; Zhu, Z.; Wei, D.; et al. Ultrasensitive Detection of SARS-CoV-2 Antibody by Graphene Field-Effect Transistors. Nano Lett. 2021, 21, 7897–7904. [Google Scholar] [CrossRef]
- Cui, T.-R.; Qiao, Y.-C.; Gao, J.-W.; Wang, C.-H.; Zhang, Y.; Han, L.; Yang, Y.; Ren, T.-L. Ultrasensitive Detection of COVID-19 Causative Virus (SARS-CoV-2) Spike Protein Using Laser Induced Graphene Field-Effect Transistor. Molecules 2021, 26, 6947. [Google Scholar] [CrossRef]
- Ji, D.; Zhao, J.; Liu, Y.; Wei, D. Electrical Nanobiosensors for Nucleic Acid Based Diagnostics. J. Phys. Chem. Lett. 2023, 14, 4084–4095. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, X.; Gu, C.; Guo, M.; Wang, Y.; Ai, Z.; Zhang, S.; Chen, Y.; Liu, W.; Wu, Y.; et al. Direct SARS-CoV-2 Nucleic Acid Detection by Y-Shaped DNA Dual-Probe Transistor Assay. J. Am. Chem. Soc. 2021, 143, 17004–17014. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ji, D.; Dai, C.; Kong, D.; Chen, Y.; Wang, L.; Guo, M.; Liu, Y.; Wei, D. Triple-Probe DNA Framework-Based Transistor for SARS-CoV-2 10-in-1 Pooled Testing. Nano Lett. 2022, 22, 3307–3316. [Google Scholar] [CrossRef]
- Ke, G.; Su, D.; Li, Y.; Zhao, Y.; Wang, H.; Liu, W.; Li, M.; Yang, Z.; Xiao, F.; Yuan, Y.; et al. An Accurate, High-Speed, Portable Bifunctional Electrical Detector for COVID-19. Sci. China Mater. 2021, 64, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.T.; Park, I.; Heiranian, M.; Taqieddin, A.; You, S.; Faramarzi, V.; Pak, A.A.; Zande, A.M.; Aluru, N.R.; Bashir, R. Ultrasensitive Detection of Dopamine, IL-6 and SARS-CoV-2 Proteins on Crumpled Graphene FET Biosensor. Adv. Mater. Technol. 2021, 6, 2100712. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.T.; Heiranian, M.; Kim, Y.; You, S.; Leem, J.; Taqieddin, A.; Faramarzi, V.; Jing, Y.; Park, I.; Van Der Zande, A.M.; et al. Ultrasensitive Detection of Nucleic Acids Using Deformed Graphene Channel Field Effect Biosensors. Nat. Commun. 2020, 11, 1543. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic Beads Combined with Carbon Black-Based Screen-Printed Electrodes for COVID-19: A Reliable and Miniaturized Electrochemical Immunosensor for SARS-CoV-2 Detection in Saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef]
- Li, J.; Wu, D.; Yu, Y.; Li, T.; Li, K.; Xiao, M.-M.; Li, Y.; Zhang, Z.-Y.; Zhang, G.-J. Rapid and Unamplified Identification of COVID-19 with Morpholino-Modified Graphene Field-Effect Transistor Nanosensor. Biosens. Bioelectron. 2021, 183, 113206. [Google Scholar] [CrossRef]
- Wang, X.; Kong, D.; Guo, M.; Wang, L.; Gu, C.; Dai, C.; Wang, Y.; Jiang, Q.; Ai, Z.; Zhang, C.; et al. Rapid SARS-CoV-2 Nucleic Acid Testing and Pooled Assay by Tetrahedral DNA Nanostructure Transistor. Nano Lett. 2021, 21, 9450–9457. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Guo, M.; Wu, Y.; Cao, B.-P.; Wang, X.; Wu, Y.; Kang, H.; Kong, D.; Zhu, Z.; Ying, T.; et al. Ultraprecise Antigen 10-in-1 Pool Testing by Multiantibodies Transistor Assay. J. Am. Chem. Soc. 2021, 143, 19794–19801. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, A.; Awwad, F.; Gelovani, J.G.; Qamhieh, N.; Ayesh, A.I. COVID-19 Detection via Silicon Nanowire Field-Effect Transistor: Setup and Modeling of Its Function. Nanomaterials 2022, 12, 2638. [Google Scholar] [CrossRef] [PubMed]
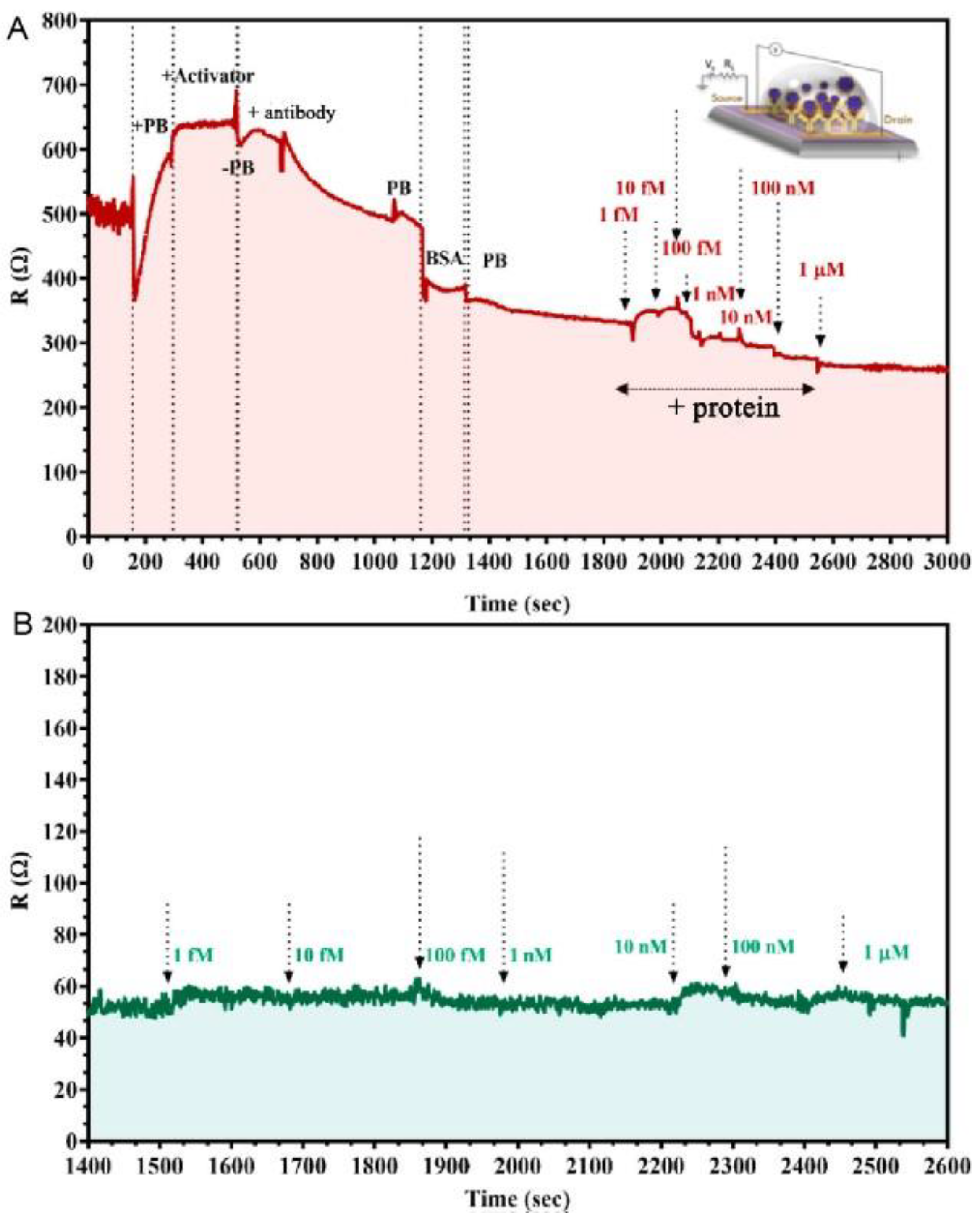
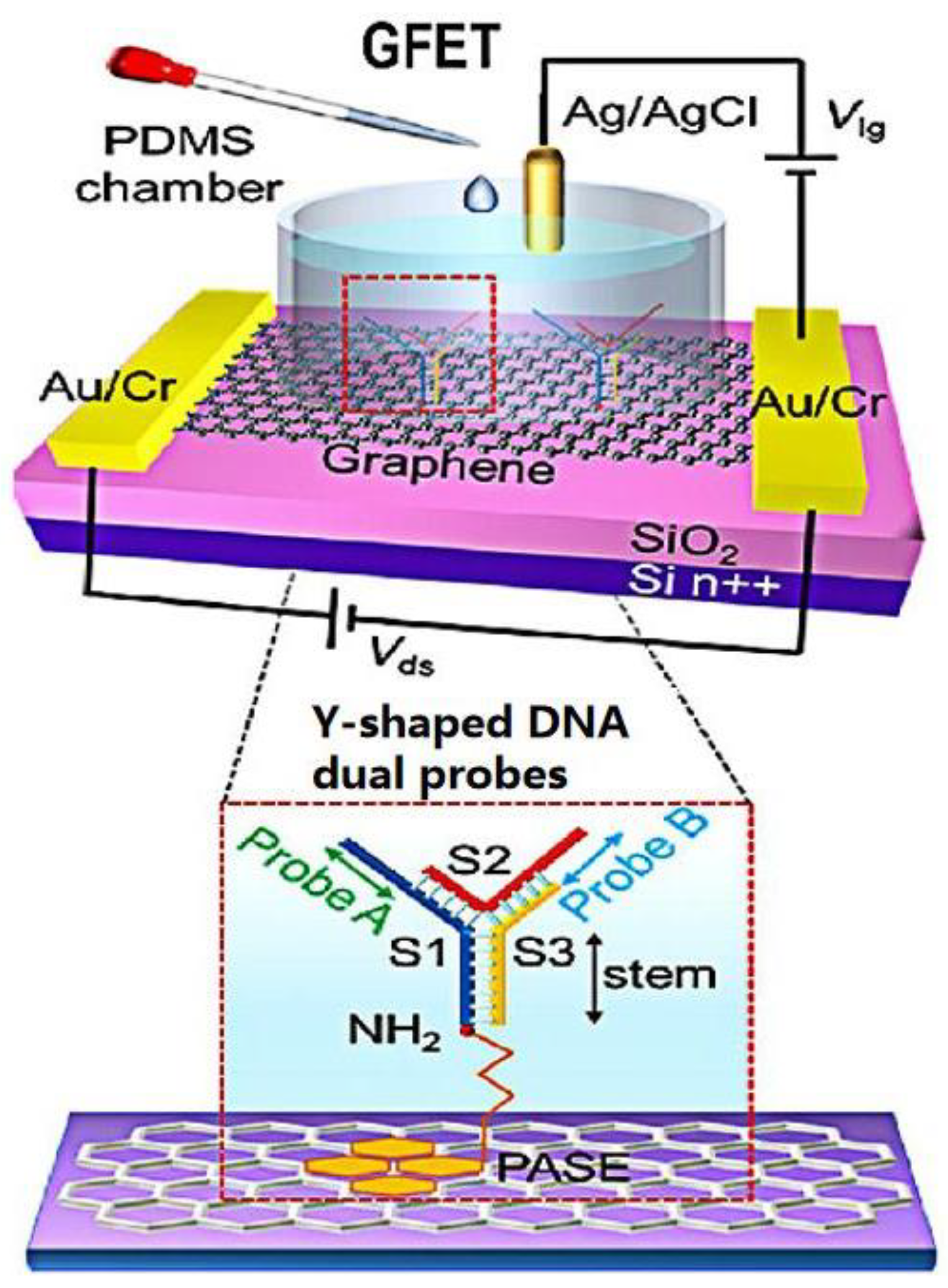
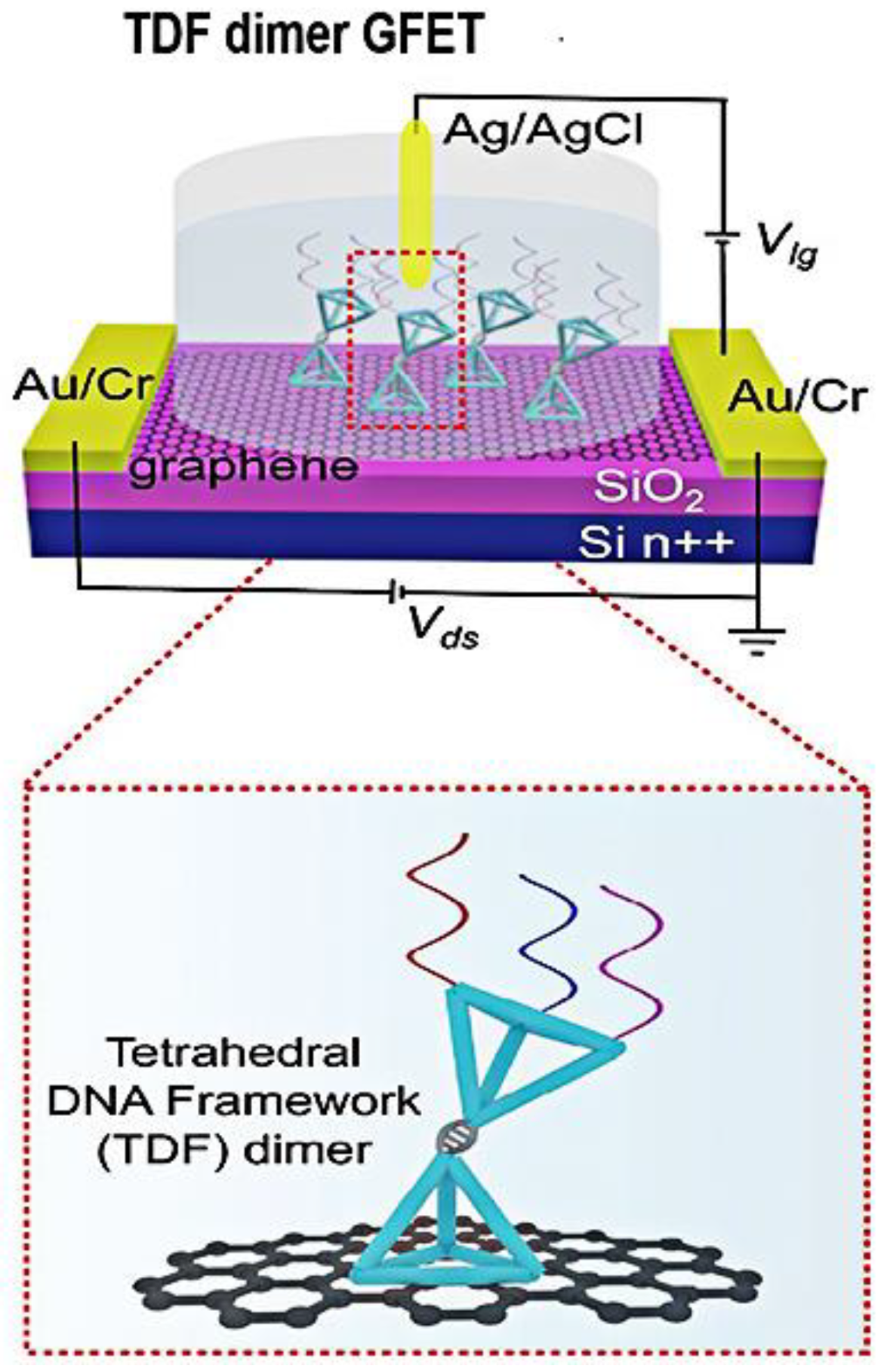

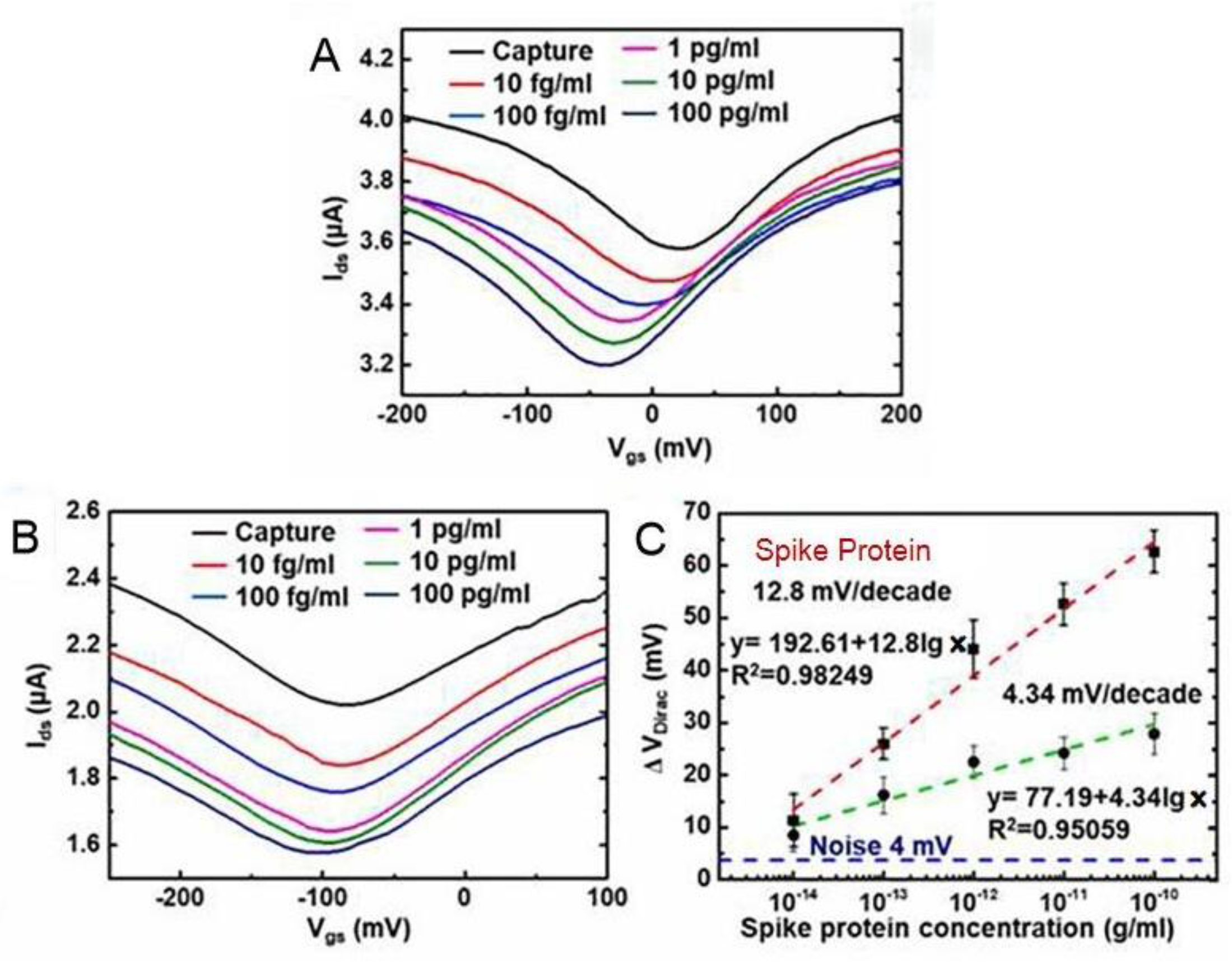

| Methods | Target | Sample or Medium | Lod | Response Time | Ref. |
|---|---|---|---|---|---|
| Paper-based electrochemical biosensor | SARS-CoV-2 antibody | Serum | ~6.4 × 10−12 M | 30 min | [12] |
| Current–voltage electrochemical assay | SARS-CoV-2 RNA | Nasopharyngeal swabs | 6.9 copy/μL | 5 min | [47] |
| Chemiluminescent Immunoassay | SARS-CoV-2 antibody | Reaction mixture | 4.6 μM | 48 min | [48] |
| qRT-PCR | SARS-CoV-2 RNA | Nasopharyngeal swabs | 11.2–21.3 copy/reaction | ~60 min | [49] |
| BN-GO gel FET biosensor | SARS-CoV-2 N-protein | Buffer | 0.00001 pg/mL | <4 min | [13] |
| GFET biosensor | SARS-CoV-2 antigen | Buffer | 0.001 pg/mL | <1 min | [50] |
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | |
| ΔR/R0 (%) | −9 | −5.8 | −2.8 | −3.6 | −5.9 | −8.9 | −6.2 | −3.1 | 6.1 |
| GFET results | + | + | + | + | + | + | + | + | + |
| Agreement | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Patient 10 | Health 1 | Health 2 | Health 3 | Health 4 | Health 5 | Health 6 | Health 7 | Health 8 | |
| ΔR/R0 (%) | −5.4 | 1.6 | −0.3 | 2 | 3.2 | 4.1 | 5.3 | 2.9 | 2.2 |
| GFET results | + | − | − | − | − | − | − | − | − |
| Agreement | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.-H.; Cao, B.-P.; Xiao, Q.; Wei, D. The Application of Graphene Field-Effect Transistor Biosensors in COVID-19 Detection Technology: A Review. Sensors 2023, 23, 8764. https://doi.org/10.3390/s23218764
Liang Q-H, Cao B-P, Xiao Q, Wei D. The Application of Graphene Field-Effect Transistor Biosensors in COVID-19 Detection Technology: A Review. Sensors. 2023; 23(21):8764. https://doi.org/10.3390/s23218764
Chicago/Turabian StyleLiang, Qin-Hong, Ban-Peng Cao, Qiang Xiao, and Dacheng Wei. 2023. "The Application of Graphene Field-Effect Transistor Biosensors in COVID-19 Detection Technology: A Review" Sensors 23, no. 21: 8764. https://doi.org/10.3390/s23218764
APA StyleLiang, Q.-H., Cao, B.-P., Xiao, Q., & Wei, D. (2023). The Application of Graphene Field-Effect Transistor Biosensors in COVID-19 Detection Technology: A Review. Sensors, 23(21), 8764. https://doi.org/10.3390/s23218764






