Classifying Tremor Dominant and Postural Instability and Gait Difficulty Subtypes of Parkinson’s Disease from Full-Body Kinematics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Movement Testing
2.1.1. Study Participants
2.1.2. Movement Assessment Protocol
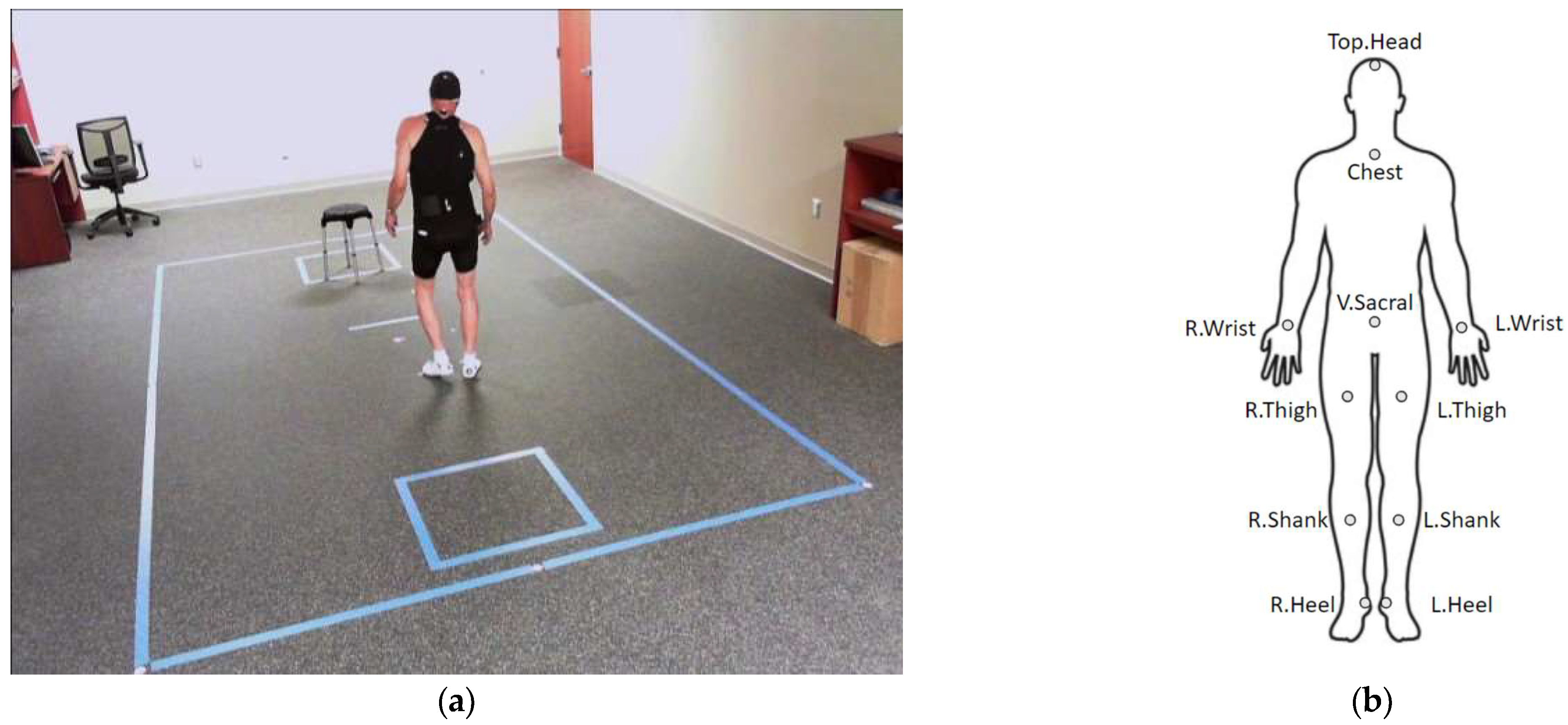
2.1.3. Motion Capture
2.2. Experiments
2.2.1. PD Subtype Calculation
2.2.2. Overall Classification Pipeline
2.2.3. Gait Features
2.2.4. Full Body Kinematics Features
2.2.5. Model Evaluation
2.2.6. Potential Biases in Classification Performance
2.2.7. Explaining Kinematic Features with Feature Analysis
3. Results
3.1. User-Independent Model Using Gait and Kinematic Features
3.1.1. Gait Features
3.1.2. Full Body Kinematics Features
3.1.3. Potential Biases in Classification Performance
3.2. User-Dependent Model Using Kinematic Features
Potential Biases in Classification Performance
3.3. Feature Relevance from Kinematics Features
3.3.1. Most Informative Features for Classification
3.3.2. Feature Correlation Analysis
3.3.3. Analysis with Restricted Feature Set
3.3.4. Analysis with Restricted Marker Set
4. Discussion
4.1. User-Independent Model Using Gait and Kinematic Features
4.1.1. Gait Features
4.1.2. Full Body Kinematics Features
4.1.3. Potential Biases in Classification Performance
4.2. User-Dependent Model Using Kinematic Features
Potential Biases in Classification Performance
4.3. Feature Relevance from Kinematics Features
4.3.1. Most Informative Features for Classification
4.3.2. Feature Correlation Analysis
4.3.3. Analysis with Restricted Marker Set
4.4. Utility of Gait Features
4.5. Utility of Kinematics Features
4.6. Limitations
4.7. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Stebbins, G.T.; Goetz, C.G.; Burn, D.J.; Jankovic, J.; Khoo, T.K.; Tilley, B.C. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov. Disord. 2013, 28, 668–670. [Google Scholar] [CrossRef]
- Herman, T.; Weiss, A.; Brozgol, M.; Giladi, N.; Hausdorff, J.M. Gait and balance in Parkinson’s disease subtypes: Objective measures and classification considerations. J. Neurol. 2014, 261, 2401–2410. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.; Postuma, R.B. Subtypes of Parkinson’s disease: What do they tell us about disease progression? Curr. Neurol. Neurosci. Rep. 2017, 17, 34. [Google Scholar]
- Vu, T.C.; Nutt, J.G.; Holford, N.H.G. Progression of Motor and Nonmotor Features of Parkinson’s Disease and Their Response to Treatment. Br. J. Clin. Pharmacol. 2012, 74, 267–283. [Google Scholar] [CrossRef]
- George, R.J.S.; Nutt, J.G.; Burchiel, K.J.; Horak, F.B. A Meta-Regression of the Long-Term Effects of Deep Brain Stimulation on Balance and Gait in PD. Neurology 2010, 75, 1292–1299. [Google Scholar] [CrossRef]
- Eisinger, R.S.; Hess, C.W.; Martinez-Ramirez, D.; Almeida, L.; Foote, K.D.; Okun, M.S.; Gunduz, A. Motor subtype changes in early Parkinson’s disease. Park. Relat. Disord. 2017, 43, 67–72. [Google Scholar]
- Giacobbe, A.; Au, K.L.K.; Nguyen, O.T.; Moore, K.; Dinh, E.; Ramirez-Zamora, A.; Okun, M.S.; Almeida, L. Geospatial Analysis of Persons with Movement Disorders Living in Underserved Regions. Tremor Other Hyperkinetic Mov. 2021, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Dall, T.M.; Storm, M.V.; Chakrabarti, R.; Drogan, O.; Keran, C.M.; Donofrio, P.D.; Henderson, V.W.; Kaminski, H.J.; Stevens, J.C.; Vidic, T.R. Supply and Demand Analysis of the Current and Future US Neurology Workforce. Neurology 2013, 81, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Evers, L.J.; Krijthe, J.H.; Meinders, M.J.; Bloem, B.R.; Heskes, T.M. Measuring Parkinson’s disease over time: The real-world within-subject reliability of the MDS-UPDRS. Mov. Disord. 2019, 34, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Regnault, A.; Boroojerdi, B.; Meunier, J.; Bani, M.; Morel, T.; Cano, S. Does the MDS-UPDRS provide the precision to assess progression in early Parkinson’s disease? Learnings from the Parkinson’s progression marker initiative cohort. J. Neurol. 2019, 266, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Johnson, A.E.; Yang, Y.; Clifford, G.D.; Clifton, D.A. Bayesian fusion of physiological measurements using a signal quality extension. Physiol. Meas. 2018, 39, 065008. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.S.; Ghahari, S.; McColl, M.A. Barriers to accessing healthcare services for people with Parkinson’s disease: A scoping review. J. Park. Dis. 2021, 11, 1537–1553. [Google Scholar] [CrossRef]
- Falconer, D.; Gow, S.; Whitney, D.; Walters, H.; Rogers, S. The Power of Access in Parkinson’s Disease Care: A Retrospective Review of Telehealth Uptake During the COVID-19 Pandemic. Front. Neurol. 2022, 13, 830196. [Google Scholar] [CrossRef]
- Lobo, V.; Branco, D.; Guerreiro, T.; Bouça-Machado, R.; Ferreira, J. Machine-Learning Models for MDS-UPDRS III Prediction: A Comparative Study of Features, Models, and Data Sources; Information Society: Ljubljana, Slovenia, 2022. [Google Scholar]
- Matias, R.; Paixão, V.; Bouça, R.; Ferreira, J.J. A perspective on wearable sensor measurements and data science for Parkinson’s disease. Front. Neurol. 2017, 8, 677. [Google Scholar] [CrossRef]
- Denk, D.; Herman, T.; Zoetewei, D.; Ginis, P.; Brozgol, M.; Cornejo Thumm, P.; Decaluwe, E.; Ganz, N.; Palmerini, L.; Giladi, N.; et al. Daily-Living Freezing of Gait as Quantified Using Wearables in People With Parkinson Disease: Comparison With Self-Report and Provocation Tests. Phys. Ther. 2022, 102, pzac129. [Google Scholar] [CrossRef]
- Brand, Y.E.; Schwartz, D.; Gazit, E.; Buchman, A.S.; Gilad-Bachrach, R.; Hausdorff, J.M. Gait detection from a wrist-worn sensor using machine learning methods: A daily living study in older adults and people with Parkinson’s disease. Sensors 2022, 22, 7094. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, M.; Jiang, X.; Shen, B.; Zhu, J.; Pan, Y.; Dong, J.; Yan, J.; Xu, P.; Zhang, W.; et al. Can Quantitative Gait Analysis Be Used to Guide Treatment of Patients with Different Subtypes of Parkinson’s Disease? Neuropsychiatr. Dis. Treat. 2020, 16, 2335. [Google Scholar] [CrossRef] [PubMed]
- Rezvanian, S.; Lockhart, T.; Frames, C.; Soangra, R.; Lieberman, A. Motor subtypes of Parkinson’s disease can be identified by frequency component of postural stability. Sensors 2018, 18, 1102. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Sun, H.; Wang, T.; Tang, M.; Bohnen, N.I.; Müller, M.; Herman, T.; Giladi, N.; Kalinin, A.; Spino, C.; et al. Model-based and model-free machine learning techniques for diagnostic prediction and classification of clinical outcomes in Parkinson’s disease. Sci. Rep. 2018, 8, 7129. [Google Scholar] [PubMed]
- Pang, H.; Yu, Z.; Yu, H.; Cao, J.; Li, Y.; Guo, M.; Cao, C.; Fan, G. Use of machine learning method on automatic classification of motor subtype of Parkinson’s disease based on multilevel indices of rs-fMRI. Park. Relat. Disord. 2021, 90, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Filtjens, B.; Ginis, P.; Nieuwboer, A.; Slaets, P.; Vanrumste, B. Automated freezing of gait assessment with marker-based motion capture and multi-stage spatial-temporal graph convolutional neural networks. J. NeuroEngineering Rehabil. 2022, 19, 48. [Google Scholar]
- Kwon, H.; Clifford, G.D.; Genias, I.; Bernhard, D.; Esper, C.D.; Factor, S.A.; McKay, J.L. An explainable spatial-temporal graphical convolutional network to score freezing of gait in parkinsonian patients. Sensors 2023, 23, 1766. [Google Scholar] [CrossRef]
- Yang, P.K.; Filtjens, B.; Ginis, P.; Goris, M.; Gilat, M.; Nieuwboer, A.; Slaets, P.; Vanrumste, B. Automated freezing of gait assessment in Parkinson’s disease using inertial measurement units and deep learning. In Proceedings of the International Society of Posture and Gait Research (ISPGR) Congress 2023, Brisbane, Australia, 9–13 July 2023. [Google Scholar]
- Diep, C.; O’Day, J.; Kehnemouyi, Y.; Burnett, G.; Bronte-Stewart, H. Gait parameters measured from wearable sensors reliably detect freezing of gait in a stepping in place task. Sensors 2021, 21, 2661. [Google Scholar] [CrossRef]
- Yungher, D.A.; Morris, T.R.; Dilda, V.; Shine, J.M.; Naismith, S.L.; Lewis, S.J.; Moore, S.T. Temporal characteristics of high-frequency lower-limb oscillation during freezing of gait in Parkinson’s disease. Park. Dis. 2014, 2014, 606427. [Google Scholar] [CrossRef]
- Rodríguez-Martín, D.; Samà, A.; Pérez-López, C.; Català, A.; Moreno Arostegui, J.M.; Cabestany, J.; Bayés, À.; Alcaine, S.; Mestre, B.; Prats, A.; et al. Home detection of freezing of gait using support vector machines through a single waist-worn triaxial accelerometer. PLoS ONE 2017, 12, e0171764. [Google Scholar]
- Huddleston, D.E.; Chen, X.; Hwang, K.; Langley, J.; Tripathi, R.; Tucker, K.; McKay, J.L.; Hu, X.; Factor, S. Neuromelanin-sensitive MRI correlates of cognitive and motor function in Parkinson disease with freezing of gait. medRxiv 2023. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.L.; Goldstein, F.C.; Sommerfeld, B.; Bernhard, D.; Perez Parra, S.; Factor, S.A. Freezing of Gait Can Persist after an Acute Levodopa Challenge in Parkinson’s Disease. NPJ Park. Dis. 2019, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Nocera, J.R.; Stegemöller, E.L.; Malaty, I.A.; Okun, M.S.; Marsiske, M.; Hass, C.J.; National Parkinson Foundation Quality Improvement Initiative Investigators. Using the Timed Up & Go test in a clinical setting to predict falling in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2013, 94, 1300–1305. [Google Scholar] [PubMed]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa I sa D ouble-E dged S word for B alance and G ait in P eople W ith P arkinson’s D isease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef]
- Kadaba, M.P.; Ramakrishnan, H.; Wootten, M. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Factor, S.A.; Steenland, N.K.; Higgins, D.S.; Molho, E.S.; Kay, D.M.; Montimurro, J.; Rosen, A.R.; Zabetian, C.P.; Payami, H. Postural instability/gait disturbance in Parkinson’s disease has distinct subtypes: An exploratory analysis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 564–568. [Google Scholar] [CrossRef]
- Bulling, A.; Blanke, U.; Schiele, B. A tutorial on human activity recognition using body-worn inertial sensors. ACM Comput. Surv. 2014, 46, 1–33. [Google Scholar]
- Bachlin, M.; Plotnik, M.; Roggen, D.; Maidan, I.; Hausdorff, J.M.; Giladi, N.; Troster, G. Wearable assistant for Parkinson’s disease patients with the freezing of gait symptom. IEEE Trans. Inf. Technol. Biomed. 2009, 14, 436–446. [Google Scholar] [CrossRef]
- Ricciardi, C.; Amboni, M.; De Santis, C.; Ricciardelli, G.; Improta, G.; Iuppariello, L.; D’Addio, G.; Barone, P.; Cesarelli, M. Classifying Different Stages of Parkinson’s Disease Through Random Forests. In Proceedings of the XV Mediterranean Conference on Medical and Biological Engineering and Computing—MEDICON 2019, Coimbra, Portugal, 26–28 September 2019; IFMBE Proceedings. Springer International Publishing: Cham, Switzerland; pp. 1155–1162. [Google Scholar] [CrossRef]
- Açıcı, K.; Erdaş, B.; Aşuroğlu, T.; Toprak, M.K.; Erdem, H.; Oğul, H. A Random Forest Method to Detect Parkinson’s Disease via Gait Analysis. In Proceedings of the Engineering Applications of Neural Networks, Athens, Greece, 25–27 August 2017; Communications in Computer and Information Science. Springer International Publishing: Cham, Switzerland; pp. 609–619. [Google Scholar] [CrossRef]
- Tahafchi, P.; Molina, R.; Roper, J.A.; Sowalsky, K.; Hass, C.J.; Gunduz, A.; Okun, M.S.; Judy, J.W. Freezing-of-Gait Detection Using Temporal, Spatial, and Physiological Features with a Support-Vector-Machine Classifier. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 2867–2870. [Google Scholar] [CrossRef]
- Shetty, S.; Rao, Y.S. SVM Based Machine Learning Approach to Identify Parkinson’s Disease Using Gait Analysis. In Proceedings of the 2016 International Conference on Inventive Computation Technologies (ICICT), Coimbatore, India, 26–27 August 2016; Volume 2, pp. 1–5. [Google Scholar] [CrossRef]
- Bakar, Z.A.; Tahir, N.M.; Yassin, I.M. Classification of Parkinson’s Disease Based on Multilayer Perceptrons Neural Network. In Proceedings of the 2010 6th International Colloquium on Signal Processing & Its Applications, Malacca, Malaysia, 21–23 May 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Challa, K.N.R.; Pagolu, V.S.; Panda, G.; Majhi, B. An Improved Approach for Prediction of Parkinson’s Disease Using Machine Learning Techniques. In Proceedings of the 2016 International Conference on Signal Processing, Communication, Power and Embedded System (SCOPES), Paralakhemundi, India, 3–5 October 2016; pp. 1446–1451. [Google Scholar] [CrossRef]
- Wan, S.; Liang, Y.; Zhang, Y.; Guizani, M. Deep Multi-Layer Perceptron Classifier for Behavior Analysis to Estimate Parkinson’s Disease Severity Using Smartphones. IEEE Access 2018, 6, 36825–36833. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, W.; Yao, Y.; Bint Ahmed, J.; Tan, Y.; Gu, D. Prediction of freezing of gait in patients with Parkinson’s disease by identifying impaired gait patterns. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 591–600. [Google Scholar] [CrossRef]
- Naghavi, N.; Wade, E. Prediction of freezing of gait in Parkinson’s disease using statistical inference and lower–limb acceleration data. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 947–955. [Google Scholar] [PubMed]
- Palmerini, L.; Rocchi, L.; Mazilu, S.; Gazit, E.; Hausdorff, J.M.; Chiari, L. Identification of characteristic motor patterns preceding freezing of gait in Parkinson’s disease using wearable sensors. Front. Neurol. 2017, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.T.; MacDougall, H.G.; Ondo, W.G. Ambulatory monitoring of freezing of gait in Parkinson’s disease. J. Neurosci. Methods 2008, 167, 340–348. [Google Scholar] [PubMed]
- Baby, M.S.; Saji, A.; Kumar, C.S. Parkinsons disease classification using wavelet transform based feature extraction of gait data. In Proceedings of the 2017 International Conference on Circuit, Power and Computing Technologies (ICCPCT), Kollam, India, 20–21 April 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–6. [Google Scholar]
- Hammerla, N.Y.; Plötz, T. Let’s (not) stick together: Pairwise similarity biases cross-validation in activity recognition. In Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing, Osaka, Japan, 7–11 September 2015; pp. 1041–1051. [Google Scholar]
- Stone, M. Cross-Validatory Choice and Assessment of Statistical Predictions. J. R. Stat. Soc. Ser. B 1974, 36, 111–147. [Google Scholar] [CrossRef]
- Wilson, E.B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 1927, 22, 209–212. [Google Scholar]
- McLean, R.A.; Sanders, W.L.; Stroup, W.W. A unified approach to mixed linear models. Am. Stat. 1991, 45, 54–64. [Google Scholar]
- Louppe, G.; Wehenkel, L.; Sutera, A.; Geurts, P. Understanding Variable Importances in Forests of Randomized Trees. In Advances in Neural Information Processing Systems; Curran Associates, Inc.: Red Hook, NJ, USA, 2013; Volume 26. [Google Scholar]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar]
- Sarafianos, N.; Boteanu, B.; Ionescu, B.; Kakadiaris, I.A. 3D Human Pose Estimation: A Review of the Literature and Analysis of Covariates. Comput. Vis. Image Underst. 2016, 152, 1–20. [Google Scholar] [CrossRef]
- Kaur, R.; Motl, R.W.; Sowers, R.; Hernandez, M.E. A Vision-Based Framework for Predicting Multiple Sclerosis and Parkinson’s Disease Gait Dysfunctions-A Deep Learning Approach. IEEE J. Biomed. Heal. Inform. 2022, 27, 190–201. [Google Scholar] [CrossRef]
- Längkvist, M.; Karlsson, L.; Loutfi, A. A Review of Unsupervised Feature Learning and Deep Learning for Time-Series Modeling. Pattern Recognit. Lett. 2014, 42, 11–24. [Google Scholar] [CrossRef]
- Von Coelln, R.; Gruber-Baldini, A.; Reich, S.; Armstrong, M.; Savitt, J.; Shulman, L. The Inconsistency and Instability of Parkinson’s Disease Motor Subtypes. Park. Relat. Disord. 2021, 88, 13–18. [Google Scholar] [CrossRef]
- Simuni, T.; Caspell-Garcia, C.; Coffey, C.; Lasch, S.; Tanner, C.; Marek, K.; PPMI Investigators. How Stable Are Parkinson’s Disease Subtypes in de Novo Patients: Analysis of the PPMI Cohort? Park. Relat. Disord. 2016, 28, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kotagal, V. Is PIGD a Legitimate Motor Subtype in Parkinson Disease? Ann. Clin. Transl. Neurol. 2016, 3, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Kim, Y.E.; Yun, J.Y.; Kim, H.J.; Jeon, B.S. Identifying the Clusters within Nonmotor Manifestations in Early Parkinson’s Disease by Using Unsupervised Cluster Analysis. PLOS ONE 2014, 9, e91906. [Google Scholar] [CrossRef]
- Salmanpour, M.R.; Shamsaei, M.; Rahmim, A. Feature Selection and Machine Learning Methods for Optimal Identification and Prediction of Subtypes in Parkinson’s Disease. Comput. Methods Programs Biomed. 2021, 206, 106131. [Google Scholar] [CrossRef]
- Albrecht, F.; Poulakis, K.; Freidle, M.; Johansson, H.; Ekman, U.; Volpe, G.; Westman, E.; Pereira, J.B.; Franzén, E. Unraveling Parkinson’s Disease Heterogeneity Using Subtypes Based on Multimodal Data. Park. Relat. Disord. 2022, 102, 19–29. [Google Scholar] [CrossRef] [PubMed]

| PD-FOG | PD-NoFOG | PP-FOG | |
|---|---|---|---|
| N | 35 | 17 | 5 |
| Age, y | 69 ± 7 | 67 ± 12 | 66 ± 6 |
| Sex | |||
| Male | 30 | 11 | 2 |
| Female | 5 | 6 | 3 |
| Disease duration, years | 10.5 ± 6.7 | 6.0 ± 3.6 | 6.0 ± 3.3 |
| LED, mg | 1429 ± 673 | 833 ± 303 | 1258 ± 640 |
| MDS-UPDRS-III (OFF) | 34.0 ± 10.6 | 30.8 ± 13.2 | 39.4 ± 7.8 |
| Hoehn and Yahr Stage (OFF) | |||
| II | 24 | 16 | 3 |
| III | 6 | 1 | 1 |
| IV | 6 | 2 | |
| NFOG-Q | 20.1 ± 4.9 | 0.0 ± 0.0 | 17.8 ± 7.5 |
| PD Subtype | FOG Scores | Total | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| PIGD | 9 | 6 | 13 | 9 | 7 | 44 |
| TD | 10 | 1 | 11 | |||
| Ind. | 1 | 1 | ||||
| Features | Testing Session | FOG Included | Mean F1 Score [%] | ||
|---|---|---|---|---|---|
| RF | rbfSVM | MLP | |||
| Gait | Walk-Thru | Yes | 61.0 ± 6.0 | 63.9 ± 6.0 | 53.0 ± 6.2 |
| No | 59.5 ± 6.1 | 57.8 ± 6.1 | 64.2 ± 5.9 | ||
| Kinematic | Walk-Thru | Yes | 66.5 ± 6.4 | 66.0 ± 6.4 | 62.8 ± 6.5 |
| No | 62.4 ± 6.6 | 79.6 ± 5.5 | 72.9 ± 6.1 | ||
| Kinematic | Turn-360° | Yes | 67.3 ± 6.1 | 77.6 ± 5.4 | 69.9 ± 6.0 |
| No | 71.3 ± 6.2 | 70.5 ± 6.2 | 64.4 ± 6.5 | ||
| Kinematic | TUG | Yes | 64.1 ± 6.4 | 69.1 ± 6.1 | 63.5 ± 6.4 |
| No | 66.3 ± 6.5 | 72.1 ± 6.2 | 68.4 ± 6.4 | ||
| Features | Testing Session | FOG Included | Mean F1 Score [%] | ||
|---|---|---|---|---|---|
| RF | rbfSVM | MLP | |||
| Kinematic | Walk-Thru | Yes | 91.9 ± 3.3 | 92.6 ± 3.1 | 75.7 ± 5.3 |
| No | 94.1 ± 2.8 | 89.9 ± 3.7 | 81.3 ± 4.8 | ||
| Kinematic | Turn-360° | Yes | 70.9 ± 5.6 | 86.0 ± 4.2 | 51.7 ± 6.1 |
| No | 92.3 ± 3.2 | 89.3 ± 3.8 | 77.5 ± 5.1 | ||
| Kinematic | TUG | Yes | 88.7 ± 3.8 | 74.7 ± 5.3 | 84.5 ± 4.4 |
| No | 95.4 ± 2.5 | 88.8 ± 3.8 | 92.0 ± 3.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, N.J.; Clifford, G.D.; Esper, C.D.; Factor, S.A.; McKay, J.L.; Kwon, H. Classifying Tremor Dominant and Postural Instability and Gait Difficulty Subtypes of Parkinson’s Disease from Full-Body Kinematics. Sensors 2023, 23, 8330. https://doi.org/10.3390/s23198330
Gong NJ, Clifford GD, Esper CD, Factor SA, McKay JL, Kwon H. Classifying Tremor Dominant and Postural Instability and Gait Difficulty Subtypes of Parkinson’s Disease from Full-Body Kinematics. Sensors. 2023; 23(19):8330. https://doi.org/10.3390/s23198330
Chicago/Turabian StyleGong, N. Jabin, Gari D. Clifford, Christine D. Esper, Stewart A. Factor, J. Lucas McKay, and Hyeokhyen Kwon. 2023. "Classifying Tremor Dominant and Postural Instability and Gait Difficulty Subtypes of Parkinson’s Disease from Full-Body Kinematics" Sensors 23, no. 19: 8330. https://doi.org/10.3390/s23198330
APA StyleGong, N. J., Clifford, G. D., Esper, C. D., Factor, S. A., McKay, J. L., & Kwon, H. (2023). Classifying Tremor Dominant and Postural Instability and Gait Difficulty Subtypes of Parkinson’s Disease from Full-Body Kinematics. Sensors, 23(19), 8330. https://doi.org/10.3390/s23198330






