Electrochemical (Bio)Sensing Devices for Human-Microbiome-Related Biomarkers
Abstract
1. Introduction
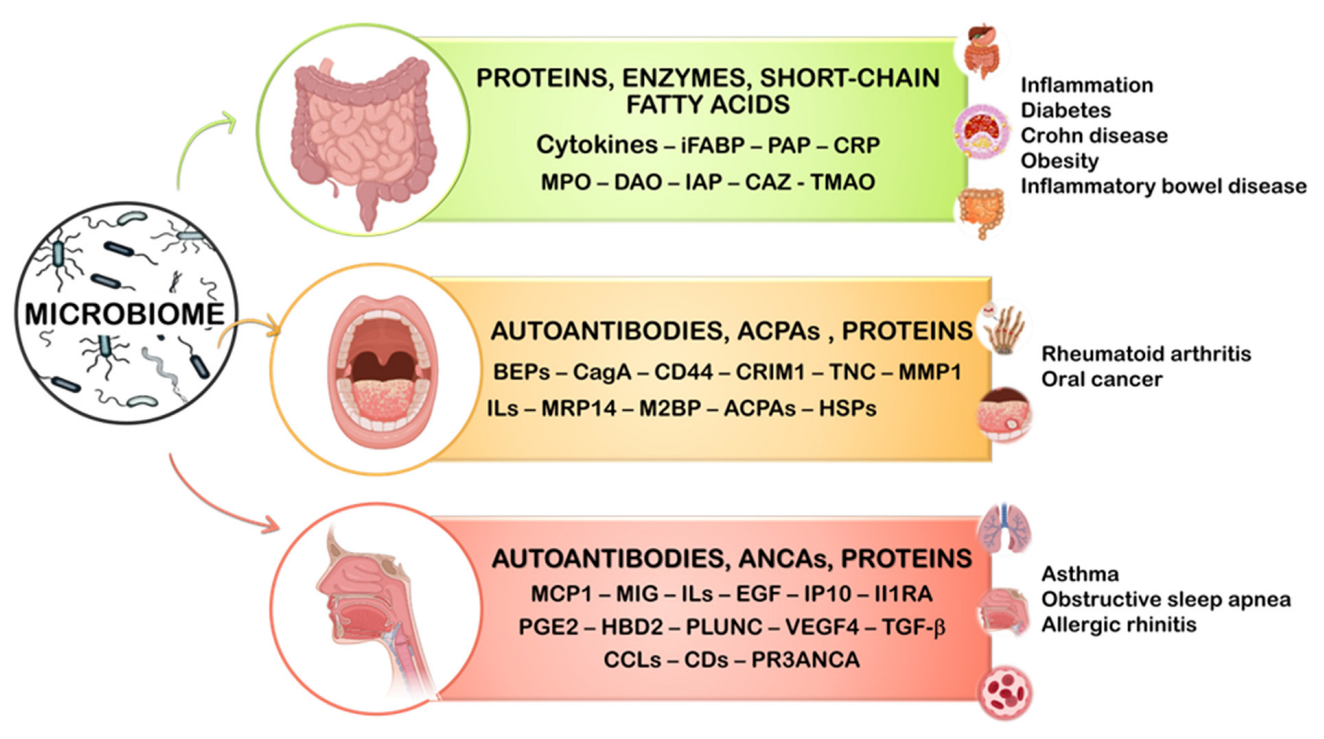
2. Gastrointestinal Microbiome
3. Oral Microbiome
4. Nasal Microbiome
5. Concluding Remarks and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Champomier Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Herrero Corral, G.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Lederberg, J.; McCray, A. ‘Ome sweet’omics-a genealogical treasury of words. Science 2001, 15, 8. [Google Scholar]
- Sosnowski, K.; Akarapipad, P.; Yoon, J.-Y. The future of microbiome analysis: Biosensor methods for big data collection and clinical diagnostics. Med. Devices Sens. 2020, 3, e10085. [Google Scholar] [CrossRef]
- Aggarwal, N.; Shohei Kitano, V.; Ying Puah, G.R.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and human health: Current understanding, engineering, and enabling technologies. Chem. Rev. 2022, in press. [CrossRef] [PubMed]
- Yoon, M.-S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef]
- Yadav, A.K.; Verma, D.; Sajwan, R.K.; Poddar, M.; Yadav, S.K.; Verma, A.K.; Solanki, P.R. Nanomaterial-based electrochemical nanodiagnostics for human and gut metabolites diagnostics: Recent advances and challenges. Biosensors 2022, 12, 733. [Google Scholar] [CrossRef]
- Fuentes-Chust, C.; Parolo, C.; Rosati, G.; Rivas, L.; Perez-Toralla, K.; Simon, S.; de Lecuona, I.; Junot, C.; Trebicka, J.; Merkoçi, A. The microbiome meets nanotechnology: Opportunities and challenges in developing new diagnostic devices. Adv. Mater. 2021, 33, 2006104. [Google Scholar] [CrossRef]
- Dietert, R.R.; Silbergeld, E.K. Biomarkers for the 21st Century: Listening to the Microbiome. Toxicol. Sci. 2015, 144, 208. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The brain-gut-microbiome axis. Cell. Mol. Gastroenter. Hepatol. 2018, 6, 133. [Google Scholar] [CrossRef]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.C.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef]
- Naik, A.; Misra, S.K. Modern sensing approaches for predicting toxicological responses of food- and drug-based bioactives on microbiomes of gut origin. J. Agric. Food Chem. 2021, 69, 6396–6413. [Google Scholar] [CrossRef] [PubMed]
- Willing, B.P.; Van Kessel, A.G. Host pathways for recognition: Establishing gastrointestinal microbiota as relevant in animal health and nutrition. Livest. Sci. 2010, 133, 82–91. [Google Scholar] [CrossRef]
- Lee, W.-J.; Hase, K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014, 10, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef]
- Pietro, C.; Verlhac, V.; Pérez Calvo, E.; Schmeisser, J.; Kluenter, A.-M. Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim. Feed Sci. Technol. 2019, 250, 9–31. [Google Scholar]
- Wang, Z.; Zhao, Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein. Cell. 2018, 9, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Brial, F.; Le Lay, A.; Dumas, M.E.; Gauguier, D. Implication of gut microbiota metabolites in cardiovascular and metabolic diseases. Cell. Mol. Life Sci. 2018, 75, 3977–3990. [Google Scholar] [CrossRef]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary bile acids and short chain fatty acids in the colon: A focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef]
- Caspani, G.; Kennedy, S.; Foster, J.S.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell. 2019, 6, 454. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.I.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661. [Google Scholar] [CrossRef]
- Gerner, E.W.; Meyskens, F.L., Jr. Polyamines and cancer: Old molecules, new understanding. Nat. Rev. Cancer 2004, 4, 781–792. [Google Scholar] [CrossRef]
- Kalnins, G.; Kuka, J.; Grinberga, S.; Makrecka-Kuka, M.; Liepinsh, E.; Dambrova, M.; Tars, K. Structure and function of CutC Choline Lyase from human microbiota bacterium Klebsiella pneumoniae. J. Biol. Chem. 2015, 290, 21732–21740. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Manghi, P.; Asnicar, F.; Pasolli, E.; Armanini, F.; Zolfo, M.; Beqhini, F.; Manara, S.; Karcher, N.; Pozzi, C.; et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 2019, 25, 667. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chu, Y.-H.; Wang, C.-C.; Wang, C.-H.; Tain, Y.-L.; Yang, H.-W. Rapid detection of gut microbial metabolite trimethylamine N-oxide for chronic kidney disease prevention. Biosensors 2021, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, G.B.V.S.; Yadav, A.; Mehlawat, K.N.; Jalandra, R.; Solanki, P.-R.; Kumar, A. Gut microbiota derived trimethylamine N-oxide (TMAO) detection through molecularly imprinted polymer based sensor. Sci. Rep. 2021, 11, 1338. [Google Scholar] [CrossRef] [PubMed]
- Mitrova, B.; Waffo, A.F.T.; Kaufmann, P.; Iobbi-Nivol, C.; Leimkühler, S.; Wollenberger, U. Trimethylamine N-oxide electrochemical biosensor with a chimeric enzyme. ChemElectroChem 2019, 6, 1732–1737. [Google Scholar] [CrossRef]
- Waffo, A.F.T.; Mitrova, B.; Tiedemann, K.; Iobbi-Nivol, C.; Leimkühler, S.; Wollenberger, U. Electrochemical trimethylamine N-oxide biosensor with enzyme-based oxygen-scavenging membrane for long-term operation under ambient air. Biosensors 2021, 11, 98. [Google Scholar] [CrossRef]
- Yi, Y.; Liang, A.; Luo, L.; Zang, Y.; Zhao, H.; Luo, A. A novel real-time TMAO detection method based on microbial electrochemical technology. Bioelectrochem. 2022, 144, 108038. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Rosser, E.C.; Piper, C.J.M.; Matei, D.E.; Blair, P.A.; Rendeiro, A.F.; Orford, M.; Alber, D.G.; Krausgruber, T.; Catalan, D.; Klein, N.; et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hidrocarbon receptor activation in regulatory B cells. Cell Metab. 2020, 31, 837–851. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Horst, R.T.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 2016, 167, 1125. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the microbiota and the immune system. Genes Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijmgoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
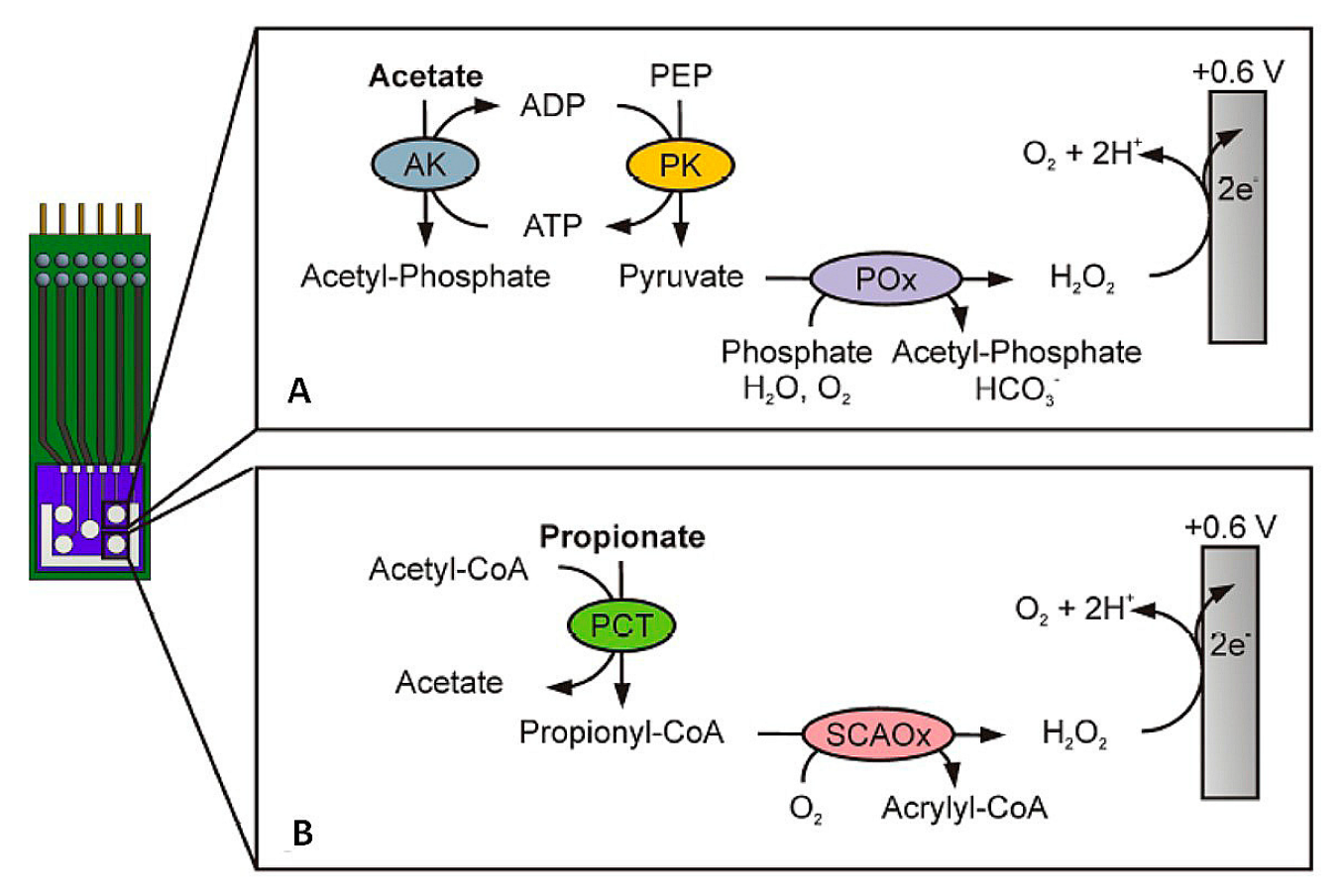
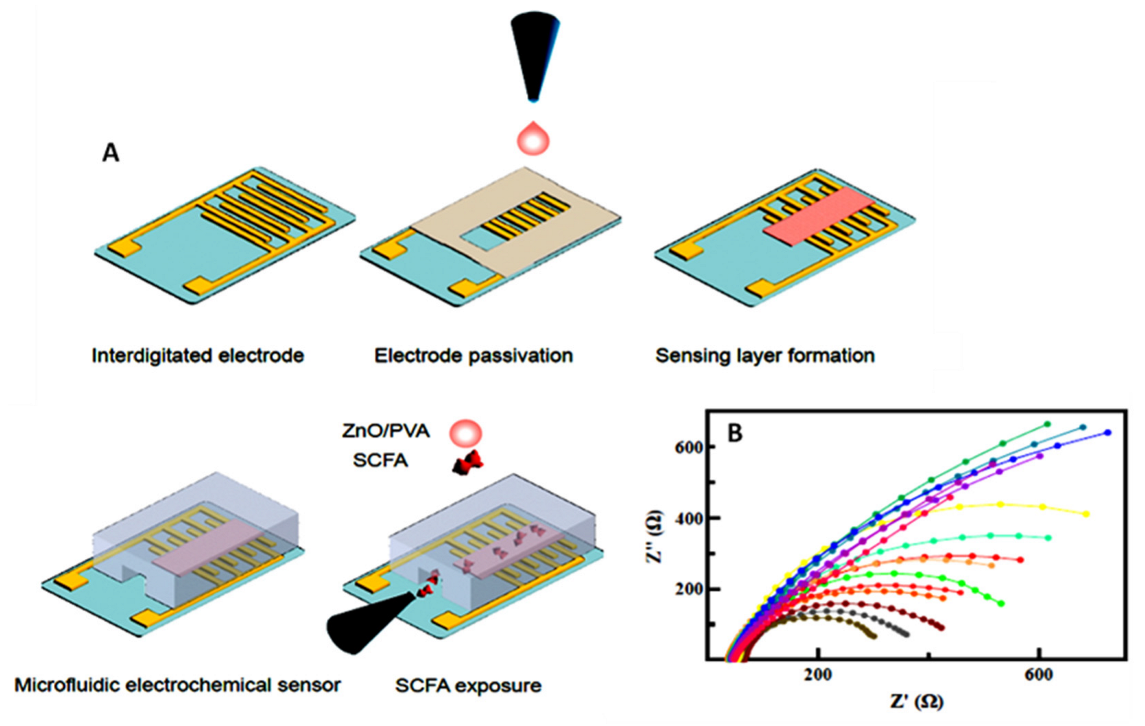
- Röhlen, D.L.; Pilas, J.; Dahmen, M.; Keusgen, M.; Selmer, T.; Schöning, M.J. Toward a hybrid biosensor system for analysis of organic and volatile fatty acids in fermentation processes. Front. Chem. 2018, 6, 284. [Google Scholar] [CrossRef] [PubMed]
- Yavarinasab, A.; Filibotte, S.; Liu, S.; Tropini, C. An impedance-based chemiresistor for the real-time detection of gut-microbiota-generated short-chain fatty acids. BioRxiv 2022, 9, 507374. [Google Scholar]
- Kaur, A.; Kim, J.R.; Michie, I.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Microbial fuel cell type biosensor for specific volatile fatty acids using acclimated bacterial communities. Biosens. Bioelectron. 2013, 47, 50–55. [Google Scholar] [CrossRef]
- Osuna-Prieto, F.J.; Martinez-Tellez, B.; Ortiz-Alvarez, L.; Di, X.; Jurado-Fasoli, L.; Xum, H.; Ceperuelo-Mallafré, V.; Núñez-Roa, C.; Kohler, I.; Segura-Carretero, A.; et al. Elevated plasma succinate levels are linked to higher cardiovascular disease risk factors in young adults. Cardiovasc. Diabetol. 2021, 20, 151. [Google Scholar] [CrossRef]
- Keiran, N.; Ceperuelo-Mallafré, V.; Calvo, E.; Hernández-Álvarez, M.I.; Ejarque, M.; Núñez-Roa, C.; Horrillo, D.; Maymó-Masip, E.; Rodríguez, M.M.; Fradera, R.; et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat. Immunol. 2019, 20, 581–592. [Google Scholar] [CrossRef]
- Astiarraga, B.; Martínez, L.; Ceperuelo-Mallafré, V.; Llauradó, G.; Terrón-Puig, M.; Rodríguez, M.M.; Casajoana, A.; Pellitero, S.; Megia, A.; Vilarrasa, N.; et al. Impaired succinate response to a mixed meal in obesity and type 2 diabetes is normalized after metabolic surgery. Diabetes Care 2020, 43, 2581–2587. [Google Scholar] [CrossRef]
- Fernández-Veledo, S.; Vendrell, J. Gut microbiota-derived succinate: Friend or foe in human metabolic diseases? Rev. Endocr. Metab. Disord. 2019, 20, 439–447. [Google Scholar] [CrossRef]
- Serena, C.; Ceperuelo-Mallafré, V.; Keiran, N.; Queipo-Ortuño, M.I.; Bernal, R.; Gomez-Huelgas, R.; Urpi-Sarda, M.; Sabater, M.; Pérez-Brocal, V.; Andrés Lacueva, C.; et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018, 12, 1642–1657. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Dawe, N.; Van Limbergen, J. The role of succinate in the regulation of intestinal inflammation. Nutrients 2019, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, N.; Louhichi, B.; Abdel-Wahab, A. Electrochemical oxidation of succinic acid in aqueous solutions using boron doped diamond anodes. Int. J. Environ. Sci. Technol. 2012, 9, 135–143. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Revisiting electrochemical biosensing in the 21st century society for inflammatory cytokines involved in autoimmune, neurodegenerative, cardiac, viral and cancer diseases. Sensors 2021, 21, 189. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tirado, E.; Agüí, L.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Biodetection techniques for quantification of chemokines. Chemosensors 2022, 10, 294. [Google Scholar] [CrossRef]
- Shekhar, S.; Yadav, A.K.; Khosla, A.; Solanki, P.R. Review—Interleukins profiling for biosensing applications: Possibilities and the future of disease detection. ECS Sens. Plus 2022, 1, 041601. [Google Scholar] [CrossRef]
- Dutta, N.; Lillehoj, P.B.; Estrela, P.; Dutta, G. Electrochemical biosensors for cytokine profiling: Recent advancements and possibilities in the near future. Biosensors 2021, 11, 94. [Google Scholar] [CrossRef]
- Ou, L.; Xia, N. Progress in nanomaterials-based electrochemical biosensors for the detection of interleukins. Int. J. Electrochem. Sci. 2022, 17, 220449. [Google Scholar] [CrossRef]
- Frias, M.; Zine, I.A.; Sigaud, N.; Lozano-Sanchez, M.; Caffio, P.; Errachid, M. Non-covalent π–π functionalized Gii-sense® graphene foam for interleukin 10 impedimetric detection. Biosens. Bioelectron. 2022, in press. [Google Scholar] [CrossRef]
- Barani, M.; Rahdar, A.; Sargazi, S.; Amiri, M.S.; Sharma, P.K.; Bhalla, N. Nanotechnology for inflammatory bowel disease management: Detection, imaging and treatment. Sens. Bio-Sens. Res. 2021, 32, 100417. [Google Scholar] [CrossRef]
- Jagannath, B.; Lin, K.-L.; Pali, M.; Sankhala, D.; Muthukumar, S.; Prasad, S. A sweat-based wearable enabling technology for real-time monitoring of IL-1β and CRP as potential markers for inflammatory bowel disease. Inflamm. Bowel Dis. 2020, 26, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Thangamuthu, M.; Santschi, C.; Martin, O.J.F. Label-free electrochemical immunoassay for C-reactive protein. Biosensors 2018, 8, 34. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, J.; Yang, T.; Deng, Y.; Gu, M.; Wang, M.; Hu, R.; Yang, Y. Electrochemical detection of C-reactive protein using functionalized iridium nanoparticles/graphene oxide as a tag. RSC Adv. 2020, 10, 9723–9729. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, G.; Smolen, J.E.; Korchak, H.M. Release of inflammatory mediators from stimulated neutrophils. N. Engl. J. Med. 1980, 303, 27–34. [Google Scholar]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]
- Kansagra, K.; Stoll, B.; Rognerud, C.; Niinikoski, H.; Ou, C.N.; Harvey, R. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G1162. [Google Scholar] [CrossRef]
- Saiki, T.; Mitsuyama, K.; Toyonaka, A.; Ishida, H.; Tanikawa, K. Detection of pro- and anti-inflammatory cytokines in stools of patients with inflammatory bowel disease. Scand. J. Gastroenterol. 1998, 33, 616–622. [Google Scholar]
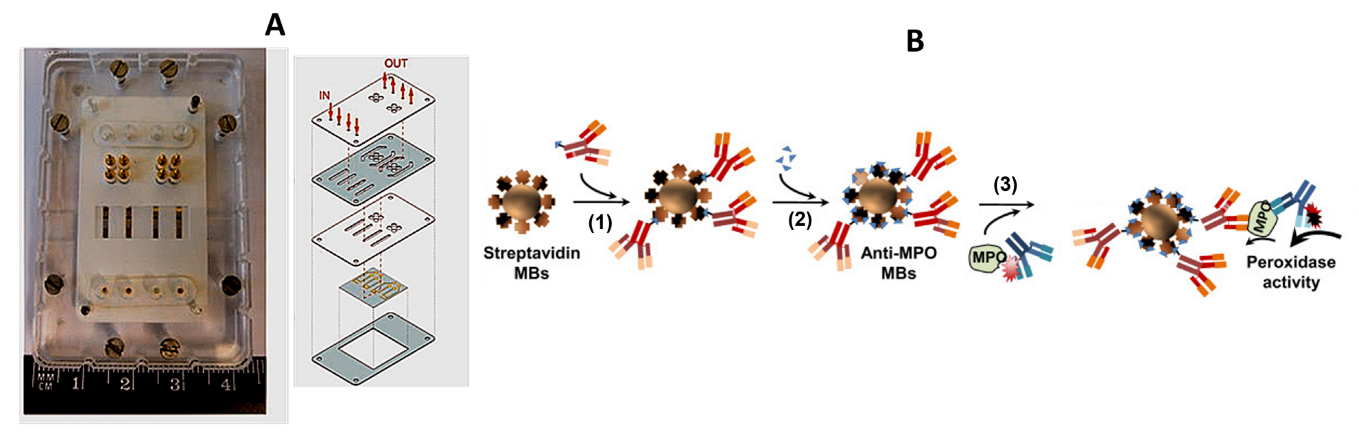
- Moral-Vico, J.; Barallat, J.; Abad, L.; Olivé-Monllau, R.; Muñoz-Pascual, F.X.; Galán Ortega, A.; del Campo, F.J.; Baldrich, E. Dual chronoamperometric detection of enzymatic biomarkers using magnetic beads and a low-cost flow cell. Biosens. Bioelectron. 2015, 69, 328–336. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, J.; Chen, J.; Zhao, Y.; Niu, Y.; Yu, C. Amperometric myeloperoxidase immunoassay based on the use of CuPdPt nanowire networks. Microchim. Acta 2018, 185, 55. [Google Scholar] [CrossRef]
- Bekhit, M.; Gorski, W. Electrochemical assays and immunoassays of the myeloperoxidase/SCN−/H2O2 system. Anal. Chem. 2019, 91, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, H.; Weng, M.; Jiang, S.; Gao, J. Diagnostic and clinical significance of serum levels of D-lactate and diamine oxidase in patients with Crohn’s disease. Gastroenterol. Res. Pract. 2019, 2019, 8536952. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Ortiz, C.; Peralta, T.; Ramos, V.; Durán, M.; Behrens, C.; Maureira, D.; Guzmán, M.A.; Bastias, C.; Ferrer, P. Standardization of a colorimetric technique for determination of enzymatic activity of diamine oxidase (DAO) and its application in patients with clinical diagnosis of histamine intolerance. World Allergy Organ. J. 2020, 13, 100457. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Bilska, J.C.; Brzozowski, B.; Magierowski, M.; Mach, T.; Magierowska, K.; Brzozowski, T. The role of intestinal alkaline phosphatase in inflammatory disorders of gastrointestinal tract. Mediators Inflamm. 2017, 2017, 9074601. [Google Scholar] [CrossRef]
- Balbaied, T.; Moore, E. Overview of optical and electrochemical alkaline phosphatase (ALP) biosensors: Recent approaches in cells culture techniques. Biosensors 2019, 9, 102. [Google Scholar] [CrossRef]
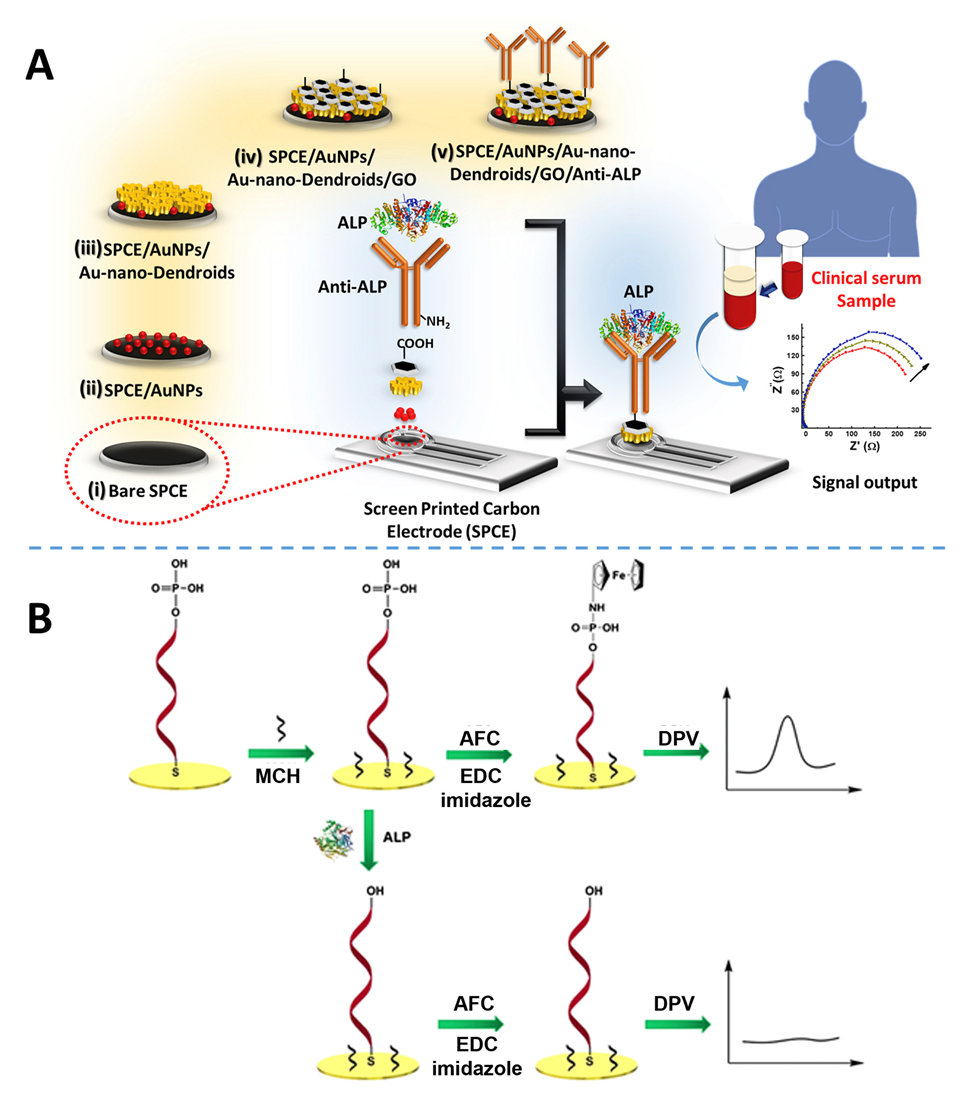
- Mahato, K.; Purohit, B.; Kumar, A.; Chandra, P. Clinically comparable impedimetric immunosensor for serum alkaline phosphatase detection based on electrochemically engineered Au-nano-dendroids and graphene oxide nanocomposite. Biosens. Bioelectron. 2020, 148, 111815. [Google Scholar] [CrossRef]
- Wang, W.; Lu, J.; Hao, L.; Yang, H.; Song, X.; Si, F. Electrochemical detection of alkaline phosphatase activity through enzyme-catalyzed reaction using aminoferrocene as an electroactive probe. Anal. Bioanal. Chem. 2021, 413, 1827. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Childers, W.S. A biosensor for detection of indole metabolites. ACS Synth. Biol. 2021, 10, 1605. [Google Scholar] [CrossRef]
- Beloborodova, N.V.; Chernevskaya, E.A.; Getsina, M.L. Indolic structure metabolites as potential biomarkers of non-infectious diseases. Curr. Pharm. Des. 2021, 27, 238. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhang, X.; Zhen, Q.; He, Y.; Chen, X.; Lyu, W.; Han, R.; Ding, M. An electrochemical sensor for indole in plasma based on MWCNTs-chitosan modified screen-printed carbon electrode. Biosens. Bioelectron. 2017, 98, 392. [Google Scholar] [CrossRef]
- Moncer, F.; Adhoum, N.; Catak, D.; Monser, L. Electrochemical sensor based on MIP for highly sensitive detection of 5-hydroxyindole-3-acetic acid carcinoid cancer biomarker in human biological fluids. Anal. Chim. Acta. 2021, 1181, 338925. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasoul, G.N.; MacKay, S.; Salim, S.Y.; Ismond, K.P.; Tamura, M.; Khalifa, C.; Mannan, E.; Lin, D.; Mandal, T.; Montgomery, R.R.; et al. Non-invasive point-of-care device to diagnose acute mesenteric ischemia. ACS Sens. 2018, 3, 2296. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, W.P.N.G.W.; Chubb, S.A.P.; Gillett, M.J.; Vasikaran, S.D. Faecal Calprotectin. Clin. Biochem. Rev. 2018, 39, 77. [Google Scholar] [PubMed]
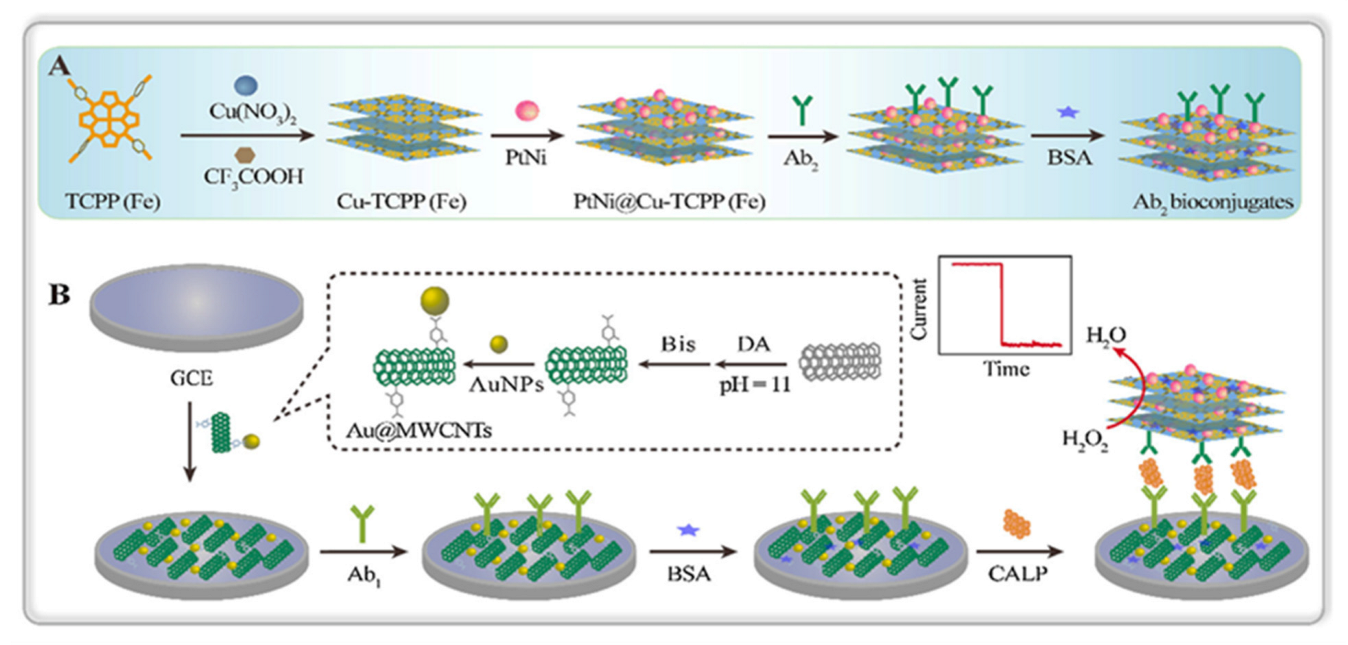
- Dong, L.; Yin, L.; Tian, G.; Wang, Y.; Pei, H.; Wu, Q.; Cheng, W.; Ding, S.; Xia, Q. An enzyme-free ultrasensitive electrochemical immunosensor for calprotectin detection based on PtNi nanoparticles functionalized 2D Cumetal organic framework nanosheets. Sens. Actuators B. Chem. 2020, 308, 127687. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2018, 195, 74. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Immune regulation by microbiome metabolites. Immunology 2018, 154, 220. [Google Scholar] [CrossRef] [PubMed]
- Housley, W.J.; Pitt, D.; Hafler, D.A. Biomarkers in multiple sclerosis. Clin. Immunol. 2015, 161, 51. [Google Scholar] [CrossRef]
- Farrokhi, V.; Nemati, R.; Nichols, F.C.; Yao, X.; Anstadt, E.; Fujiwara, M.; Grady, J.; Wakefield, D.; Castro, W.; Donaldson, J.; et al. Bacterial lipodipeptide, Lipid 654, is a microbiome associated biomarker for multiple sclerosis. Clin. Transl. Immunol. 2013, 2, e8. [Google Scholar] [CrossRef]
- Bergot, A.-S.; Giri, R.; Thomas, R. The microbiome and rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 2019, 33, 101497. [Google Scholar] [CrossRef]
- Hammad, D.B.M.; Hider, S.L.; Liyanapathirana, V.C.; Tonge, D.P. Molecular characterization of circulating microbiome signatures in rheumatoid arthritis. Front. Cell. Infect. Microbiol. 2020, 9, 440. [Google Scholar] [CrossRef]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumathol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef] [PubMed]
- Mobed, A.; Dolati, S.; Shakouri, S.K.; Eftekharsadat, B.; Izadseresht, B. Recent advances in biosensors for detection of osteoarthritis andrheumatoid arthritis biomarkers. Sens. Actuators A Phys. 2021, 331, 112975. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122. [Google Scholar] [PubMed]
- Sivadasan, P.; Kumar Gupta, M.; Sathe, G.H.; Balakrishnan, L.; Palit, P.; Gowda, H.; Suresh, A.; Kuriakos, M.A.; Sirdeshmukh, E. Human salivary proteome—A resource of potential biomarkers for oral cancer. J. Proteom. 2015, 127, 89–95. [Google Scholar] [CrossRef]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal pathogens as risk factors of cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease—Is there cause for consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef]
- Tar, I.; Csősz, É.; Végh, E.; Lundberg, K.; Kharlamova, N.; Soós, B.; Szekanecz, Z.; Márton, I. Salivary citrullinated proteins in rheumatoid arthritis and associated periodontal disease. Sci. Rep. 2021, 11, 13525. [Google Scholar] [CrossRef]
- Rabelo Buzalaf, M.A.; de Cássia Ortiz, A.; Souza Carvalho, T.; Moura Fideles, S.O.; Araújo, T.T.; Mascarenhas Moraes, S.; Rabelo Buzalaf, N.; Navas Reis, F. Saliva as a diagnostic tool for dental caries, periodontal disease and cancer: Is there a need for more biomarkers? Expert Rev. Mol. Diagn. 2020, 20, 543. [Google Scholar] [CrossRef]
- Nguyen, A.T.M.; Akhter, R.; Garde, S.; Scott, C.; Twigg, S.M.S.; Colagiuri, S.; Ajwani, S.; Eberhard, J. The association of periodontal disease with the complications of diabetes mellitus. A systematic review diabetes research and clinical practice. Diabetes Res. Clin. Pract. 2020, 165, 108244. [Google Scholar] [CrossRef]
- Chattopadhyayn, I.; Panda, M. Recent trends of saliva omics biomarkers for the diagnosis and treatment of oral cancer. J. Oral Biosci. 2019, 61, 84–94. [Google Scholar] [CrossRef]
- Schmidt, B.L.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M.; Queiroz, E.L.; Nightingale, K.; Kerr, A.R.; De Lacure, M.D.; Veeramachaneni, R.; et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS One 2014, 2, e98741-53. [Google Scholar] [CrossRef]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. Oral microbiome: A new biomarker reservoir for oral and oropharyngeal cancers. Theranostics 2017, 7, 4313–4321. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, P.; Eslami, H.; Yousefi, M.; Asgharzadeh, M.; Kafil, S.H. Role of oral microbiome on oral cancers, a review. Biomed. Pharmacol. 2016, 84, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J. Electrochemical biosensors for the detection of matrix metalloproteinases. Int. J. Electrochem. Sci. 2022, 17, 221034. [Google Scholar] [CrossRef]
- Ruiz-Vega, G.; García-Robaina, A.; Ben Ismail, M.; Pasamar, H.; García-Berrocoso, T.; Montaner, J.; Zourob, M.; Othmane, A.; del Campo, F.J.; Baldrich, E. Detection of plasma MMP-9 within minutes. Unveiling some of the clues to develop fast and simple electrochemical magneto-immunosensors. Biosens. Bioelectron. 2018, 115, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, B.; ben Hassine, A.; Valverde, A.; Serafín, V.; Montero-Calle, A.; Raouafi, N.; Camps, J.; Arenas, M.; Barderas, R.; Yáñez-Sedeño, P.; et al. Electrochemical immunoplatform to assist in the diagnosis and classification of breast cancer through the determination of matrix-metalloproteinase-9. Talanta 2021, 225, 122054. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Rizantal Delgado, R.Z.; Frías-López, J. The oral microbiome and cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, A.; Okuma, Y. Perspective on immune oncology with liquid biopsy, peripheral blood mononuclear cells, and microbiome with non-invasive biomarkers in cancer patients. Clin. Transl. Oncol. 2018, 20, 966–974. [Google Scholar] [CrossRef]
- Mani, V.; Beduk, T.; Khushaim, W.; Ceylan, A.-E.; Timur, S.; Wolfbeis, O.S.; Salama, K.N. Electrochemical sensors targeting salivary biomarkers: A comprehensive review. TrAC, Trends Anal. Chem. 2021, 135, 116164. [Google Scholar] [CrossRef]
- McCrae, L.E.; Ting, W.-T.; Howlader, M.M.R. Advancing electrochemical biosensors for interleukin-6 detection. Biosens. Bioelectron.: X 2023, 13, 100288. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Brooks, E.; Li, Y.; Denny, P.; Ho, C.-M.; Qi, F.W.; Shi, W.; Wolinsky, L.; Wu, B.; Wong, D.T.W.; et al. Detection of picomolar levels of interleukin-8 in human saliva by SPR. Lab Chip 2005, 5, 1017–1023. [Google Scholar] [CrossRef]
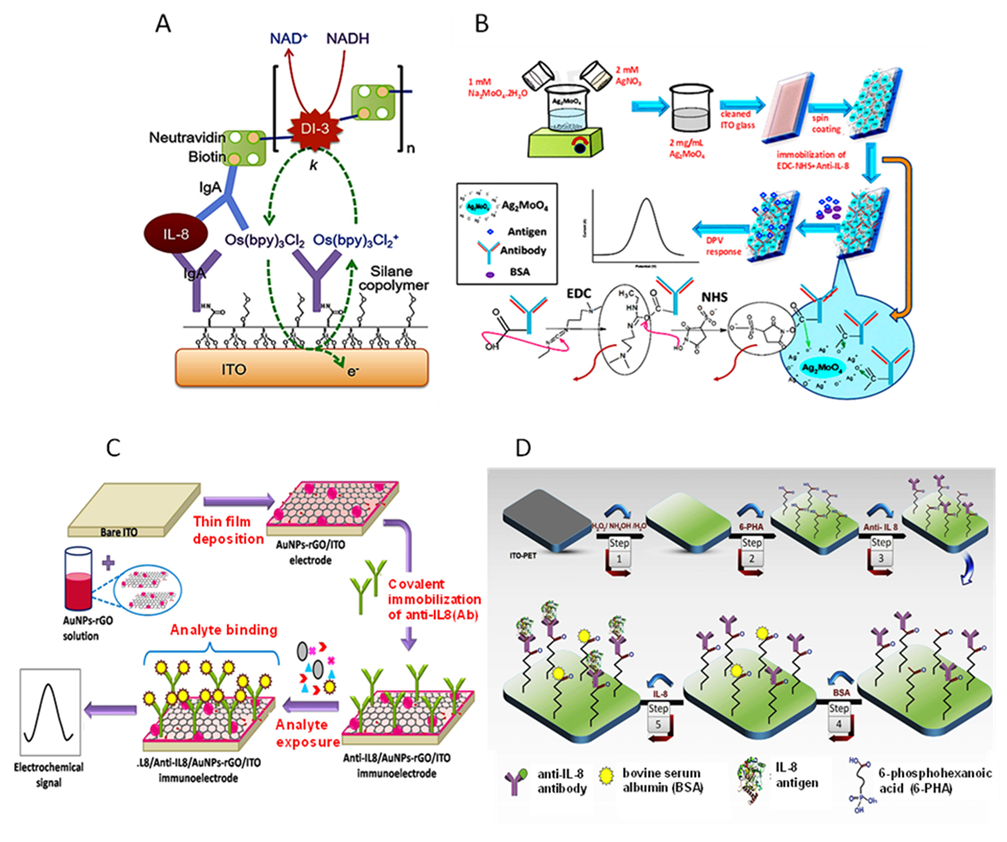
- Bhatia, A.; Na, H.S.; Nandhakumar, P.; Yu, B.; Jon, S.; Chung, J.; Yang, H. Electrochemical detection of interleukin-8 in human saliva using a polyenzyme label based on diaphorase and neutravidin. Sens. Actuators B Chem. 2021, 326, 128979. [Google Scholar] [CrossRef]
- Pachauri, N.; Lakshmi, G.B.V.S.; Sri, S.; Gupta, P.K.; Solanki, P.R. Silver molybdate nanoparticles based immunosensor for the non-invasive detection of Interleukin-8 biomarker. Mater. Sci. Eng. C 2020, 113, 110911. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Shukla, A.; Kaswan, J.; Arora, K.; Ramirez-Vick, J.; Singh, P.; Singh, S.P. Anti-IL8/AuNPs-rGO/ITO as an immunosensing platform for noninvasive electrochemical detection of oral cancer. ACS Appl. Mater. Interf. 2017, 9, 27462–27474. [Google Scholar] [CrossRef] [PubMed]
- Aydın, M.; Aydın, E.B.; Sezgintürk, M.K. A highly selective electrochemical immunosensor based on conductive carbon black and star PGMA polymer composite material for IL-8 biomarker detection in human serum and saliva. Biosens. Bioelectron. 2018, 117, 720. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Sezginturk, M.K. An impedimetric immunosensor for highly sensitive detection of IL-8 in human serum and saliva samples: A new surface modification method by 6-phosphonohexanoic acid for biosensing applications. Anal. Biochem. 2018, 554, 44. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Sezginturk, M.K. Fabrication of electrochemical immunosensor for detection of interleukin 8 biomarker via layer-by-layer self-assembly process on cost-effective fluorine tin oxide electrode. Electroanalysis 2021, 33, 1596. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Lakshminarayanan, V. Adsorption kinetics of phosphonic acids and proteins on functionalized Indium tin oxide surfaces using electrochemical impedance spectroscopy. Electrochim. Acta 2016, 197, 1–9. [Google Scholar] [CrossRef]
- Aydin, E.B.; Sezgintürk, M.K. A disposable and ultrasensitive ITO based biosensor modified by 6-phosphonohexanoic acid for electrochemical sensing of IL-1β in human serum and saliva. Anal. Chim. Acta. 2018, 1039, 41–50. [Google Scholar] [CrossRef]
- Guerrero, S.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Design of electrochemical immunosensors using electro-click chemistry. Application to the detection of IL-1β cytokine in saliva. Bioelectrochemistry 2020, 133, 107484. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Campuzano, S.; Ruiz-Valdepeñas Montiel, V.; Gamella, M.; Pingarrón, J.M. Electrochemical bioplatforms for the simultaneous determination of interleukin (IL)-8 mRNA and IL-8 protein oral cancer biomarkers in raw saliva. Biosens. Bioelectron. 2016, 77, 543–548. [Google Scholar] [CrossRef]
- Sánchez-Tirado, E.; Salvo, C.; González-Cortés, A.; Yáñez-Sedeño, P.; Langa, F.; Pingarrón, J.M. Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double-walled carbon nanotubes. Anal. Chim. Acta 2017, 959, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Bellando-Randone, S.; Russo, E.; Venerito, V.; Matucci-Cerinic, M.; Iannone, F.; Tangaro, S.; Amedei, A. Exploring the oral microbiome in rheumatic diseases, state of art and future prospective in personalized medicine with an AI approach. J. Pers. Med. 2021, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Bender, P.; Burgin, W.B.; Sculean, A.; Eick, S. Serum antibody levels against Porphyromonas gingivalis in patients with and without rheumatoid arthritis: A systematic review and meta-analysis. Clin. Oral Invest. 2017, 21, 33–42. [Google Scholar] [CrossRef]
- Selvam, S.P.; Chinnadayyala, S.R.; Cho, S. Electrochemical nanobiosensor for early detection of rheumatoid arthritis biomarker: Anti- cyclic citrullinated peptide antibodies based on polyaniline (PANI)/MoS2-modified screen-printed electrode with PANI-Au nanomatrix-based signal amplification. Sens. Actuators B Chem. 2021, 333, 129570. [Google Scholar] [CrossRef]
- Guerrero, S.; Sánchez-Tirado, E.; Martínez-García, G.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical biosensor for the simultaneous determination of rheumatoid factor and anti-cyclic citrullinated peptide antibodies in human serum. Analyst 2020, 145, 4680. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.-H.; Tsao, Y.-T.; Shen, C.-J.; Tsai, C.-Y.; Cheng, C.-M. Small-volume detection: Platform developments for clinically-relevant applications. J. Nanobiotechnol. 2021, 19, 114. [Google Scholar] [CrossRef]
- Martensson, A.; Greiff, L.; Lamei, S.S.; Lindstedt, M.; Olofsson, T.C.; Vasquez, A.; Cervin, A. Effects of a honeybee lactic acid bacterial microbiome on human nasal symptoms, commensals, and biomarkers. Int. Forum Allergy Rhinol. 2016, 6, 957–963. [Google Scholar] [CrossRef]
- Hassan-Nixon, H.A.G.; Singh, N.; Cass, A.E.G. A sensitive impedimetric immunosensor for the detection of Interleukin-8 in nasal epithelial lining fluid of asthma patients. Biosens. Bioelectron. X. 2022, 10, 100118. [Google Scholar]
- Chawes, B.L.; Edwards, M.J.; Shamji, B.; Walker, C.; Nicholson, G.C.; Tan, A.J.; Folsgaard, N.V.; Bonnelykke, K.; Bisgaard, H.; Hansel, T.T. A novel method for assessing unchallenged levels of mediators in nasal epithelial lining fluid. J. Allergy Clin. Immunol. 2010, 125, 1387–1389. [Google Scholar] [CrossRef]
- Li, J.-X.; Wang, Z.-Z.; Zhai, G.-T.; Chen, C.-L.; Zhu, K.-Z.; Yu, Z.; Liu, Z. Untargeted metabolomic profiling identifies disease-specific and outcome-related signatures in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2022, 150, 727–735. [Google Scholar] [CrossRef]
- Mahdavinia, M.; Keshavarzian, A.; Tobin, M.C.; Landay, A.L.; Schleimer, R.P. A comprehensive review of the nasal microbiome in chronic rhinosinusitis (CRS). Clin. Exp. Allergy 2016, 46, 21–41. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Xu, H.; Yi, H.; Guan, J.; Yin, S. Metabolomics and microbiome profiling as biomarkers in obstructive sleep apnoea: A comprehensive review. Eur. Resp. Rev. 2021, 30, 200220. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Strobel, W.; Christ, M.; Todd, J.; Estis, J.; Wildi, K.; Thalmann, G.; Hilti, J.; Brutsche, M. Twerenbold, R.; et al. Comprehensive biomarker profiling in patients with obstructive sleep apnea. Clin. Biochem. 2015, 48, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Huang, J.; Wei, H.; Cao, C.; Feng, S.; Guo, Q.; Goldys, E.M.; Li, R.; Liu, G. Graphene oxide thin film with dual function integrated into a nanosandwich device for in vivo monitoring of interleukin-6. ACS Appl. Mater. Interfaces 2017, 9, 41659–41668. [Google Scholar] [CrossRef]
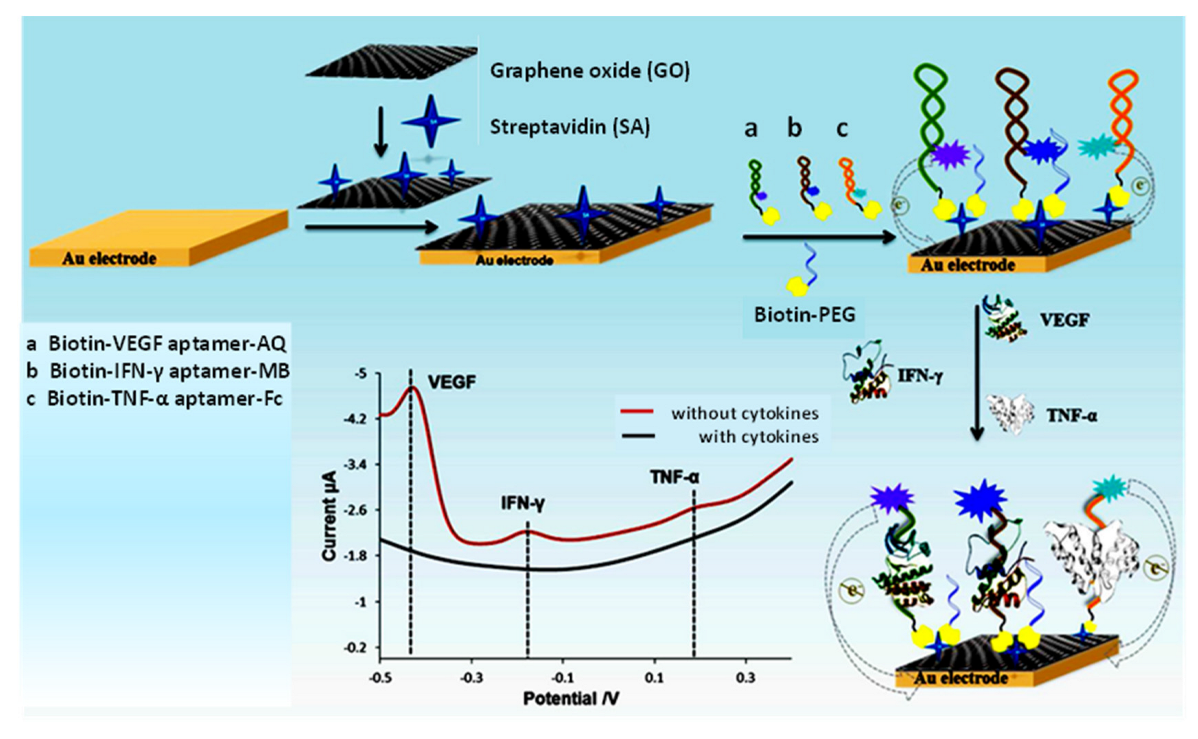
- Shen, Z.; Ni, S.; Yang, W.; Sun, W.; Yang, G.; Liu, G. Redox probes tagged electrochemical aptasensing device for simultaneous detection of multiple cytokines in real time. Sens. Actuators B Chem. 2021, 336, 129747. [Google Scholar] [CrossRef]
- Ni, S.; Qiao, L.; Shen, Z.; Gao, Y.; Liu, G. Physical absorption vs covalent binding of graphene oxide on glassy carbon electrode towards a robust aptasensor for ratiometric electrochemical detection of vascular endothelial growth factor (VEGF) in serum. Electrochim. Acta 2020, 331, 135321. [Google Scholar] [CrossRef]
- Johari-Ahar, M.; Karami, P.; Ghanei, M.; Afkham, A.; Bagheri, H. Development of a molecularly imprinted polymer tailored on disposable screen-printed electrodes for dual detection of EGFR and VEGF using nanoliposomal amplification strategy. Biosens. Bioelectron. 2018, 107, 26–33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Tirado, E.; Agüí, L.; González-Cortés, A.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical (Bio)Sensing Devices for Human-Microbiome-Related Biomarkers. Sensors 2023, 23, 837. https://doi.org/10.3390/s23020837
Sánchez-Tirado E, Agüí L, González-Cortés A, Campuzano S, Yáñez-Sedeño P, Pingarrón JM. Electrochemical (Bio)Sensing Devices for Human-Microbiome-Related Biomarkers. Sensors. 2023; 23(2):837. https://doi.org/10.3390/s23020837
Chicago/Turabian StyleSánchez-Tirado, Esther, Lourdes Agüí, Araceli González-Cortés, Susana Campuzano, Paloma Yáñez-Sedeño, and José Manuel Pingarrón. 2023. "Electrochemical (Bio)Sensing Devices for Human-Microbiome-Related Biomarkers" Sensors 23, no. 2: 837. https://doi.org/10.3390/s23020837
APA StyleSánchez-Tirado, E., Agüí, L., González-Cortés, A., Campuzano, S., Yáñez-Sedeño, P., & Pingarrón, J. M. (2023). Electrochemical (Bio)Sensing Devices for Human-Microbiome-Related Biomarkers. Sensors, 23(2), 837. https://doi.org/10.3390/s23020837










