The Effect of Using a Rehabilitation Robot for Patients with Post-Coronavirus Disease (COVID-19) Fatigue Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garg, M.; Maralakunte, M.; Garg, S.; Dhooria, S.; Sehgal, I.; Bhalla, A.S.; Vijayvergiya, R.; Grover, S.; Bhatia, V.; Jagia, P.; et al. The Conundrum of ‘Long-COVID-19’: A Narrative Review. Int. J. Gen. Med. 2021, 14, 2491–2506. [Google Scholar] [CrossRef] [PubMed]
- Kovačić, D.; Jotanović, J.; Laković, J. The possible role of molecular mimicry in SARS-CoV-2-mediated autoimmunity: An immunobiochemical basis. JMS 2021, 90, e560. [Google Scholar] [CrossRef]
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Abrahao-Machado, L.F.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar] [PubMed]
- Zasadzka, E.; Tobis, S.; Trzmiel, T.; Marchewka, R.; Kozak, D.; Roksela, A.; Pieczyńska, A.; Hojan, K. Application of an EMG-Rehabilitation Robot in Patients with Post-Coronavirus Fatigue Syndrome (COVID-19)-A Feasibility Study. Int. J. Environ. Res. Public Health 2022, 19, 10398. [Google Scholar] [CrossRef] [PubMed]
- Vink, M.; Vink-Niese, A. Could Cognitive Behavioural Therapy Be an Effective Treatment for Long COVID and Post COVID-19 Fatigue Syndrome? Lessons from the Qure Study for Q-Fever Fatigue Syndrome. Healthcare 2020, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Tansey, C.M.; Louie, M.; Loeb, M.; Gold, W.L.; Muller, M.P.; de Jager, J.; Cameron, J.I.; Tomlinson, G.; Mazzulli, T.; Walmsley, S.L.; et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch. Intern. Med. 2007, 167, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Diagnostic and Pharmacological Potency of Creatine in Post-Viral Fatigue Syndrome. Nutrients 2021, 13, 503. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing the Longterm Effects of COVID-19. Available online: https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742 (accessed on 29 March 2023).
- Wostyn, P. COVID-19 and chronic fatigue syndrome: Is the worst yet to come? Med. Hypotheses 2021, 146, 110469. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Nagy, E.N.; Elimy, D.A.; Ali, A.Y.; Ezzelregal, H.G.; Elsayed, M.M. Influence of Manual Diaphragm Release Technique Combined with Inspiratory Muscle Training on Selected Persistent Symptoms in Men with Post-COVID-19 Syndrome: A Randomized Controlled Trial. J. Rehabil. Med. 2022, 54, jrm00330. [Google Scholar] [CrossRef] [PubMed]
- Araújo, B.T.S.; Barros, A.E.V.R.; Nunes, D.T.X.; Remígio de Aguiar, M.I.; Mastroianni, V.W.; de Souza, J.A.F.; Fernades, J.; Campos, S.L.; Brandão, D.C.; Dornelas de Andrade, A. Effects of continuous aerobic training associated with resistance training on maximal and submaximal exercise tolerance, fatigue, and quality of life of patients post-COVID-19. Physiother. Res. Int. 2023, 28, e1972. [Google Scholar] [CrossRef] [PubMed]
- Nopp, S.; Moik, F.; Klok, F.A.; Gattinger, D.; Petrovic, M.; Vonbank, K.; Koczulla, A.R.; Ay, C.; Zwick, R.H. Outpatient Pulmonary Rehabilitation in Patients with Long COVID Improves Exercise Capacity, Functional Status, Dyspnea, Fatigue, and Quality of Life. Respiration 2022, 101, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Swarnakar, R.; Yadav, S.L. Rehabilitation in long COVID-19: A mini-review. World J. Methodol. 2022, 12, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Kim, H.J.; Kwon, B.S.; Park, J.-W.; Lee, H.J.; Yoo, A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Carod-Artal, F.J. Síndrome post-COVID-19: Epidemiología, criterios diagnósticos y mecanismos patogénicos implicados. Rev. Neurol. 2021, 72, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Steffen, T.M.; Hacker, T.A.; Mollinger, L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys. Ther. 2002, 82, 128–137. [Google Scholar] [CrossRef]
- Raîche, M.; Hébert, R.; Prince, F.; Corriveau, H. Screening older adults at risk of falling with the Tinetti balance scale. Lancet 2000, 356, 1001–1002. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- MacDermid, J.; Solomon, G.; Valdes, K. Clinical Assessment Recommendations, 3rd ed.; American Society of Hand Therapists: Mount Laurel, NJ, USA, 2015; ISBN 9780692525159. [Google Scholar]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Linacre, J.M.; Heinemann, A.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The structure and stability of the functional independence measure. Arch. Phys. Med. Rehabil. 1994, 75, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Pezzola, A.; Urbano, M.; Guglielmelli, E. Assessing Effectiveness and Costs in Robot-Mediated Lower Limbs Rehabilitation: A Meta-Analysis and State of the Art. J. Healthc. Eng. 2018, 2018, 7492024. [Google Scholar] [CrossRef] [PubMed]
- Toots, A.; Littbrand, H.; Lindelöf, N.; Wiklund, R.; Holmberg, H.; Nordström, P.; Lundin-Olsson, L.; Gustafson, Y.; Rosendahl, E. Effects of a High-Intensity Functional Exercise Program on Dependence in Activities of Daily Living and Balance in Older Adults with Dementia. J. Am. Geriatr. Soc. 2016, 64, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Villafañe, J.H.; Taveggia, G.; Galeri, S.; Bissolotti, L.; Mullè, C.; Imperio, G.; Valdes, K.; Borboni, A.; Negrini, S. Efficacy of Short-Term Robot-Assisted Rehabilitation in Patients With Hand Paralysis After Stroke: A Randomized Clinical Trial. Hand 2018, 13, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mayr, A.; Quirbach, E.; Picelli, A.; Kofler, M.; Smania, N.; Saltuari, L. Early robot-assisted gait retraining in non-ambulatory patients with stroke: A single blind randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, H.; Bosomworth, H.; Krebs, H.I.; van Wijck, F.; Howel, D.; Wilson, N.; Aird, L.; Alvarado, N.; Andole, S.; Cohen, D.L.; et al. Robot assisted training for the upper limb after stroke (RATULS): A multicentre randomised controlled trial. Lancet 2019, 394, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bustamante Valles, K.; Montes, S.; Madrigal, M.d.J.; Burciaga, A.; Martínez, M.E.; Johnson, M.J. Technology-assisted stroke rehabilitation in Mexico: A pilot randomized trial comparing traditional therapy to circuit training in a Robot/technology-assisted therapy gym. J. Neuroeng. Rehabil. 2016, 13, 83. [Google Scholar] [CrossRef]
- Dehem, S.; Gilliaux, M.; Stoquart, G.; Detrembleur, C.; Jacquemin, G.; Palumbo, S.; Frederick, A.; Lejeune, T. Effectiveness of upper-limb robotic-assisted therapy in the early rehabilitation phase after stroke: A single-blind, randomised, controlled trial. Ann. Phys. Rehabil. Med. 2019, 62, 313–320. [Google Scholar] [CrossRef]
- Daunoraviciene, K.; Adomaviciene, A.; Grigonyte, A.; Griškevičius, J.; Juocevicius, A. Effects of robot-assisted training on upper limb functional recovery during the rehabilitation of poststroke patients. Technol. Health Care 2018, 26, 533–542. [Google Scholar] [CrossRef]
- Kawasaki, S.; Ohata, K.; Yoshida, T.; Yokoyama, A.; Yamada, S. Gait improvements by assisting hip movements with the robot in children with cerebral palsy: A pilot randomized controlled trial. J. Neuroeng. Rehabil. 2020, 17, 87. [Google Scholar] [CrossRef]
- Pool, D.; Valentine, J.; Taylor, N.F.; Bear, N.; Elliott, C. Locomotor and robotic assistive gait training for children with cerebral palsy. Dev. Med. Child Neurol. 2021, 63, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, M.A.; Öneş, K.; Gökşenoğlu, G. Early term effects of robotic assisted gait training on ambulation and functional capacity in patients with spinal cord injury. Turk. J. Med. Sci. 2019, 49, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Hertz, N.; Wiese, E. Good advice is beyond all price, but what if it comes from a machine? J. Exp. Psychol. Appl. 2019, 25, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Tobis, S.; Piasek, J.; Cylkowska-Nowak, M.; Suwalska, A. Robots in Eldercare: How Does a Real-World Interaction with the Machine Influence the Perceptions of Older People? Sensors 2022, 22, 1717. [Google Scholar] [CrossRef] [PubMed]
- Lennon, O.; Ryan, C.; Helm, M.; Moore, K.; Sheridan, A.; Probst, M.; Cunningham, C. Psychological Distress among Patients Attending Physiotherapy: A Survey-Based Investigation of Irish Physiotherapists’ Current Practice and Opinions. Physiother. Can. 2020, 72, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Bastemeijer, C.M.; van Ewijk, J.P.; Hazelzet, J.A.; Voogt, L.P. Patient values in physiotherapy practice, a qualitative study. Physiother. Res. Int. 2021, 26, e1877. [Google Scholar] [CrossRef] [PubMed]
- Patsaki, I.; Gerovasili, V.; Sidiras, G.; Karatzanos, E.; Mitsiou, G.; Papadopoulos, E.; Christakou, A.; Routsi, C.; Kotanidou, A.; Nanas, S. Effect of neuromuscular stimulation and individualized rehabilitation on muscle strength in Intensive Care Unit survivors: A randomized trial. J. Crit. Care 2017, 40, 76–82. [Google Scholar] [CrossRef]



| Intervention Group n = 42 | Control Group n = 39 | p-Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 67.38 | 8.46 | 64.92 | 11.74 | 0.280 |
| Height | 169.86 | 9.00 | 170.95 | 9.31 | 0.593 |
| Weight | 81.55 | 12.89 | 75.21 | 13.97 | 0.036 |
| BMI | 28.19 | 3.52 | 25.64 | 3.89 | 0.003 |
| FIM | 77.71 | 21.63 | 83.59 | 24.14 | 0.138 |
| Berg | 27.67 | 15.16 | 30.28 | 16.08 | 0.303 |
| HGS | 13.20 | 10.40 | 15.69 | 11.29 | 0.359 |
| Tinetti | 12.81 | 8.32 | 15.03 | 7.78 | 0.223 |
| 6MW | 115.81 | 112.09 | 134.41 | 121.36 | 0.491 |
| Barthel | 10.48 | 3.97 | 11.00 | 3.10 | 0.762 |
| Mean flexion strength | 5.04 | 4.77 | 5.91 | 5.30 | 0.105 |
| Peak flexion strength | 16.22 | 10.35 | 18.40 | 12.34 | 0.397 |
| Mean extension strength | 4.75 | 3.83 | 5.95 | 4.51 | 0.157 |
| Peak extension strength | 15.31 | 9.11 | 16.65 | 10.52 | 0.674 |
| Intervention Group n = 42 | Control Group n = 39 | ANOVA p-Value | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time | Group | Group * Time | |
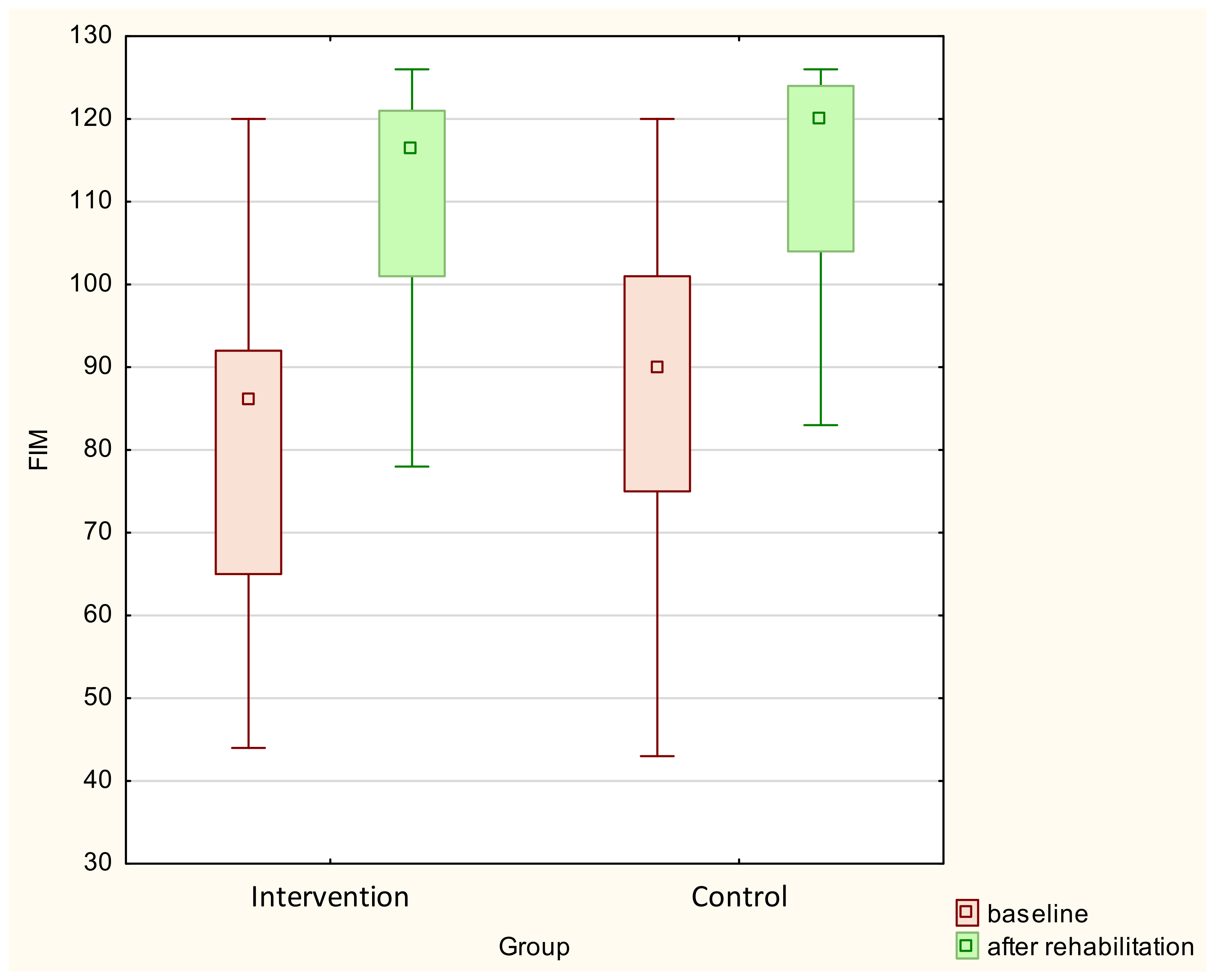
| FIM | 79.76 ± 18.32 | 110.60 ± 13.64 | 84.13 ± 24.15 | 108.62 ± 25.09 | <0.001 | 0.78 | 0.076 |
| FIM self-care | 25.95 ± 8.68 | 37.24 ± 5.53 | 28.31 ± 10.55 | 36.31 ± 9.00 | <0.001 | 0.679 | 0.055 |
| FIM sphincter control | 9.48 ± 2.94 | 12.71 ± 1.98 | 10.03 ± 3.33 | 12.38 ± 3.11 | <0.001 | 0.851 | 0.097 |
| FIM transverse | 11.43 ± 4.59 | 18.33 ± 3.27 | 12.44 ± 5.83 | 18.05 ± 4.57 | <0.001 | 0.434 | 0.325 |
| FIM locomotion | 5.45 ± 3.08 | 10.74 ± 2.72 | 6.26 ± 3.79 | 10.95 ± 3.15 | <0.001 | 0.699 | 0.142 |
| FIM motor subscale | 52.31 ± 17.07 | 79.02 ± 12.30 | 57.03 ± 20.83 | 77.69 ± 19.22 | <0.001 | 0.64 | 0.047 |
| FIM communication | 11.57 ± 2.34 | 13.10 ± 1.28 | 11.33 ± 2.31 | 12.87 ± 2.26 | <0.001 | 0.57 | 0.975 |
| FIM social cognition | 15.88 ± 3.15 | 18.48 ± 2.23 | 15.77 ± 3.84 | 18.05 ± 4.39 | <0.001 | 0.69 | 0.649 |
| FIM cognition subscale | 27.45 ± 5.07 | 31.57 ± 3.34 | 27.10 ± 5.83 | 30.92 ± 6.59 | <0.001 | 0.638 | 0.778 |
| Berg | 27.67 ± 15.16 | 44.57 ± 12.83 | 30.28 ± 16.08 | 45.18 ± 14.25 | <0.001 | 0.592 | 0.412 |
| HGS | 13.20 ± 10.40 | 18.08 ± 11.12 | 15.69 ± 11.29 | 20.22 ± 12.06 | <0.001 | 0.342 | 0.768 |
| Tinetti | 12.81 ± 8.32 | 22.38 ± 5.89 | 15.03 ± 7.78 | 23.00 ± 5.91 | <0.001 | 0.324 | 0.225 |
| 6MW | 115.81 ± 112.09 | 240.02 ± 123.58 | 134.41 ± 121.36 | 256.69 ± 123.94 | <0.001 | 0.483 | 0.919 |
| Barthel | 10.48 ± 3.97 | 17.95 ± 2.38 | 11.00 ± 3.10 | 17.44 ± 4.23 | <0.001 | 0.996 | 0.123 |
| Mean flexion strength | 5.04 ± 4.77 | 7.42 ± 5.64 | 5.91 ± 5.30 | 6.97 ± 4.94 | 0.003 | 0.838 | 0.233 |
| Peak flexion strength | 16.22 ± 10.35 | 21.83 ± 11.52 | 18.40 ± 12.34 | 20.90 ± 11.16 | <0.001 | 0.79 | 0.116 |
| Mean extension strength | 4.75 ± 3.83 | 7.36 ± 4.73 | 5.95 ± 4.51 | 16.65 ± 10.52 | <0.001 | 0.633 | 0.118 |
| Peak extension strength | 15.31 ± 9.11 | 30.39 ± 26.08 | 6.99 ± 4.91 | 35.18 ± 29.92 | <0.001 | 0.391 | 0.572 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzmiel, T.; Marchewka, R.; Pieczyńska, A.; Zasadzka, E.; Zubrycki, I.; Kozak, D.; Mikulski, M.; Poświata, A.; Tobis, S.; Hojan, K. The Effect of Using a Rehabilitation Robot for Patients with Post-Coronavirus Disease (COVID-19) Fatigue Syndrome. Sensors 2023, 23, 8120. https://doi.org/10.3390/s23198120
Trzmiel T, Marchewka R, Pieczyńska A, Zasadzka E, Zubrycki I, Kozak D, Mikulski M, Poświata A, Tobis S, Hojan K. The Effect of Using a Rehabilitation Robot for Patients with Post-Coronavirus Disease (COVID-19) Fatigue Syndrome. Sensors. 2023; 23(19):8120. https://doi.org/10.3390/s23198120
Chicago/Turabian StyleTrzmiel, Tomasz, Renata Marchewka, Anna Pieczyńska, Ewa Zasadzka, Igor Zubrycki, Dominika Kozak, Michał Mikulski, Anna Poświata, Sławomir Tobis, and Katarzyna Hojan. 2023. "The Effect of Using a Rehabilitation Robot for Patients with Post-Coronavirus Disease (COVID-19) Fatigue Syndrome" Sensors 23, no. 19: 8120. https://doi.org/10.3390/s23198120
APA StyleTrzmiel, T., Marchewka, R., Pieczyńska, A., Zasadzka, E., Zubrycki, I., Kozak, D., Mikulski, M., Poświata, A., Tobis, S., & Hojan, K. (2023). The Effect of Using a Rehabilitation Robot for Patients with Post-Coronavirus Disease (COVID-19) Fatigue Syndrome. Sensors, 23(19), 8120. https://doi.org/10.3390/s23198120







