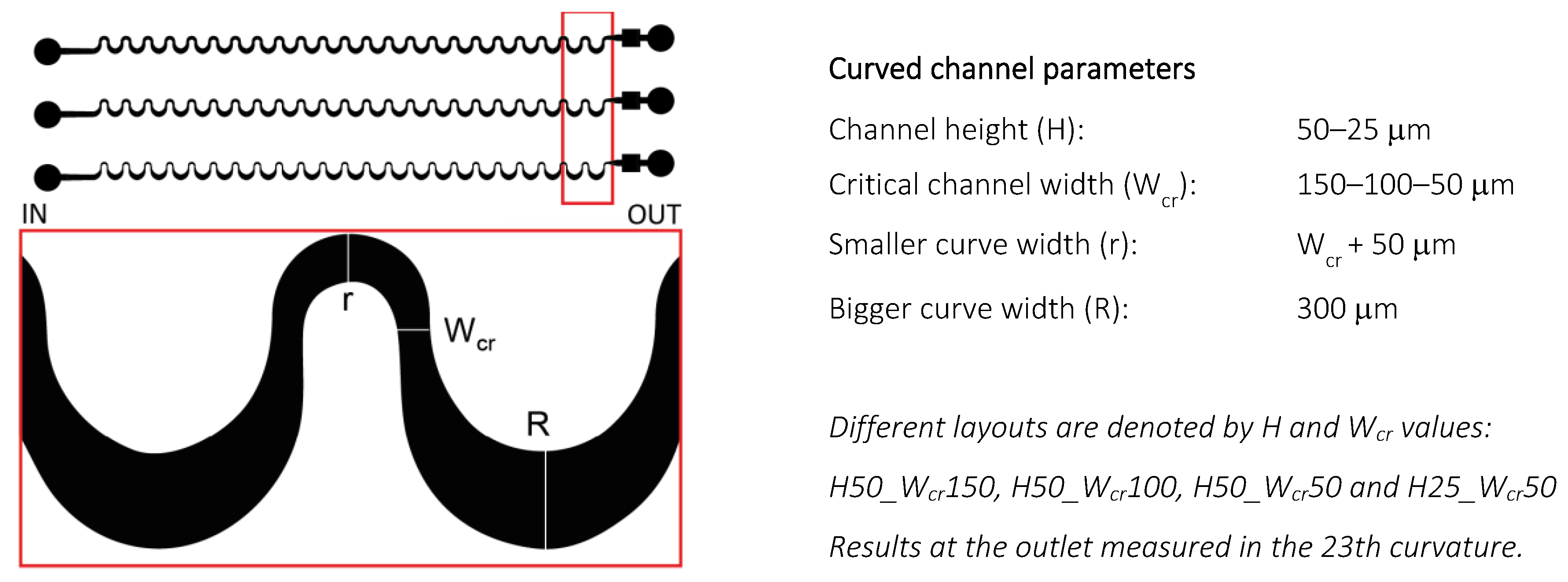
1. Introduction
The very first observation of flow-generated particle positioning was made by Segere et al. in 1961, when the annual arrangement of particles was experienced in an initially uniformly diluted suspension at 0.6 times the tube’s radius, in the laminar, parabolic flow of a straight tubular channel [
1].
Several channel cross-sections [
2,
3,
4,
5] were examined in the literature by different research groups over the years in order to observe the interstitial migration of particles. The main goal was to find stable lateral equilibrium positions, to reliably separate or focus different particles according to their size, to increase the distance between successive particles, and to apply these microfluidic structures for cell-level studies. By testing the flow conditions of a designed geometry, such as asymmetric curved serpentine [
6,
7], curved [
8,
9], and spiral channels [
10,
11,
12,
13], quick successes can be achieved in the field of inertial focusing with rigid particles, although in the case of biological samples, several effects complicate the process, such as the varied morphology, rigidity, and deformability of the cells; the viscosity contrast between the medium and cell interior; the shape assumed by the cell in flow; and the cell–cell interactions. Moreover, an organelle’s characteristic movement can be further influenced by the environmental effects and the characteristic properties of the chosen chip material in which the experiment takes place.
In case of the passive, channel-geometry-dependent, hydrodynamic-based separation methods, no external power is required to achieve the lateral migration of the particles. Different channel geometries are applied for enabling particle separation. In a curved, asymmetric channel geometry, the phenomenon was demonstrated by Di Carlo et al. [
6] that the curvature ratio of channel geometry (δ), channel Reynolds number (
Rec), particle diameter (
a), and hydraulic diameter (
Dh) strictly parameterize the evoked force balance in the flow. At low Reynolds numbers, the balance of the lift forces and geometry-evoked counter-rotating Dean vortices induced Dean drag forces determining the lateral-focusing position of a given bead size in the channel cross-section.
The effect of a weak Dean flow can help lateral focusing, although in the case of the Dean drag force becoming dominant, mixing can occur instead. Accordingly, a particle size to channel cross-section ratio was defined (a/Dh > 0.07), above which lateral focusing is possible.
The asymmetric serpentine-type microfluidic channel designs enable inertial focusing and are preferred for flow cytometry especially for high-throughput fluorescence-activated cell sorting (FACS). John Oakey et al. [
14] combined an asymmetric curved channel with a high aspect ratio with a straight channel to reduce lateral focus positions to a single point. In a tortuous channel, the beads migrated laterally to one side, but in fact two equilibrium positions were formed above each other along the height of the channel. In a straight channel, on the other hand, two equilibrium positions could be isolated laterally at the same height. Competing lift forces helped to migrate the applied 10.2 µm diameter fluorescent beads to their lateral position, and hydrodynamic repulsion aided the longitudinal spacing at a flow rate of 100 µL/min. By precisely controlling the particle position and spacing at high linear particle velocities (>1 m/s), higher separation throughput and cleaner data collection can be achieved.
In medical diagnostics, efforts were made for being able to manipulate targets present in the sub-micron range, due to the size of bacterial cells being in the range of 1–3 µm. Viruses are smaller, e.g., the size of influenza viruses is around 80–100 nm [
15]; the size of SARS-CoV2 ranges between 70 and 90 nm [
16] (p.19). The use of passive hydrodynamic separation methods in the sub-micron (<1 µm) range is a serious challenge, since the elastic forces scale with the particle volume, and in this size range, particles are effected by the increasing phenomena of Brownian motion [
17].
The recent work of Lei Wang et al. [
18] has proven that this passive method is even capable to concentrate and focus particles under the size of 2 µm, such as bacteria, sub-cellular organelles, and even viruses. Fluorescent spheres were used in the size range of 2 µm–200 nm and 2 µm beads could be separated from 920 nm in asymmetric serpentine-type microfluidic channel with a channel width of 20 µm and a height of 10 µm—in the smaller curvature—at an 80 µL/min flow rate. Even 920 nm particles could be segregated from 200 nm in a tightened channel cross-section of 10 µm × 5 µm. In each case, the bigger particles became focused, while the smaller ones remained diffuse, proving the fact that the segregation of nano- and bioparticles can be possible using adequate flow parameters. The comprehensive study even detailed how sample density can degrade the degree of focusing, and how the altering target-particle rigidity affects the focusing position in continuous flow. In the case of GFP-modified cyanobacteria and spheroid fluorescent beads having the same size (2 µm), the bacteria-focusing peak was 1.05 µm closer to the centerline compared to the rigid particle, which could be explained by the shape or deformation of the biological species. The possible deterioration of biological samples due to evolving shear forces at extremely high flow rates has to be reckoned with.
Henceforth, the material of the microfluidic platform must be chosen according to the task to be performed. Polydimethylsiloxane (PDMS) is preferred by many labs for rapid prototyping because of its non-toxicity, chemical inertness, optical transparency, good adhesion for multiple substrates, cost effectiveness, and rapid and easy fabrication. Channel deformation, however, due to high-pressure application, seems to be a disadvantage. A solution may be to choose another material with greater stiffness (characterized by Young’s modulus), such as polycarbonate (2 GPa), thermoset polyester (~1.2 GPa), polyurethane methacrylate (91 MPa), or Norland Adhesive 81 (325 MPa), instead of PDMS (10:1, 2.5 MPa) to overcome the given problem areas, or at least perform the experiment on a hybrid platform: PDMS combined with glass (Pyrex Glass 63 GPa) or silicon (130 GPa) [
19].
In this report, the measurements were executed in a PDMS-glass hybrid platform. In our previous work [
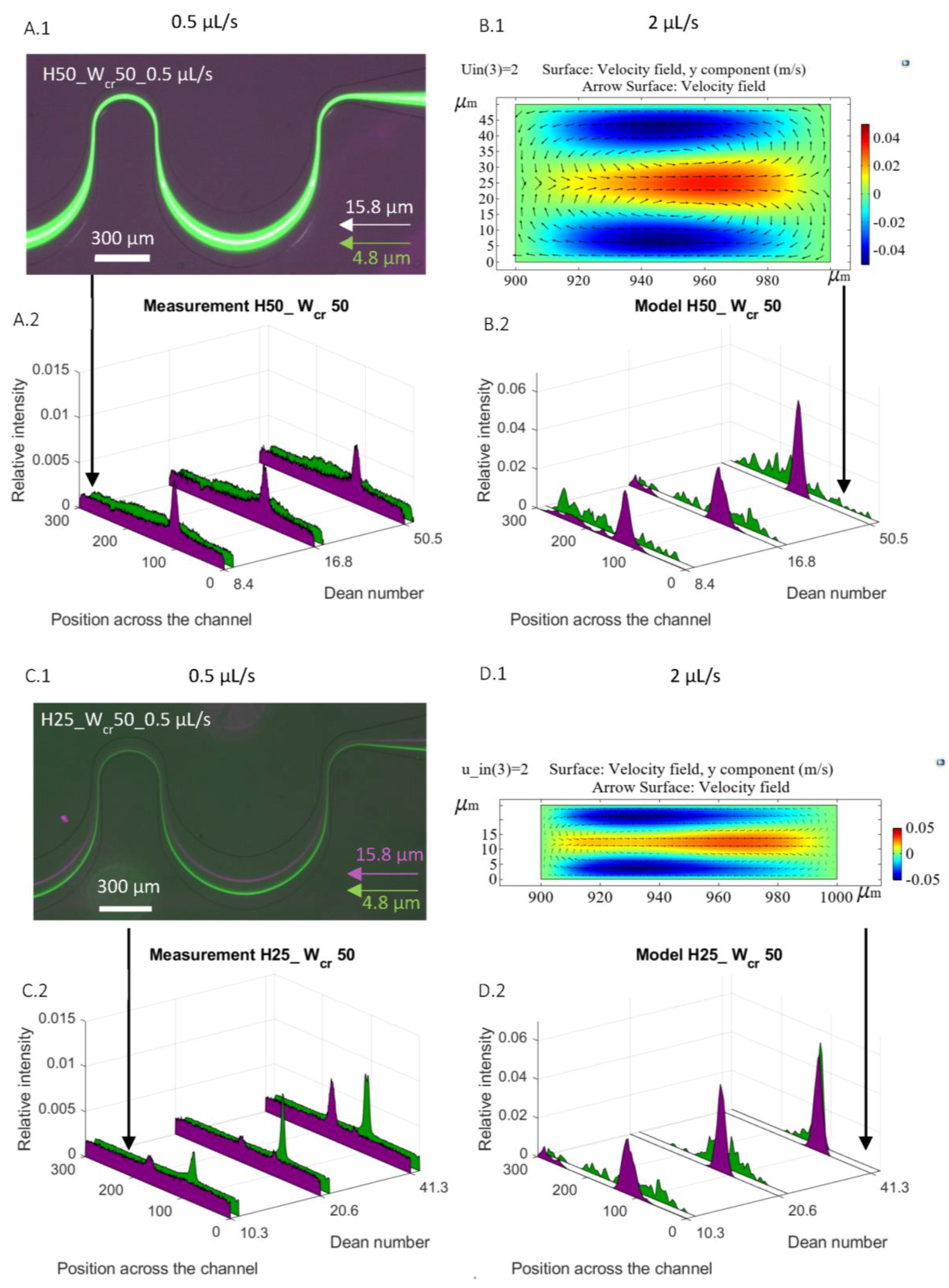
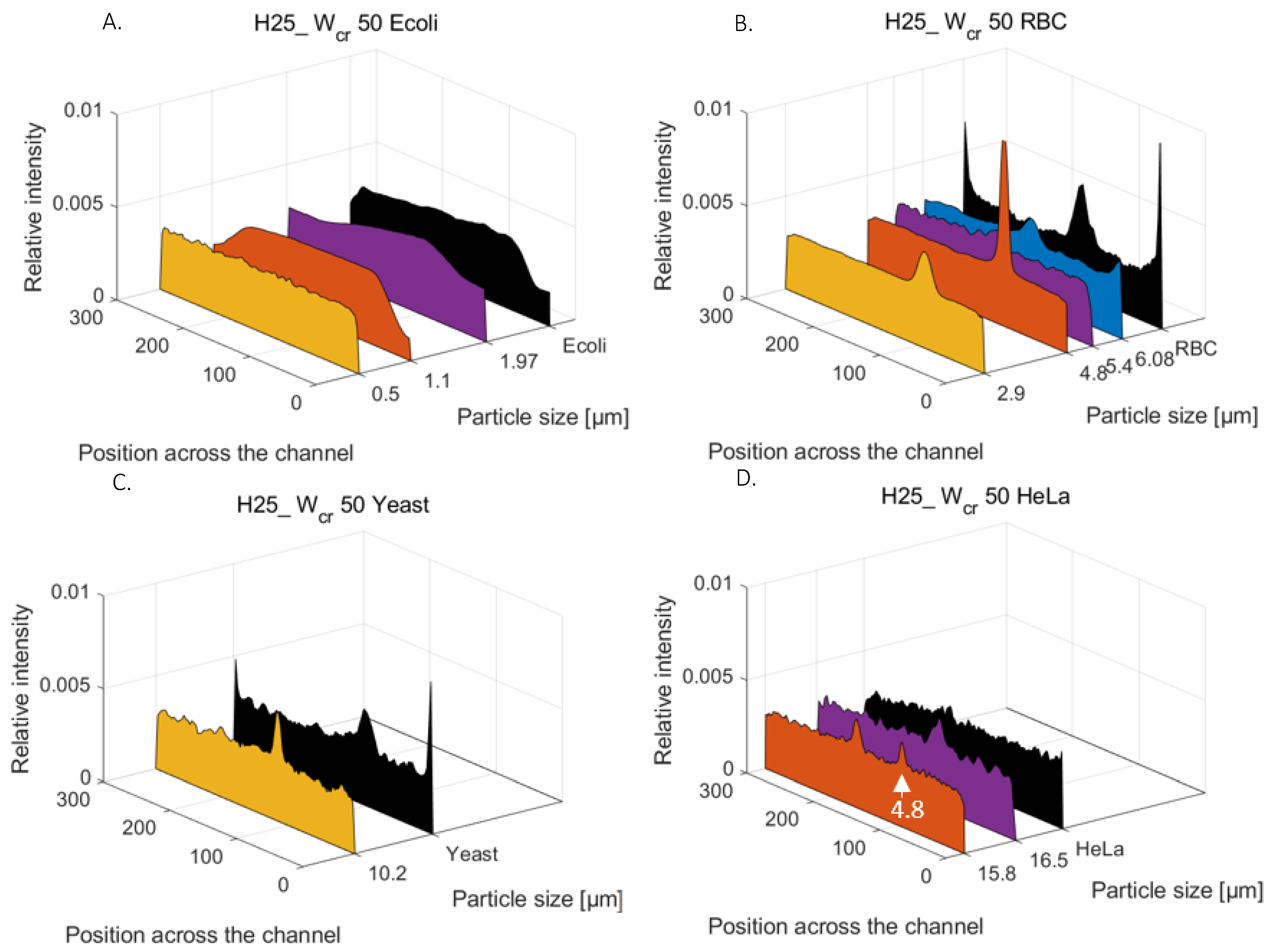
20], experiments were prepared to understand how the microfluidic channel’s parametric change (height and critical width) can affect the focusing efficiency of smaller beads; what are the minimal flow rates for particle focusing of the specific parameters; and how extending the length of the curved channel would affect the focusing process. The purpose of the current report is to give a detailed explanation in the interpretation of the particle-size-dependent focusing phenomenon. On one hand, clarification needed to be obtained for why the phenomenon of focusing of smaller beads (under Ø 4.8 µm) did not occur in curved channels with square-shaped restrictions (H50 W
cr50), and what could help to achieve the effective focusing of these Ø 4.8 and 15.8 µm spherical beads in a rectangular channel (H25 W
cr50), interpreting the experimental results through numerical simulation. On the other hand, the aim is to examine the focusing efficiency and alterations in focusing positions when using biological particles owning different morphologies rather than the spherical polystyrene beads.
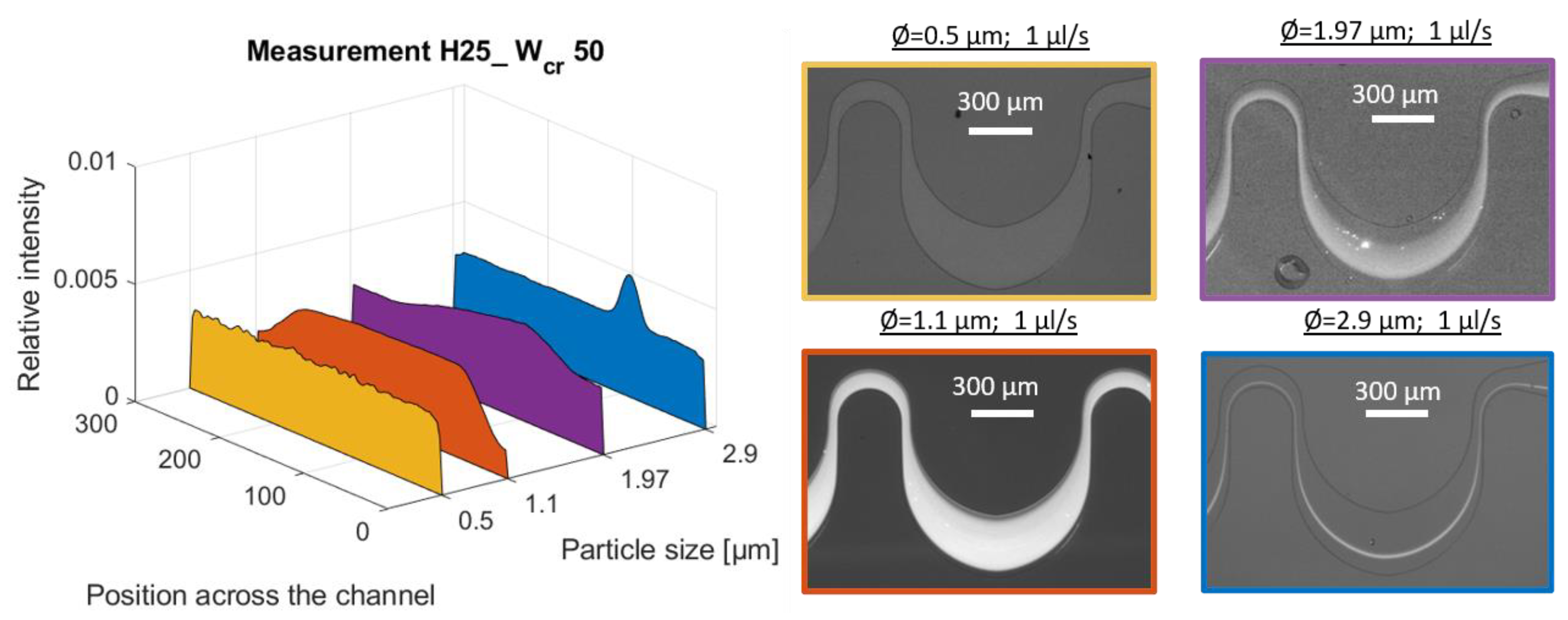
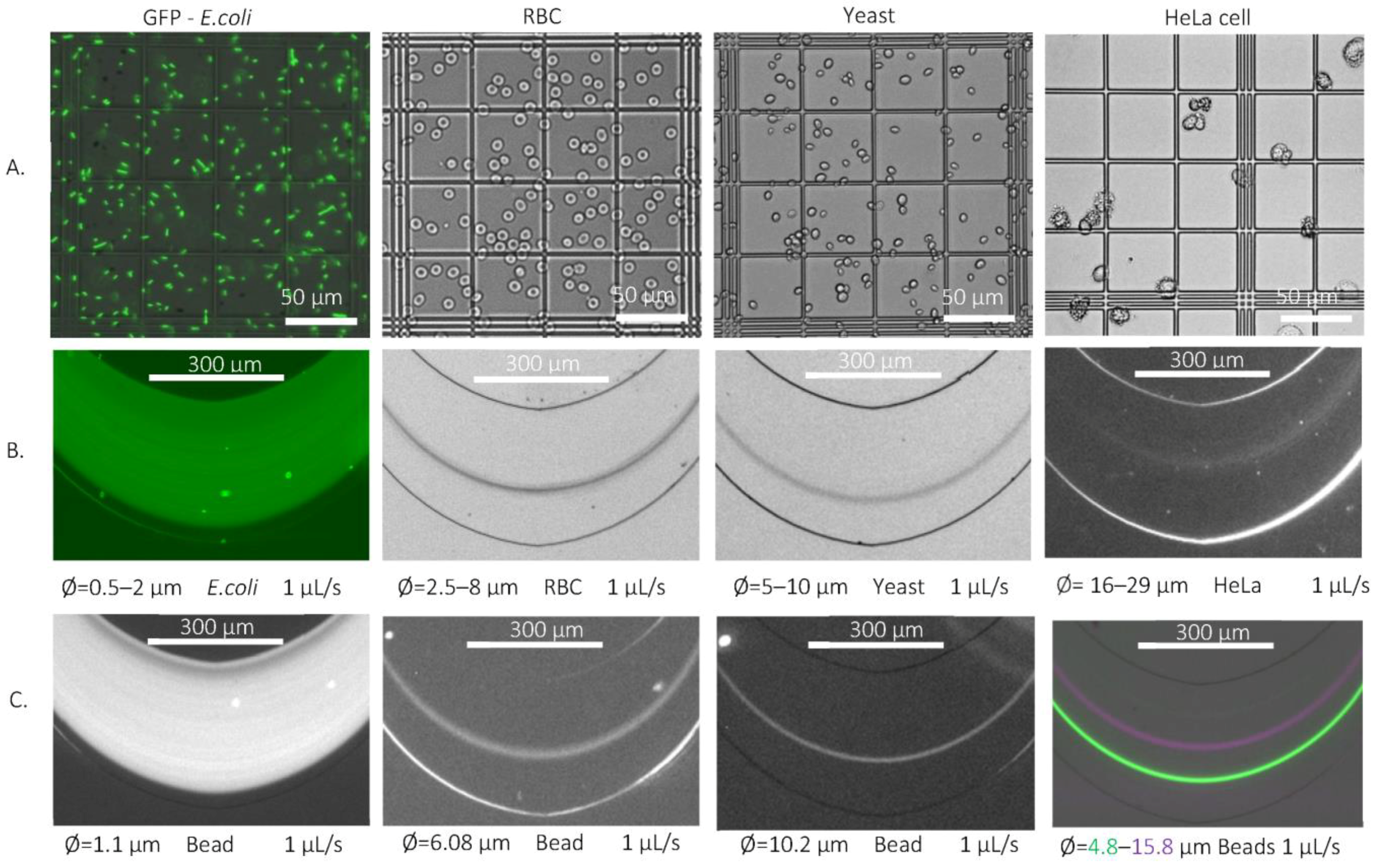
Primarily fluorescent particles with different bead diameters (Ø = 0.5, 1.1, 1.97, 2.9, 4.8, 5.4, 6.08, 10.2, 15.8 and 16.5 µm) were used in the suggested curved channel (H25_Wcr50) at different flow rates (0.5, 1 and 2 µL/s). Due to the possible targeted cell sizes being between 0.5µm (E. coli) and 10–20µm (blood cells or tumor cells—CTCs), test polystyrene beads were chosen as equivalent models with bead sizes that covered this range. The flow rates were defined to ensure adequate flow conditions for inertial focusing, without deteriorating the cell membranes caused by the shear forces emerging in the case of high flow velocities. The analyzed size-dependent focusing was represented by a precise map of the equilibrium positions of the spherical beads at the end of the channel, giving a good benchmark for the behavior of the multi-dimensional particles. The lateral positions of living cells were also evaluated in relation to the lateral position of spherical particles of similar size, such as E. coli bacteria (rod-shaped), red blood cells (discoid-biconcave-shaped), Saccharomyces cerevisiae (round- or ovoid-shaped yeast), and HeLa cancer cell-line cells (soft-irregular-shaped). The focusing efficiencies were studied by fluorescent or dark-field imaging the spherical rigid particles and real biological cells having the same size range to better understand the role of particle morphology in hydrodynamic focusing phenomena.
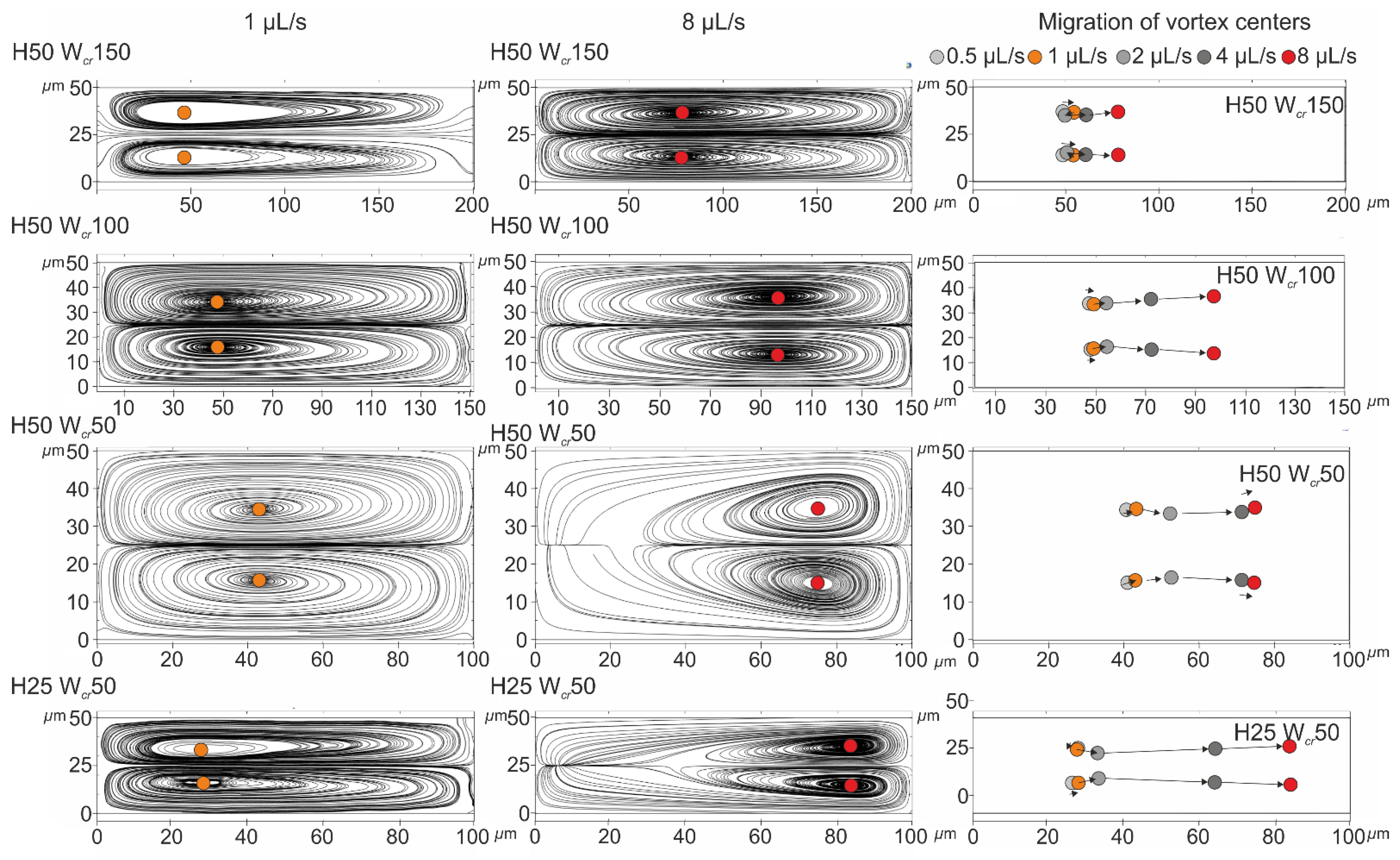
The hydrodynamic background of particle positioning was comprehended by demonstrating the formation and migration of Dean vortex centers using finite element modelling by COMSOL Multiphysics [
21].
2. Theoretical Background
2.1. Hydrodynamics of Focusing Multi-Dimensional Particles
The Reynolds number (Re) was introduced to characterize the flow in a microfluidic channel, based on the ratio between the internal friction of the medium [
22,
23]:
(
um—max channel velocity;
DH—hydraulic diameter;
η—dynamic viscosity;
ρ—density of the medium); and to characterize the forces acting on the particles in a closed microfluidic channel,
(Re
p—particle Reynolds number;
a—diameter of the particle); in which the hydraulic diameter (
DH) can be given by the Equation (3) below:
(
w—channel’s width;
h—height).
In Poiseuille flow, the “two-staged” process of inertial migration occurs due to the balance of the inertial lift (
, the wall-induced lift (
, and shear-gradient-induced lift forces (
[
24]:
(
—dimensionless share rate;
α-dimensionless share gradient;
—function of lateral position).
The equilibrium position can be further tuned by increasing the Reynolds number—particles tend to migrate closer to the walls—or by introducing a less dominant force at a low Reynolds number: rotation-induced lift force (
) triggered either by the shape of the particle or by geometrical considerations on the channel:
(
—angular velocity;
ur—vectors of relative particle velocity).
In an asymmetric curved channel, a secondary rotational flow—Dean flow—can be triggered, which further reduces the lateral equilibrium positions in inertial focusing. Such a geometry was presented by di Carlo et al. [
6], where the magnitude of the rotational flow velocity and the resulting drag force further tuned these equilibrium positions. The evolution of this secondary rotational flow can be characterized by a dimensionless number: the Dean number, which indicates the strength of the Dean flow, and can be calculated as [
7]:
(
—the radius of the curvature).
The phenomenon evolving in the curved channel was explained in more detail in our previous publication [
20]. To study the concept, rigid spherical polystyrene beads were used, which do not take on a special form of movement in continuous flow. What surrounds our curiosity is: what other effects influence the focusing positions of soft, deformable biological cells?
Jinghong Su et al. [
24] presented a series of detailed three-dimensional numerical simulations studying the change in focusing equilibrium positions influenced by particle shape in straight channels having different channel cross-sections, and at altered channel Reynolds numbers (50–400). In these calculations, the non-spherical particle form factor (
L/
D), the aspect ratio of the particle main size to the channel height (
), the aspect ratio of the channel cross-section (
W/
H), and the lift coefficient (
—the moment of inertia tensor of the particle—were also considered when the Cartesian (overlapping) grid method was executed.
(
—normal diameter identical size between the spherical and non-spherical particle).
The equilibrium positions can be found in a given channel cross-section based on the rate of inertial migration and spatial distribution of average lift force coefficient at a steady-state condition. Therefore, equivalent bead sizes had been determined between spherical and non-spherical particles; the classification was made by: the nominal diameter (), axial length diameter (), and rotational diameter (). These parameters optimally describe the equivalent diameter as: at = 50, but at = 200 in case of square-channel cross-sections; and at = 50, at = 100; but >, when is higher (in rectangular channels), in that case, particle equilibrium positions are shifted closer to the centerline.
Whenever L/D increases, the equilibrium position of non-spherical particles is approaching the walls, although at = 150–200, when L/D = 3 and 4, this trend is experienced to be reversed.
Moreover, special movement forms can be observed, such as
Oscillation, the magnitude, and also amplitude increases with L/D, such as at rod-like particles;
Rotation is characterized by an extreme lift force coefficient and also increases with L/D; the angular velocities () are in phase; and in the case of ellipsoidal particles, is nearly independent of ;
Tumbling motion: oblate ellipsoid in linear shear flow around the vortex axis, but with increasing , L/D slowly decreases.
2.2. Morphology and Movement of Rod-Shape Cells (E. coli) in Flow
The comprehension of bacterial motility compared to hydrodynamics-based predictions can be crucial to see the whole picture in terms of microfluidics. Motile bacteria commonly move via flagella [
25] as a result of some environmental impact influenced by pH, viscosity, chemical environment, surface roughness, and so on.
Viola Tokárová et al. [
26] examined five different species of bacteria (
Vibrio natriegens,
Magnetococcus marinus,
Pseudomonas putida,
Vibrio fischeri, and
Escherichia coli), with different sizes and flagellum structures, in order to better understand their movement characteristics in chambers, linear-, angled-, and serpentine-type channels having different diameters. Cell motility was represented by density maps, spatial distribution, bacterial trajectories in 2D and 3D visualization. Based on their observations, three types of behavior were distinguished and grouped as (1) accumulators: bacteria swimming in a distance of a few tens of nanometers to the wall, interspersed with steric interactions; (2) parallel movers: hydrodynamic forces retaining bacteria in a specific region, a few micrometers from the surface; (3) escapers: the bacteria leaving the wall due to hydrodynamic interactions. The cell body aspect ratio and length of the flagellum contributes greatly to the classification.
E. coli has a rod-like shape, owning a high aspect ratio, and has several flagella. Based on hydrodynamic principles, and observations of species-specific movements, E. coli was classified as a parallel swimmer, but in a smaller proportion of cases, “wall escaper” behavior could be also identified; rarely, circular movements were also shown until they attached to the walls.
In a tortuous channel with different local channel restrictions (5, 10, 15 µm), it was observed that the hydrodynamics-driven movement was more characteristic in a wider channel, although in a narrower channel, their behavior was dominantly based on local steric interactions between the wall and the flagella. In channels characterized by a restricted region of 10 µm width, the two effects did not cooperate, parallel swimmers got easily trapped, and the ratio of the characteristic shape had a greater role. E. coli—which has the lowest ratio of the cell body and the flagella—could not overpass, and started to show circular movements and got easily trapped at the corners.
Based on Jinghong Su’s [
24] observations in the case of
E. coli, the cell aspect ratio
L/
D = 2/0.5 = 4. Based on inertial migration at
Re = 50, the equivalent diameter compared to spherical ones could be estimated by the axial length of diameter, and the
E. coli tended to oscillate in flow. It should be noted that, in our recent study, serpentine-type channels were used without corners, and the critical channel’s cross-sections were parameterized by 25 µm height and 50 µm width; accordingly, the hydrodynamics-driven movement of
E. coli was dominant.
2.3. Morphology and Movement of Disc-Shape Cells (Red Blood Cells—RBC) in Flow
Thomas M. Geislinger et al. [
27] conducted thorough research on the effects of hydrodynamic forces acting on rigid and deformable particles moving in the flow. RBCs are deformable objects, surrounded by a lipid bilayer, owning a discoid biconcave shape. In hydrodynamic flow, several motion forms of RBCs were observed: (1) tumbling motion at low shear rates, (2) tank-treading motion at higher shear rates, or (3) swinging. The difference between the viscosity of the medium and the internal cytoplasm of the cell, as well as the excess area, also influenced the characteristics of the motion. Considering the degree of shearing effect, RBCs can take on such varied forms in flow as: biconcave discoid shape, slipper shape, or parachutes.
Many questions still arise, such as how the phenomenon can be described as accurately as possible with mathematical models, and to what extent rotational lift forces contribute to the success of focusing besides shear- and wall-induced lift forces in Poiseuille flow. At a higher hematocrit level, the effective viscosity decreases with the increased shear force; moreover, the cell–cell interactions and the significant restriction of the channel cross-section (8 µm) can, thus, drastically change the cell arrangement and dynamic shape of the cells.
The observations of di Carlo [
6] showed that ordering RBCs was also possible (dilution 2 V/V%) in a straight and curved serpentine channel; however, at even at higher flow rates (
Re = 60), cell viability was barely affected, and they all behaved as rigid particles in the flow. In a 50 µm square channel, the rotational alignment of RBCs was also observed setting the disk axis parallel to the closest wall.
Based on Jinghong Su’s [
24] observations, at a cell aspect ratio
L/D = 4:15 with a diameter of 7.5 µm and thickness of 2 µm,
in our channel should be around 7.5/25 = 0.3; its equivalent diameter compared to spherical beads can be represented by its rotational diameter, but at higher
or
Re, the diameter can be slightly larger.
In human blood, the Reynolds number can be characterized between
Re < 0.01,
Re~1, and
Re ≈ 4000 in the capillaries, arteries, and aorta, respectively [
27].
Making a classification based on the shear stress, tumbling motion is characteristic at low stress and rolling motion at higher ones, but still, the stabilized cell membrane maintains its chase, even at tank-treading dynamics or off-shear-plane tumbling [
28]. When shear-induced viscous stress is evolved, bouncing in circumferential directions can balance the surface stresses. In order to reduce the viscosity of the medium and cell–cell interaction, the used blood sample was also diluted in our case.
2.4. Morphology and Movement of Spherical Cells (Yeast and HeLa) in Flow
Eliezer Kainan et al. [
29] performed inertial focusing and sorting of
Saccharomyces cereviae in a curved channel at higher Reynolds numbers (
Re = 215, 1.5 mL/min) to segregate yeast based on age-related cell size. The degree of budding is a good indicator of cell aging. A high-density yeast cell culture after several days showed that ~32% of the population were newborn, ~67% had 0–2 scars, ~33% had 3
scars, and less than 5% had 11
scars. Adult yeasts were classified with the size of 5.4–11 µm (3–10 scars) and the younger ones with 2.3–4.7 µm (0–2 scars). The linear growth rate was about 0.8
0.1 µm/scar, the produced chitin-rich bud scars were fluorescently labelled, and the histogram of forward scatter (FCS) of yeast populations was measured from the collected yeast populations at the end of curved channel. The collection was completed with a high throughput (10
7 yeast/min), and without any cell damage despite the quite high shear stress (691 Pa). The smaller, younger cells left the centered ports enriched with ~12%, compared to the presence of young yeasts in the original mixture, having a scar number of 1.3 ± 1.6; and the larger, adult cells left the channel at a concave port with an enrichment of ~46%, having an average budding number of 3.4 ± 1.7. PDMS-glass hybrid platforms were used for the performed test.
During our measurements, the same strains were used, and the diameter of the used yeast approximately fell between 5 and 10 µm, but the age distribution of the cells was ignored. Only their hydrodynamic behavior and their focusing position were taken into account.
5. Conclusions
The phenomenon of Dean-flow-affected lateral focusing was investigated in a periodically inhomogeneous curvilinear microfluidic channel with special focus on the behavior of biological target cells, since several biomedical applications are intended to position or separate these species by their size and morphology in flow.
Primarily, the channel-geometry- and flow-rate-dependent formation of Dean vortices was simulated by finite element modelling applying COMSOL Multiphysics FEM software. The flow-rate-dependent migration of the vortex centers was determined for a better comprehension of the evolution of the equilibrium-focusing states of particles in a microfluidic environment. Based on the numerical calculations for flow velocity distributions, the size dependent particle movements were also estimated to predict their lateral-focusing positions at the outlet of the microfluidic system using the particle-tracing unit of the COMSOL program.
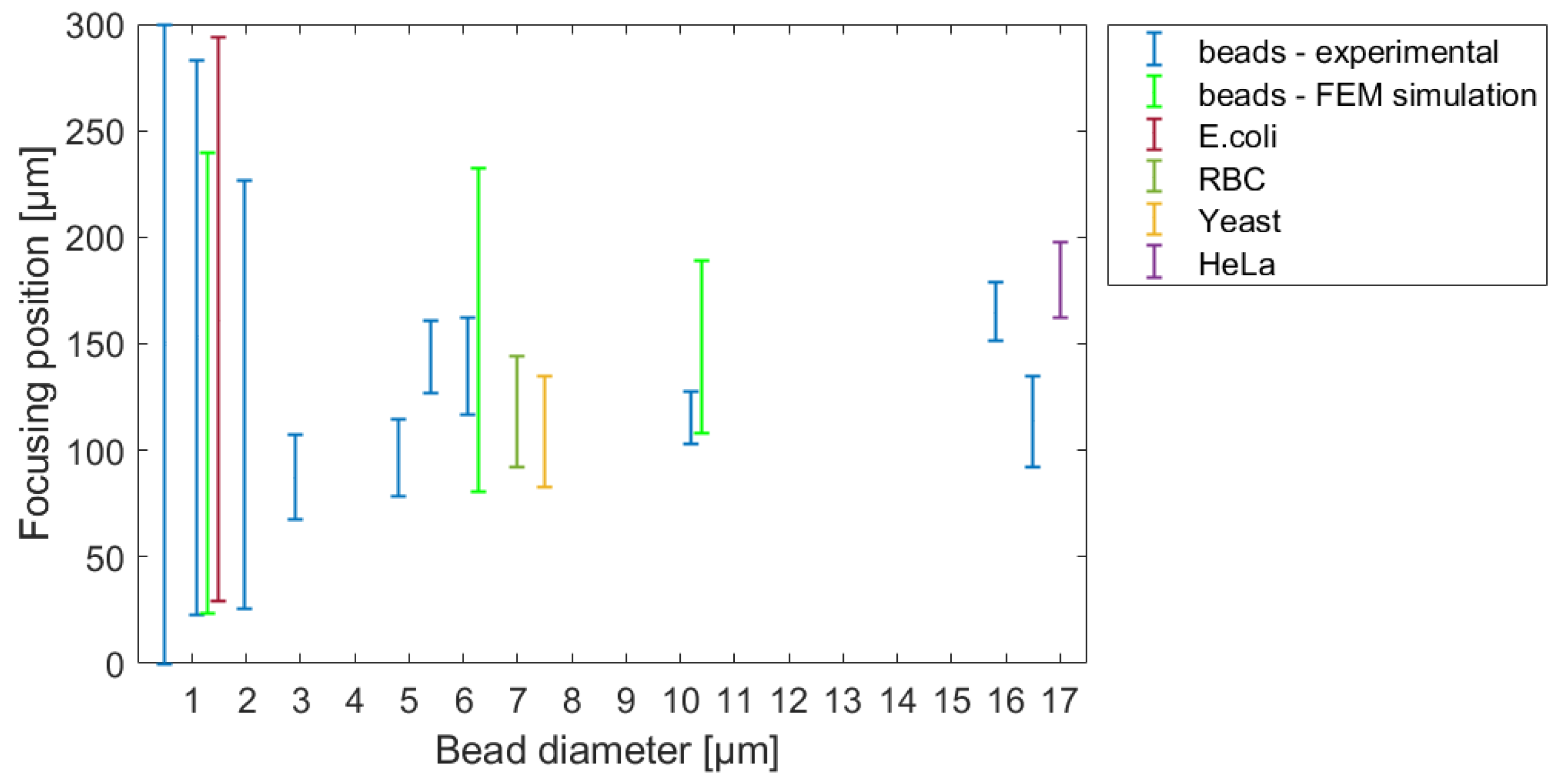
To validate our theoretical results, the tracing behavior of real biological cells and polystyrene beads with different sizes was studied in variously parameterized microfluidic systems. A comprehensive map of equilibrium lateral-focusing positions were determined for rigid polystyrene beads having a diameter in the range of Ø 0.5–16.5 μm, and for representative biological cells such as
E. coli bacteria, yeast, red blood cells, and HeLa cancer cells. The recorded trajectories of the analysed beads and cells are compared in the movie titled A_Banyai_LatFocus_Experimental.mp4 in the
Supplementary Materials. Compared to rigid spherical beads, these cells can adopt different forms of movement in flow due to their various degrees of flexibility, multi-dimensional sizes, and shapes, which can modify their lateral-focusing position. This study suggested that rigid particles can serve as an adequate model for individual biological species and an equivalent diameter can be defined for a prediction of the lateral-focusing positions of the analyzed cells in the specific microfluidic structure.
Figure 7 represents the evolution of focusing positions in the investigated microfluidic channel for different spherical-bead diameters and demonstrates the behaviors of the applied target cells.
To validate the reliability of the FEM simulation, a probable focusing range in the cross-section of the channel was defined and compared to the measured focusing positions in the case of bead diameters of 1.1 µm, 6.8 µm, and 10.2 µm, respectively. The modelled probable focusing ranges are also indicated in
Figure 7. The central positions of the modelled particle distribution were in accordance with the experimental case. The modelled spread of the particles—variation in the numerical simulation—was higher due to the limited number (1000) of the test particles.
In the literature review, various powerful microfluidic-cell-separation solutions [
4,
18,
29] were presented. Their channel geometry and cross-sectional parameters mainly determine the possible cell sizes and morphologies to be separated and focused. Accordingly, the feasibility of a developed separation method depends on the compatibility of the applied biological sample and the designed microfluidic system. The crucial aspects of their applicability are the prevention of cell deterioration caused by evolving shear forces at higher flows and the inhomogeneity of the cell population affecting the success of its lateral focusing. In the case of larger HeLa cells, a much larger size distribution and shape variability were observed, so pre-filtration or an additional separation method could be necessary. The aim of the present work was to highlight the importance of the target morphology regarding the efficiency of the separation.
The comprehension of the positioning, separation, or filtration of cells in microfluidic systems was the motivation of the domestic project “Rapid urine bacteria analyzer—RUBA”, which aimed to develop a point-of-care (PoC) device to detect and analyze pathogenic bacteria or blood cells in urine. Due to the low concentration of bacteria in a sample, the efficient concentration of the target cells is crucial. In urine, squamous cells, fungi, sperm, and blood can be found in various sizes among the shaped elements, with particles within the 1–60 µm size range. Accordingly, various filtration and separation methods were tested and investigated, such as weir-type cross-flow filtration, deterministic lateral displacement (DLD), and inertial lateral focusing in the presented asymmetric curved serpentine structure. The interesting behavior of the different cells (E. coli, RBC, and spherical cells such as yeasts and HeLa) in a microfluidic channel was tested to understand the effects of size and shape. The proper combination of the individual structural microfluidic elements requires serious consideration, although this could provide a suitable solution for fine-tuning the filtration process. Our results could be utilized in the development of such point-of-care devices as that in the focus of 77 Elektronika Ltd. and, moreover, in promising future applications such as the separation and capturing of circulating tumor cells (CTCs).