Low-Cost Photoreactor to Monitor Wastewater Pollutant Decomposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Design Hypothesis
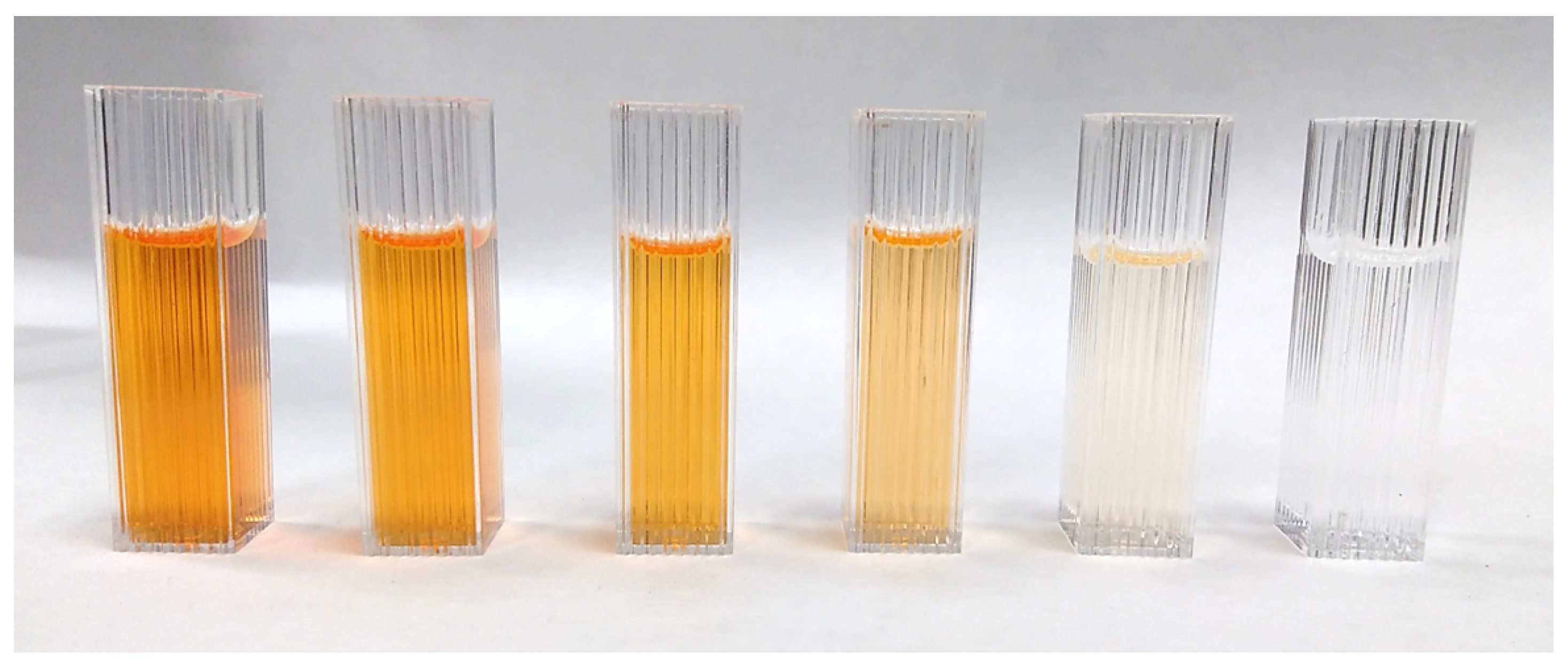
2.2. Dye Degradation Experiments
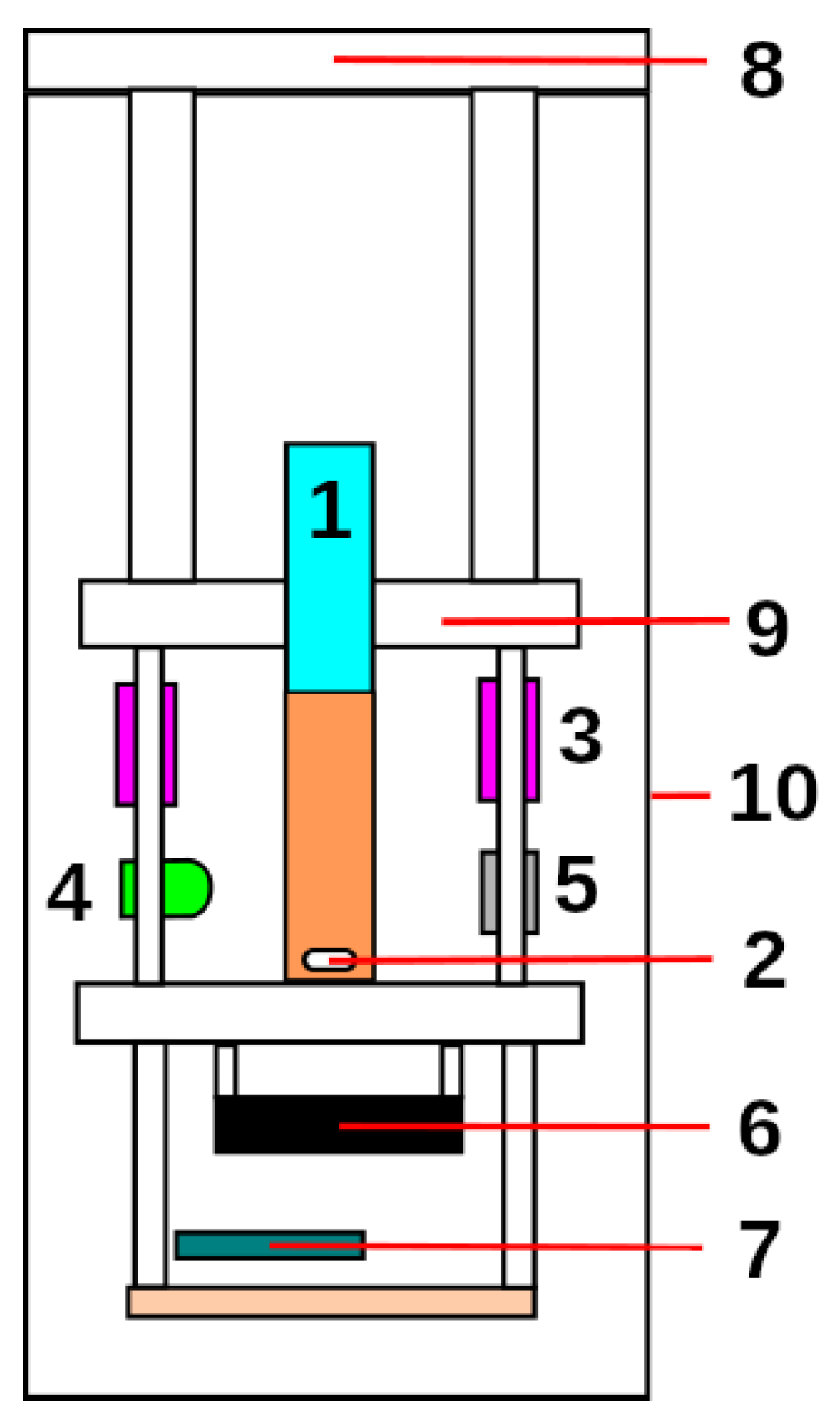
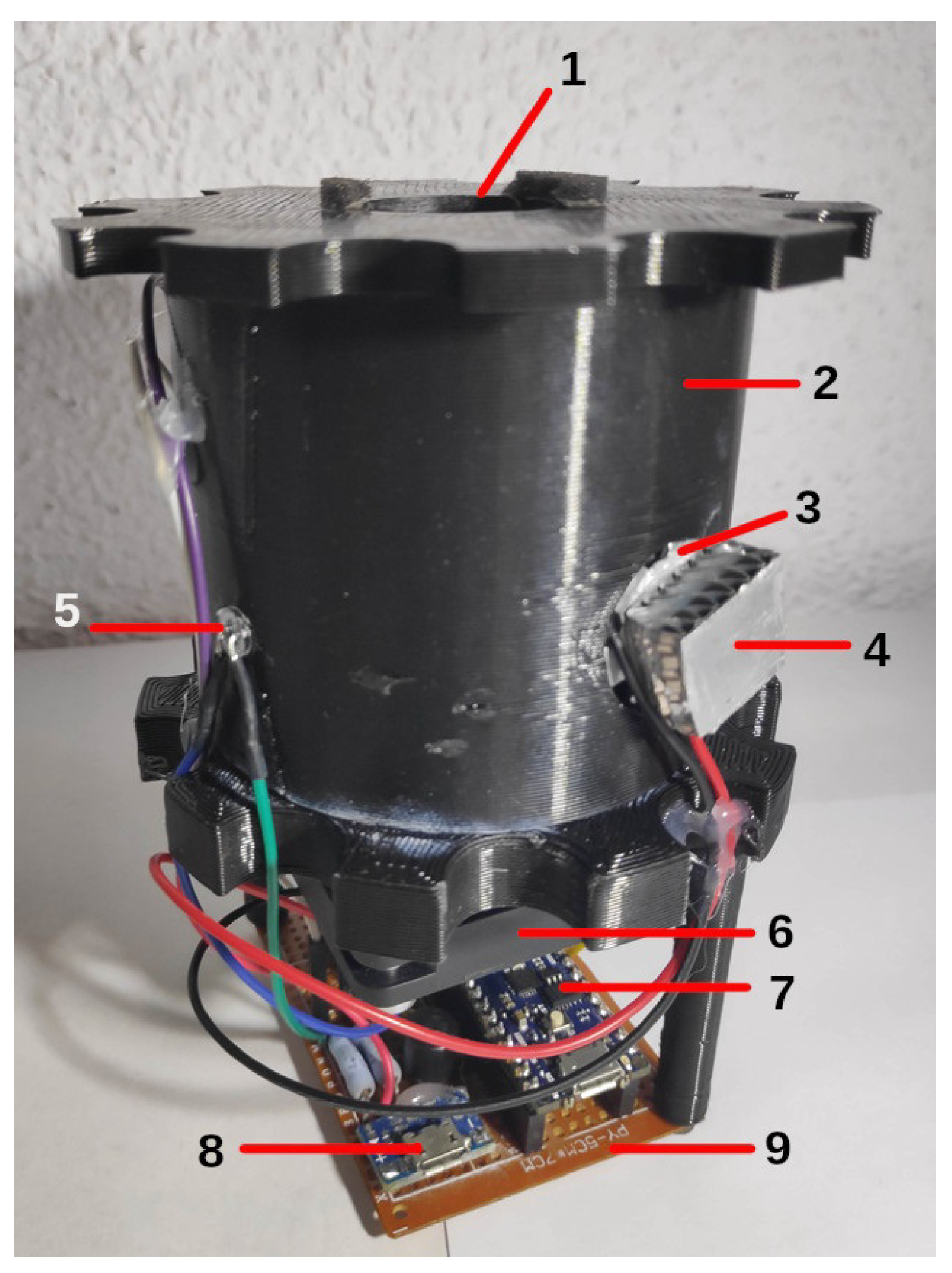
2.3. Reactor Architecture
- Transmittance LED. The Lumex SSL-LX5093UEGC [22] LED was chosen because of the higher absorption wavelength of the methyl red solution used in the experiments to appreciate the small changes in concentration. This solution had a spectral absorption curve with a maximum of 500 nm, which matched the peak wavelength emission of the LED. In addition, the narrow aperture angle of the lens allowed the beam to be concentrated and more light to reach the sensor.
- Ultraviolet LED. As a source of ultraviolet light, two 380–390 nm Epiled UV LEDs of 1W power and a wide viewing angle were chosen to cover the maximum surface area of the test tube. Two aluminum heatsinks were incorporated into the LEDs to increase the surface area and reduce their working temperature.
- Operational Amplifier. An operational amplifier was necessary to condition the signal coming from the LDR sensor. A Burr-Brown OPA241 [23] with rail-to-rail output was chosen to take advantage of the input range of the Arduino’s ADC converter. It also had to be a single supply to use the supply voltage available. Finally, its high CMMR, high input impedance, low bias current, and low noise were other key features to incorporate into the design.
- Microcontroller. Arduino is a platform that develops open hardware prototyping boards and offers multiple options depending on the field of application. In our case, it was the main component that controlled all the subsystems that made the reactor work. An Arduino Nano Every was used because all these operations do not require considerable processing power. Moreover, it was not necessary to have a large number of inputs and outputs, and a compact and minimalist design was also one of the objectives. The Arduino Nano Every board is based on the ATMega4809 microcontroller (MCU). This MCU provides the following features: 46 KB of Flash, 6 KB of SRAM, a 256 bytes EEPROM, 20 MHz clock frequency, SPI connectivity, I2C, micro USB, 8 digital inputs with a 10-bit DAC and 14 digital outputs.
- Stirrer. It was necessary to have a compact and quiet motor for the stirrer function. For this purpose, a computer axial fan of 50 × 50 mm at 5 V was used [24], to which a steel washer was attached to the shaft with cyanoacrylate adhesive, so that square neodymium magnets with opposite poles could be attached. This fan also suited the need for an active cooling solution.
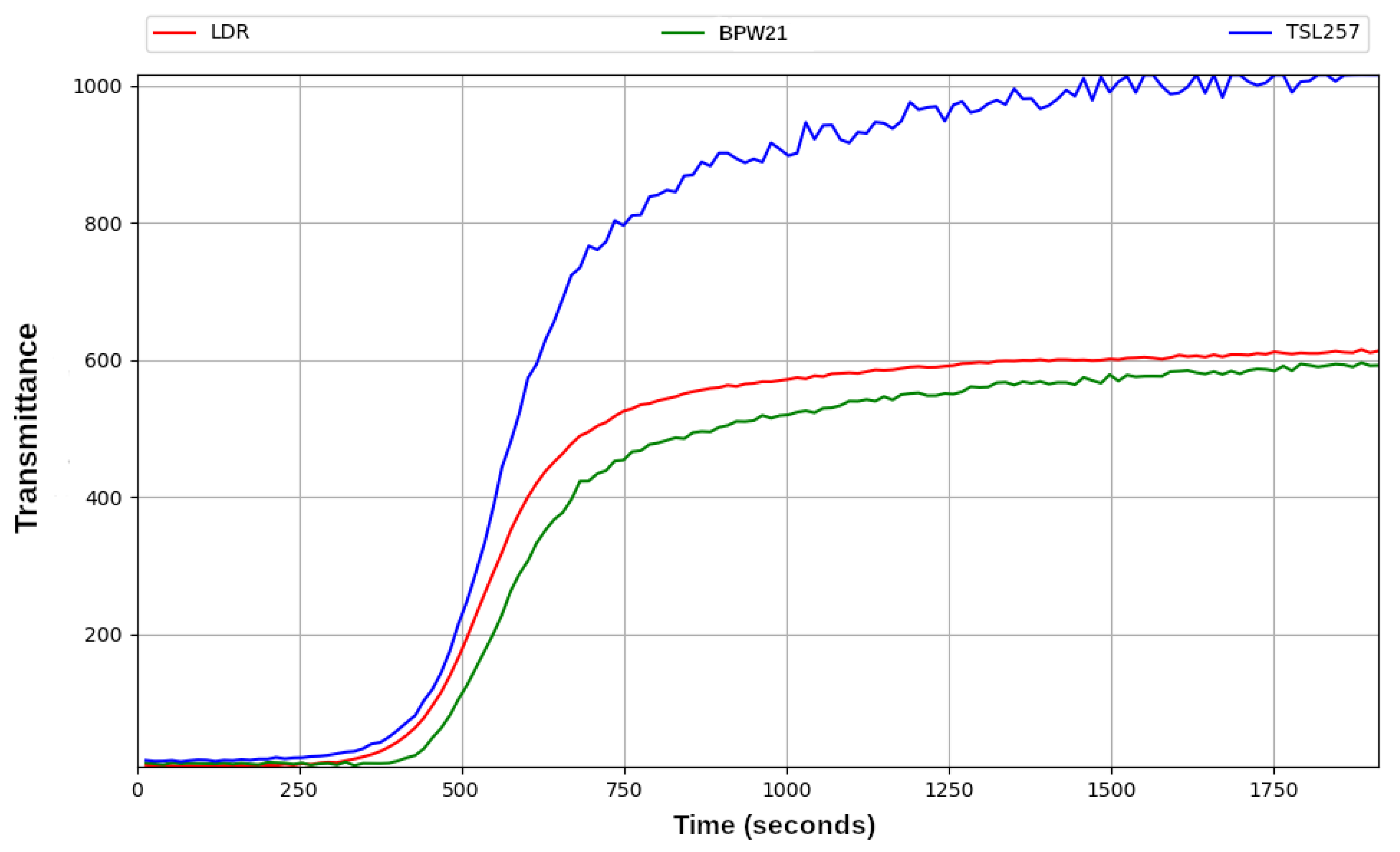
- Photosensor. At the beginning of this work, the aim was to determine what kind of sensor would be suitable. To this end, the responses of various sensors to little light transmittance variations were studied. The sensors tested were a cadmium sulfide photoresistor (LDR) [25]; a BPW21 photodiode [26], which required an external signal amplifier circuit; and a TSL235R photodiode with an integrated amplifier circuit. Finally, the sensor chosen was the LDR, as will be explained in Section 3.
2.3.1. Electronic Circuit
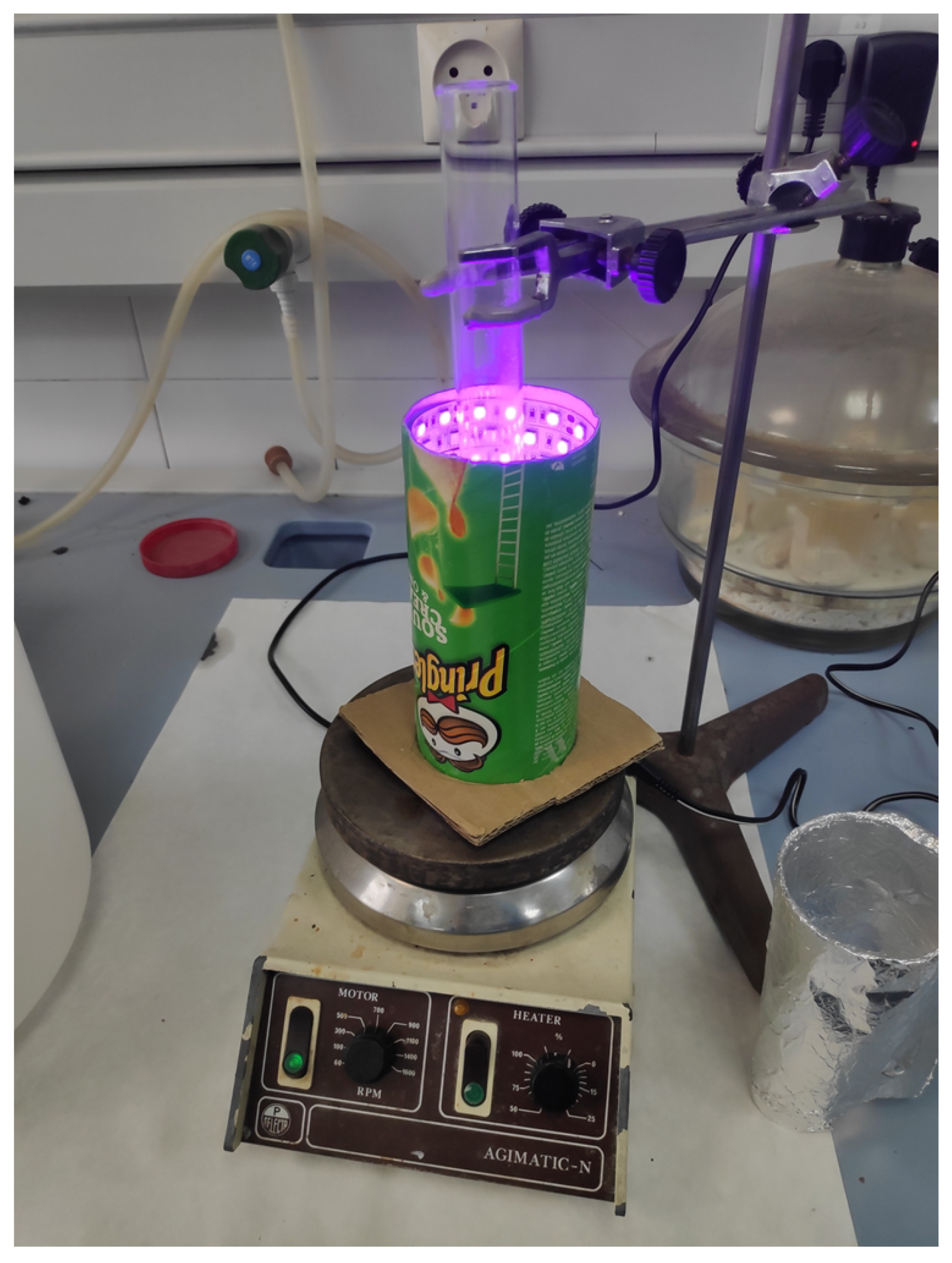
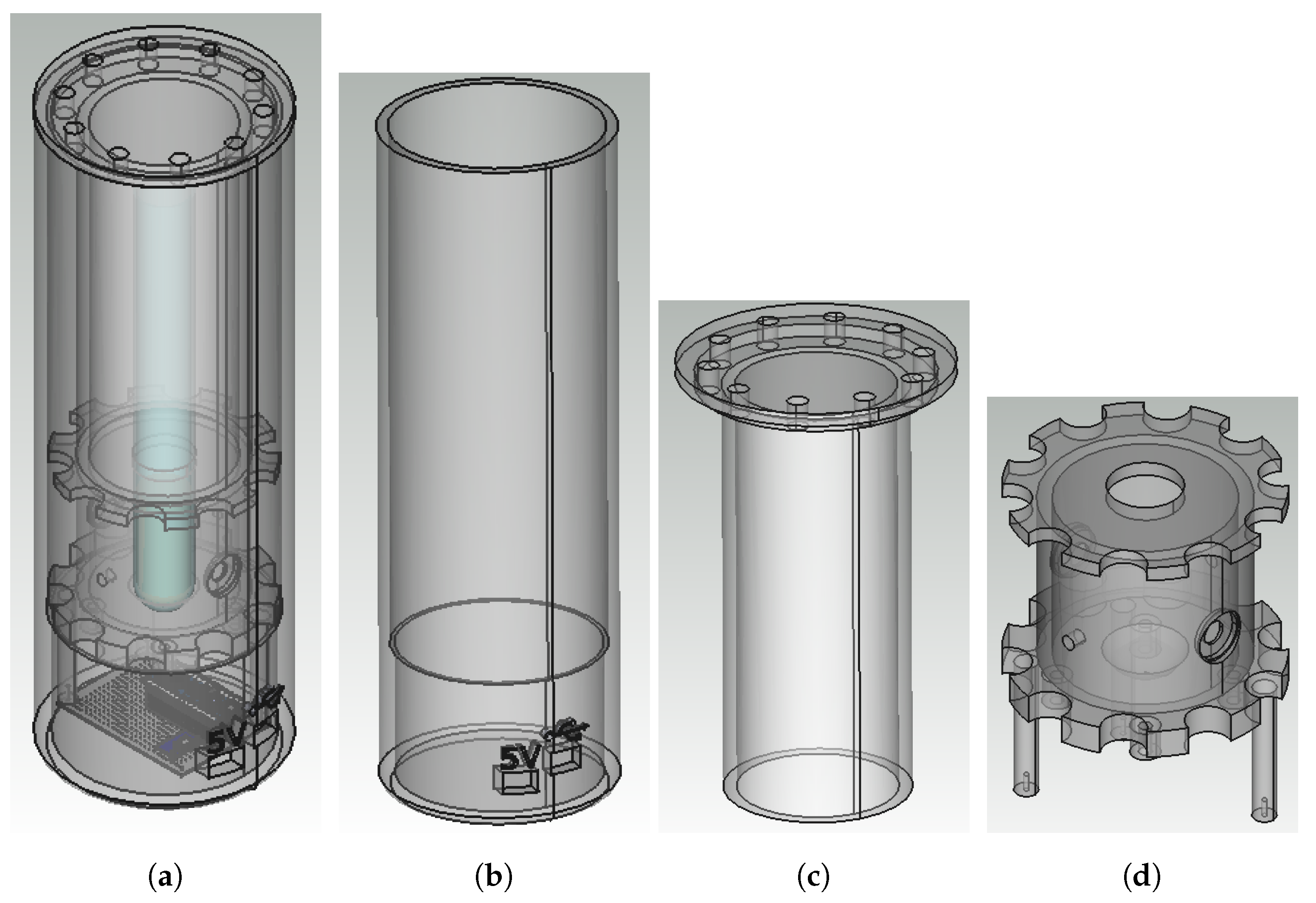
2.3.2. Reactor Casing
2.3.3. Device Firmware
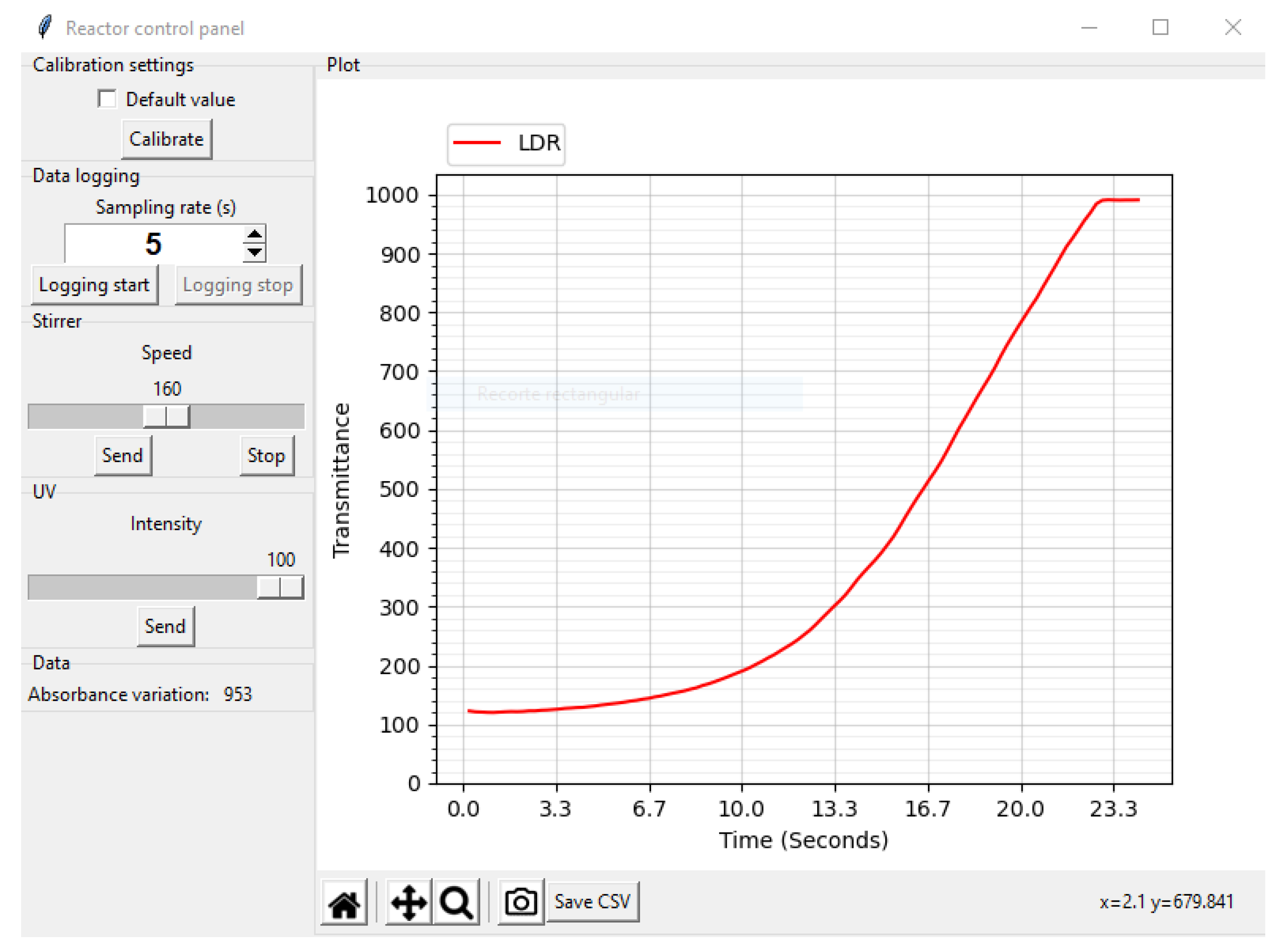
2.4. Data Acquisition Software
3. Evaluation and Results
3.1. Prototype
3.2. Kinetic Improvement of the Photodegradation Process
3.3. Monitoring the Photocatalysis Process with a High Noise Level
3.4. Comparison of Three Sensors for Transmittance Measurement
4. Conclusions and Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez-López, P.; Lázaro, N.; Alvarado-Beltrán, C.G.; Pineda, A.; Balu, A.M.; Luque, R. One-Pot Cu/TiO2 Nanoparticles Synthesis for Trans-Ferulic Acid Conversion into Vanillin. Molecules 2019, 24, 3985. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Vráblová, M.; Smutná, K.; Koutník, I.; Prostějovský, T.; Žebrák, R. Surface Plasmon Resonance Imaging Sensor for Detection of Photolytically and Photocatalytically Degraded Glyphosate. Sensors 2022, 22, 9217. [Google Scholar] [CrossRef] [PubMed]
- Demetz, D.; Sutor, A. Inductive Tracking Methodology for Wireless Sensors in Photoreactors. Sensors 2021, 21, 4201. [Google Scholar] [CrossRef] [PubMed]
- Cloete, N.A.; Malekian, R.; Nair, L. Design of Smart Sensors for Real-Time Water Quality Monitoring. IEEE Access 2016, 4, 3975–3990. [Google Scholar] [CrossRef]
- McCullagh, C.; Skillen, N.; Adams, M.; Robertson, P.K. Photocatalytic reactors for environmental remediation: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1002–1017. [Google Scholar] [CrossRef]
- Abdel-Maksoud, Y.; Imam, E.; Ramadan, A. TiO2 solar photocatalytic reactor systems: Selection of reactor design for scale-up and commercialization—Analytical review. Catalysts 2016, 6, 138. [Google Scholar] [CrossRef]
- Sundar, K.P.; Kanmani, S. Progression of photocatalytic reactors and it’s comparison: A review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Sannino, D. Main parameters influencing the design of photocatalytic reactors for wastewater treatment: A mini review. J. Chem. Technol. Biotechnol. 2020, 95, 2608–2618. [Google Scholar] [CrossRef]
- Wang, D.; Mueses, M.A.; Márquez, J.A.C.; Machuca-Martínez, F.; Grčić, I.; Moreira, R.P.M.; Puma, G.L. Engineering and modeling perspectives on photocatalytic reactors for water treatment. Water Res. 2021, 202, 117421. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Ray, A.K.; Beenackers, A.A. Development of a new photocatalytic reactor for water purification. Catal. Today 1998, 40, 73–83. [Google Scholar] [CrossRef]
- Lin, H.; Valsaraj, K.T. Development of an optical fiber monolith reactor for photocatalytic wastewater treatment. J. Appl. Electrochem. 2005, 35, 699–708. [Google Scholar] [CrossRef]
- Natarajan, K.; Natarajan, T.S.; Bajaj, H.; Tayade, R.J. Photocatalytic reactor based on UV-LED/TiO2 coated quartz tube for degradation of dyes. Chem. Eng. J. 2011, 178, 40–49. [Google Scholar] [CrossRef]
- Abhang, R.; Kumar, D.; Taralkar, S. Design of photocatalytic reactor for degradation of phenol in wastewater. Int. J. Chem. Eng. Appl. 2011, 2, 337. [Google Scholar] [CrossRef]
- Jamali, A.; Vanraes, R.; Hanselaer, P.; Van Gerven, T. A batch LED reactor for the photocatalytic degradation of phenol. Chem. Eng. Process. Process Intensif. 2013, 71, 43–50. [Google Scholar] [CrossRef]
- Shahrezaei, F.; Mansouri, Y.; Zinatizadeh, A.A.L.; Akhbari, A. Process modeling and kinetic evaluation of petroleum refinery wastewater treatment in a photocatalytic reactor using TiO2 nanoparticles. Powder Technol. 2012, 221, 203–212. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic reactors with suspended and immobilized TiO2: Comparative efficiency evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Casado, C.; Timmers, R.; Sergejevs, A.; Clarke, C.T.; Allsopp, D.W.E.; Bowen, C.R.; Van Grieken, R.; Marugán, J. Design and validation of a LED-based high intensity photocatalytic reactor for quantifying activity measurements. Chem. Eng. J. 2017, 327, 1043–1055. [Google Scholar] [CrossRef]
- Brooks, A.D.; Yeung, K.; Lewis, G.G.; Phillips, S.T. A strategy for minimizing background signal in autoinductive signal amplification reactions for point-of-need assays. Anal. Methods 2015, 7, 7186–7192. [Google Scholar] [CrossRef]
- Badr, Y.; Abd El-Wahed, M.; Mahmoud, M. Photocatalytic degradation of methyl red dye by silica nanoparticles. J. Hazard. Mater. 2008, 154, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Lumex. SSL-LX5093UEGC Datasheet. Available online: https://www.lumex.com/datasheet/SSL-LX5093UEGC (accessed on 28 December 2022).
- Burr-Brown. OPA241 Datasheet. Available online: https://www.ti.com/lit/ds/symlink/opa241.pdf (accessed on 28 December 2022).
- Sunon. DC Brushless Fan & Blower Datasheet. Available online: http://www.farnell.com/datasheets/1512037.pdf (accessed on 28 December 2022).
- Technologies, E. Photoconductive Cell VT90N2 Datasheet. Available online: http://www.datasheet.es/PDF/947344/VT90N2-pdf.html (accessed on 28 December 2022).
- Vishay. Silicon Photodiode for the Visible Spectral Range BPW 21 Datasheet. Available online: https://www.vishay.com/docs/81519/bpw21r.pdf (accessed on 28 December 2022).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Flores, A.; García, A.; Pineda, A.; Brox, M.; Gersnoviez, A.; Cañete-Carmona, E. Low-Cost Photoreactor to Monitor Wastewater Pollutant Decomposition. Sensors 2023, 23, 775. https://doi.org/10.3390/s23020775
Ruiz-Flores A, García A, Pineda A, Brox M, Gersnoviez A, Cañete-Carmona E. Low-Cost Photoreactor to Monitor Wastewater Pollutant Decomposition. Sensors. 2023; 23(2):775. https://doi.org/10.3390/s23020775
Chicago/Turabian StyleRuiz-Flores, Alberto, Araceli García, Antonio Pineda, María Brox, Andrés Gersnoviez, and Eduardo Cañete-Carmona. 2023. "Low-Cost Photoreactor to Monitor Wastewater Pollutant Decomposition" Sensors 23, no. 2: 775. https://doi.org/10.3390/s23020775
APA StyleRuiz-Flores, A., García, A., Pineda, A., Brox, M., Gersnoviez, A., & Cañete-Carmona, E. (2023). Low-Cost Photoreactor to Monitor Wastewater Pollutant Decomposition. Sensors, 23(2), 775. https://doi.org/10.3390/s23020775











