A Highly Sensitive and Selective Optical Sensor for the On-Line Detection of Cesium in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Salts
2.2. Spectroscopic Measurements
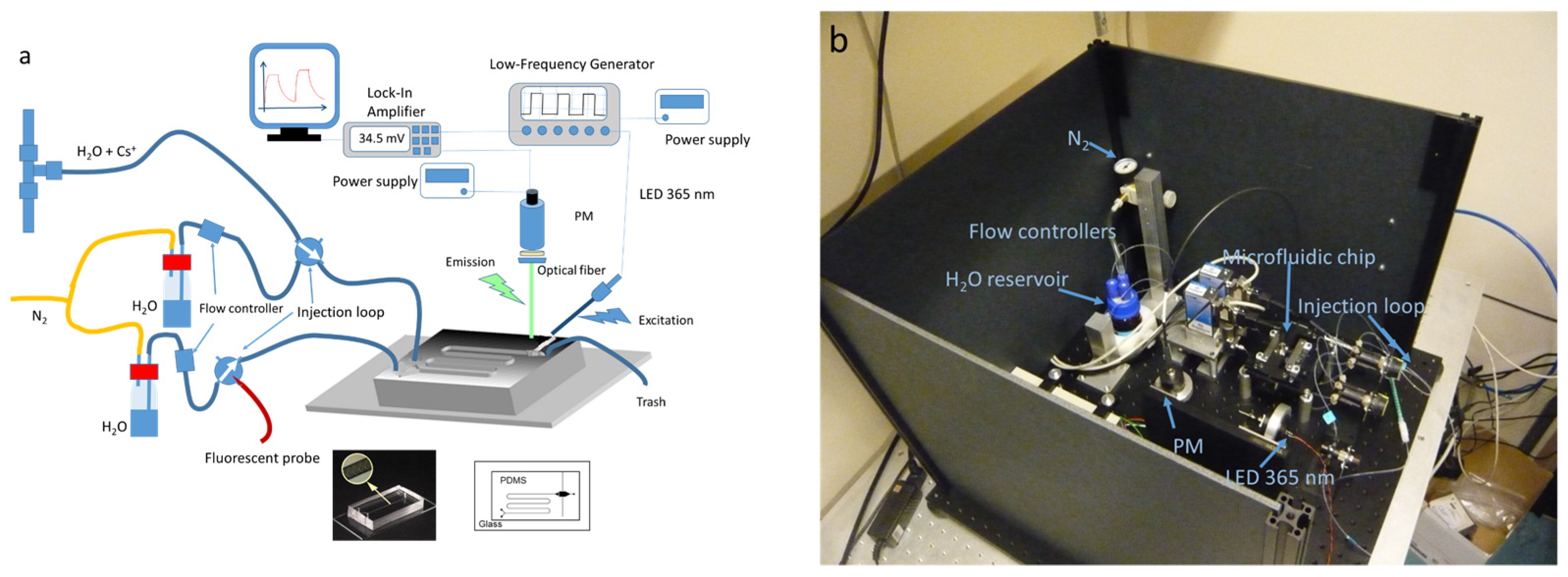
2.3. Microfluidic Device
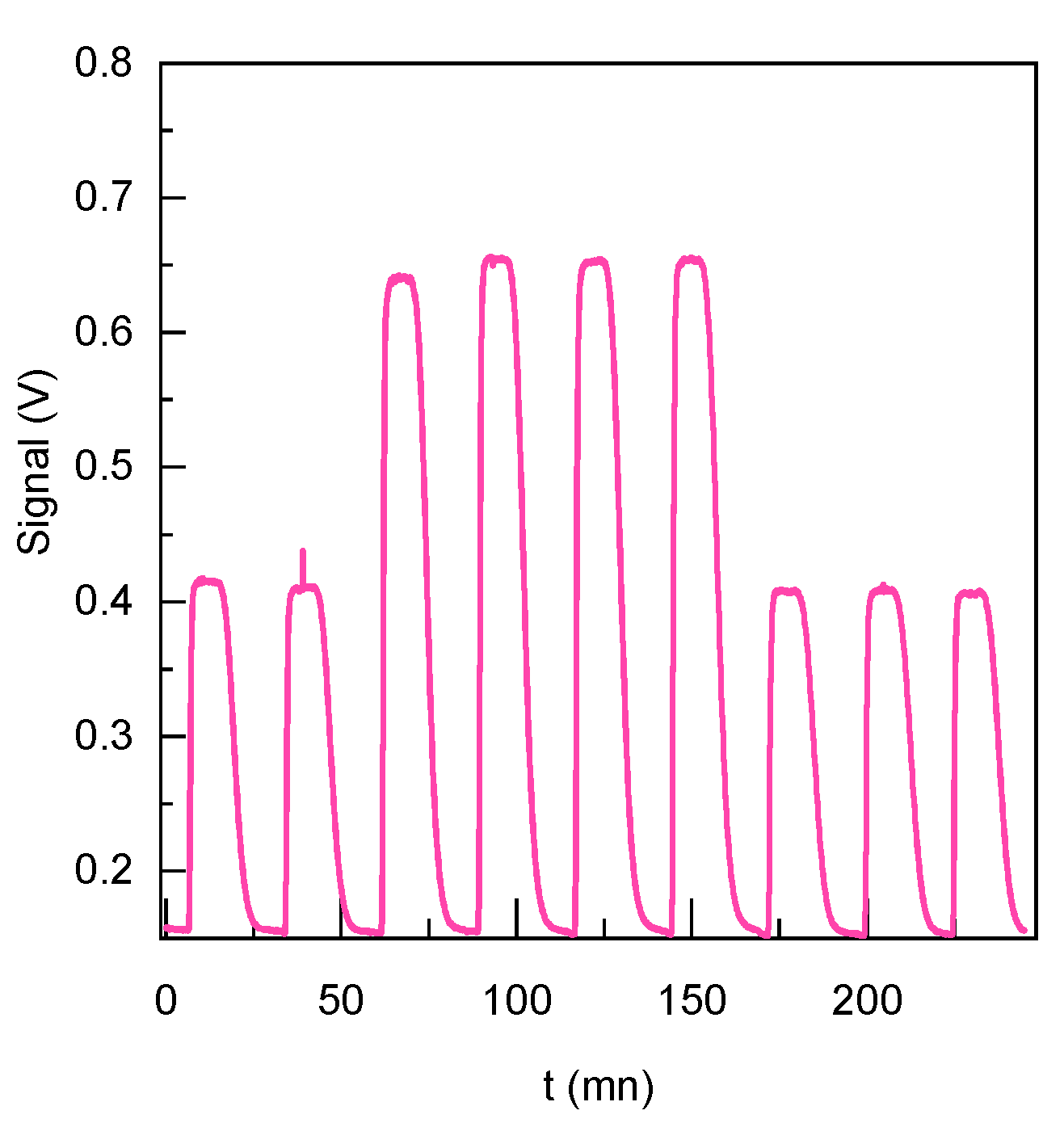
3. Results and Discussion
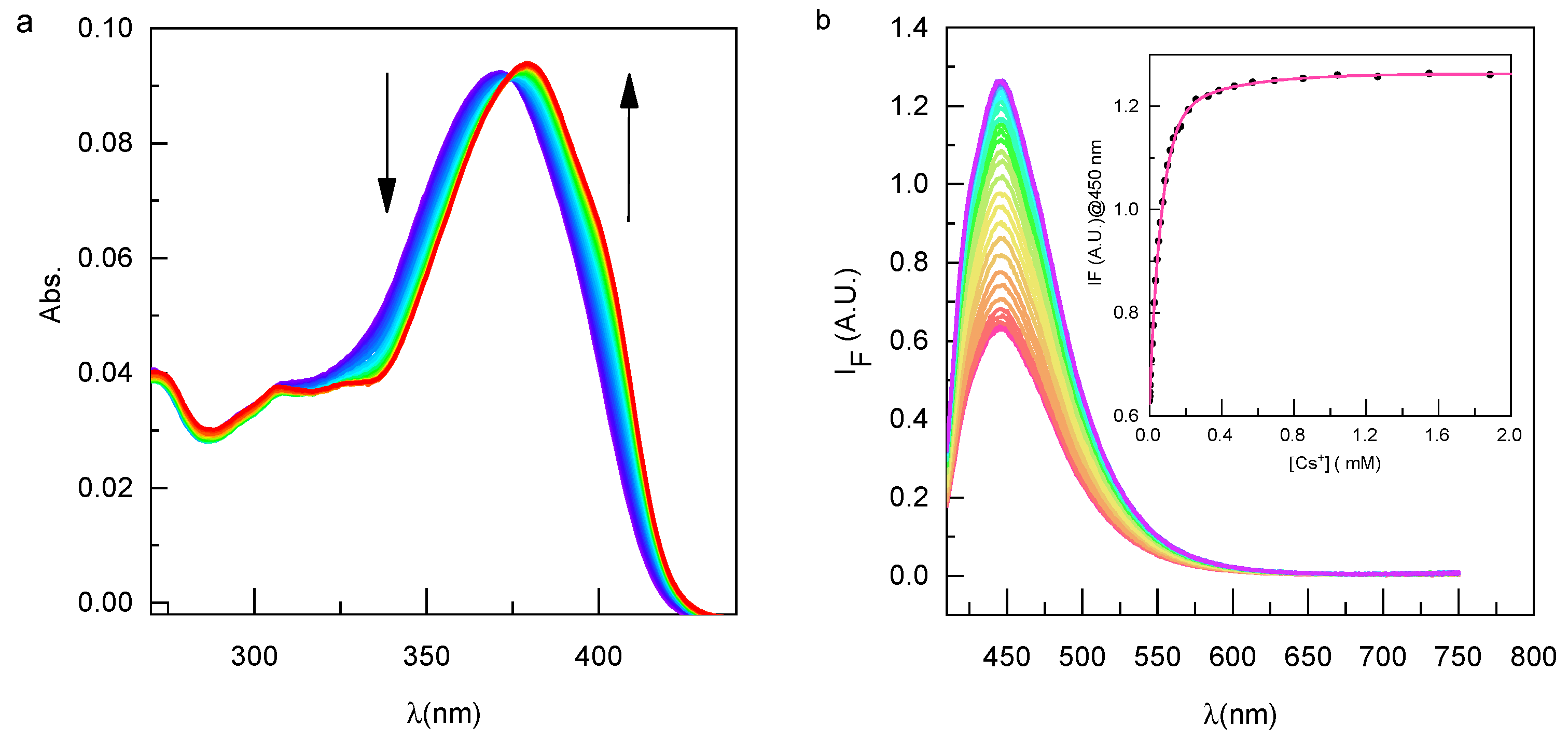
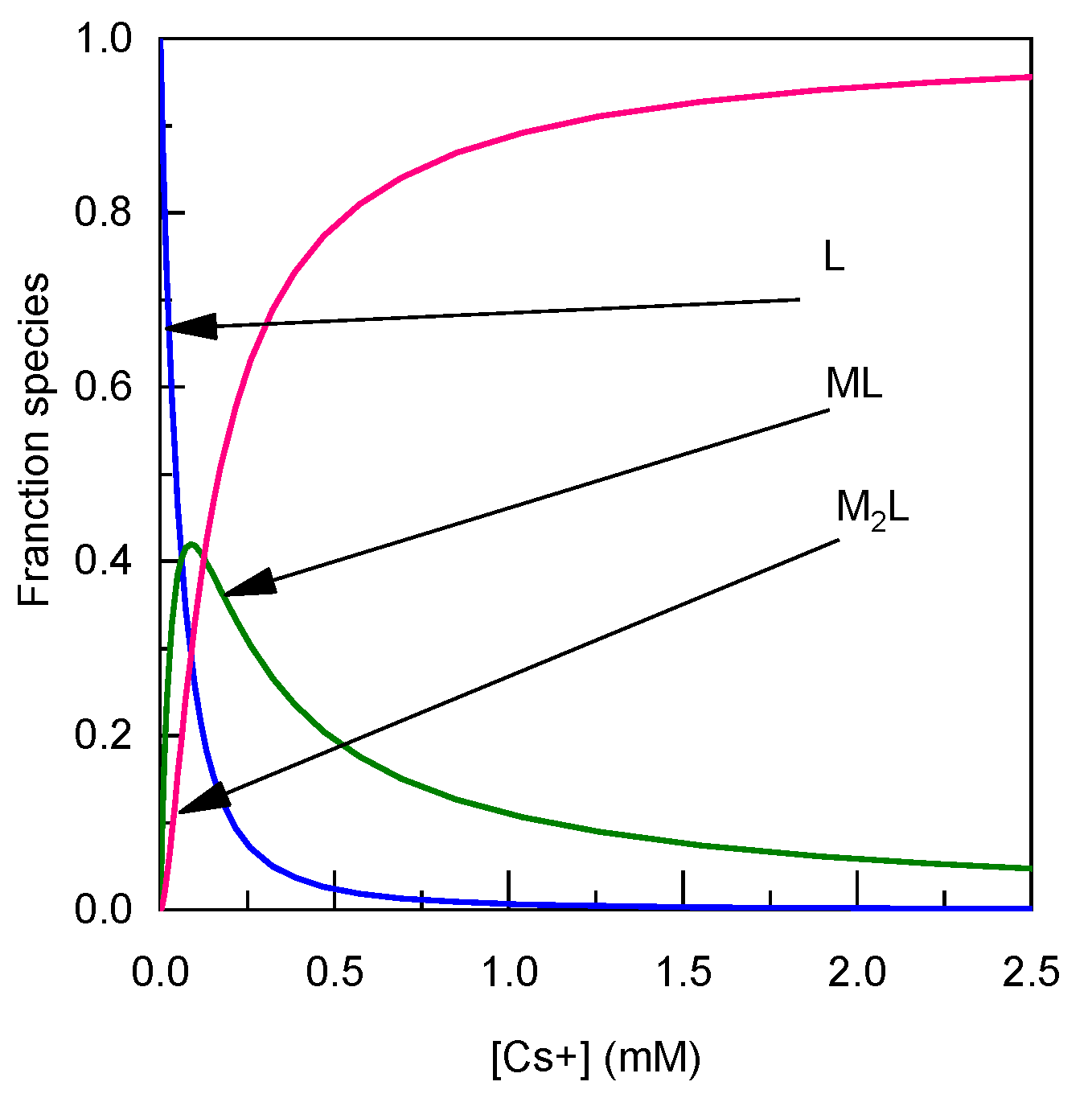
3.1. Complexing Properties with Cesium
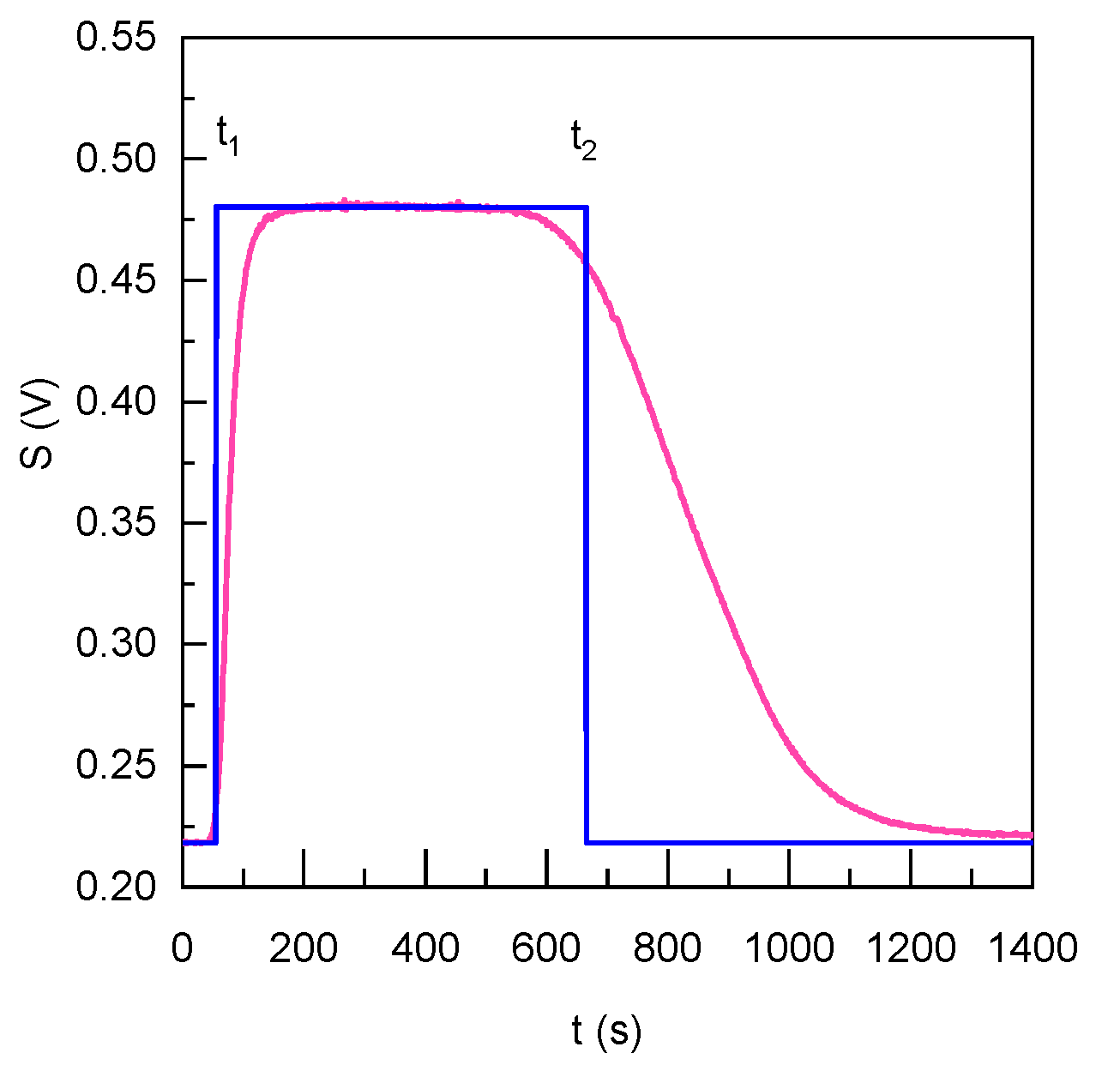
3.2. Microfluidic Device
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turekian, K.K.; Wedepohl, K.H. Distribution of the Elements in Some Major Units of the Earth’s Crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, D.; Li, X.; Zhong, J.; Xu, X. In Situ Crystallization Synthesis of CsPbBr(3) Perovskite Quantum Dot-Embedded Glasses with Improved Stability for Solid-State Lighting and Random Upconverted Lasing. ACS Appl. Mater. Interfaces 2018, 10, 18918–18926. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.K.; Phillips, W.D. Ultracold atoms and precise time standards. Philos Trans. A Math Phys. Eng. Sci. 2011, 369, 4078–4089. [Google Scholar] [CrossRef]
- Murphy, M.K.; Piper, R.K.; Greenwood, L.R.; Mitch, M.G.; Lamperti, P.J.; Seltzer, S.M.; Bales, M.J.; Phillips, M.H. Evaluation of the new cesium-131 seed for use in low-energy x-ray brachytherapy. Med. Phys. 2004, 31, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, P.; Zanoni, L.Z. Clinical effects of cesium intake. Biol. Trace Elem. Res. 2010, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Svane, M.; Hagström, M.; Davidsson, K.O.; Boman, J.; Pettersson, J.B.C. Cesium as a Tracer for Alkali Processes in a Circulating Fluidized Bed Reactor. Energy Fuels 2006, 20, 979–985. [Google Scholar] [CrossRef]
- Groll, H.; Schnürer-Patschan, C.; Kuritsyni, Y.; Niemax, K. Wavelength modulation diode laser atomic absorption spectrometry in analytical flames. Spectrochim. Acta Part B At. Spectrosc. 1994, 49, 1463–1472. [Google Scholar] [CrossRef]
- Ohno, T.; Muramatsu, Y. Determination of radioactive cesium isotope ratios by triple quadrupole ICP-MS and its application to rainwater following the Fukushima Daiichi Nuclear Power Plant accident. J. Anal. At. Spectrom. 2014, 29, 347–351. [Google Scholar] [CrossRef]
- Thornton, B.; Ohnishi, S.; Ura, T.; Odano, N.; Fujita, T. Continuous measurement of radionuclide distribution off Fukushima using a towed sea-bed gamma ray spectrometer. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2013, 79, 10–19. [Google Scholar] [CrossRef]
- Cyniak, J.S.; Kocobolska, L.; Bojdecka, N.; Gajda-Walczak, A.; Kowalczyk, A.; Wagner, B.; Nowicka, A.M.; Sakurai, H.; Kasprzak, A. Synthesis of pi-extended and bowl-shaped sumanene-ferrocene conjugates and their application in highly selective and sensitive cesium cations electrochemical sensors. Dalton Trans. 2023, 52, 3137–3147. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, L.; Wang, S.L.; Yuan, W.L.; Zhang, G.H.; Zhu, Q.H.; Chen, H.; He, L.; Tao, G.H. Ultralow-cost portable device for cesium detection via perovskite fluorescence. J. Hazard. Mater. 2022, 425, 127981. [Google Scholar] [CrossRef] [PubMed]
- Joffrion, J.B.; Mills, D.; Clower, W.; Wilson, C.G. On-Chip Microplasmas for the Detection of Radioactive Cesium Contamination in Seawater. Micromachines 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Fu, L.; Wang, H.; Zou, R.; Zhang, Y.; Li, X.; Wu, A. Colorimetric detection of Cs+ based on the nonmorphological transition mechanism of gold nanoparticles in the presence of Prussian blue. New J. Chem. 2020, 44, 2241–2246. [Google Scholar] [CrossRef]
- Nguy, T.P.; Kilinc, V.; Hayakawa, R.; Henry-de-Villeneuve, C.; Raimundo, J.-M.; Wakayama, Y.; Charrier, A. Affinity driven ion exchange EG-OFET sensor for high selectivity and low limit of detection of cesium in seawater. Sens. Actuators B 2022, 351, 130956. [Google Scholar] [CrossRef]
- Khan, S.A.; Alam, M.Z.; Mohasin, M.; Ahmad, S.; Salma, U.; Parveen, H.; Mukhtar, S.; Al-Anazi, M.; Alotaibi, F.A.; Abdelaziz, M.A. Ultrasound-Assisted Synthesis of Chalcone: A Highly Sensitive and Selective Fluorescent Chemosensor for the Detection of Fe(3+) in Aqueous Media. J. Fluoresc. 2023. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Kumar, N.; Leray, I.; Depauw, A. Chemically derived optical sensors for the detection of cesium ions. Coord. Chem. Rev. 2016, 310, 1–15. [Google Scholar] [CrossRef]
- Guria, U.N.; Mahapatra, A.K.; Ghosh, A.K.; Bindal, R.C. Fluorescent chemosensor for lethal cesium detection using thin film membrane. Sep. Sci. Technol. 2019, 54, 1687–1696. [Google Scholar] [CrossRef]
- Ji, H.F.; Dabestani, R.; Brown, G.M. A supramolecular fluorescent probe, activated by protons to detect cesium and potassium ions, mimics the function of a logic gate. J. Am. Chem. Soc. 2000, 122, 9306–9307. [Google Scholar] [CrossRef]
- Seo, H.S.; Karim, M.M.; Lee, S.H. Selective fluorimetric recognition of cesium ion by 15-crown-5-anthracene. J. Fluoresc. 2008, 18, 853–857. [Google Scholar] [CrossRef]
- Radaram, B.; Mako, T.; Levine, M. Sensitive and selective detection of cesium via fluorescence quenching. Dalton Trans. 2013, 42, 16276–16278. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, V.; Henry-de-Villeneuve, C.; Nguy, T.P.; Wakayama, Y.; Charrier, A.M.; Raimundo, J.M. Novel and Innovative Interface as Potential Active Layer in Chem-FET Sensor Devices for the Specific Sensing of Cs(). ACS Appl. Mater. Interfaces 2019, 11, 47635–47641. [Google Scholar] [CrossRef]
- Jung, S.H.; Park, J.S.; Choi, Y.; Kim, S.K.; Jung, J.H. Calix[4]arene-based fluorescent probe and the adsorption capacity of its electrospun nanofibrous film for the cesium cation as an adsorbent. Supramol. Chem. 2016, 29, 139–145. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, H.; Lee, J.K.; Lee, S.H.; Lim, H.B.; Kim, J.S. Cesium ion-selective electrodes based on 1,3-alternate thiacalix 4 biscrown-6,6. Talanta 2004, 64, 975–980. [Google Scholar] [CrossRef]
- Chitry, F.; Pellet-Rostaing, S.; Nicod, L.; Gass, J.L.; Foos, J.; Guy, A.; Lemaire, M. New cesium-selective hydrophilic ligands: UV measures of their interactions toward Cs and Cs/Na separation by nanofiltration complexation. J. Phys. Chem. A 2000, 104, 4121–4128. [Google Scholar] [CrossRef]
- ArnaudNeu, F.; Asfari, Z.; Souley, B.; Vicens, J. Binding properties of calix[4]-bis-crowns towards alkali cations. New J. Chem. 1996, 20, 453–463. [Google Scholar]
- Benco, J.S.; Nienaber, H.A.; Dennen, K.; McGimpsey, W.G. A fluoroionophore for detection of potassium ions: 9-anthryl-substituted azacrown ether covalently linked to a 1,3-alternate calix[4]arene. J. Photochem. Photobiol. A 2002, 152, 33–40. [Google Scholar] [CrossRef]
- Kim, S.K.; Sessler, J.L.; Gross, D.E.; Lee, C.H.; Kim, J.S.; Lynch, V.M.; Delmau, L.H.; Hay, B.P. A Calix[4]arene Strapped Calix[4]pyrrole: An Ion-Pair Receptor Displaying Three Different Cesium Cation Recognition Modes. J. Am. Chem. Soc. 2010, 132, 5827–5836. [Google Scholar] [CrossRef]
- Leray, I.; Asfari, Z.; Vicens, J.; Valeur, B. Photophysics of calix 4 biscrown-based ditopic receptors of caesium containing one or two dioxocoumarin fluorophores. J. Fluoresc. 2004, 14, 451–458. [Google Scholar] [CrossRef]
- Souchon, V.; Leray, I.; Valeur, B. Selective detection of cesium by a water-soluble fluorescent molecular sensor based on a calix 4 arene-bis(crown-6-ether). Chem. Commun. 2006, 4224–4226. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X. Sensing of inorganic ions in microfluidic devices. Sens. Actuators B 2021, 329, 129171. [Google Scholar] [CrossRef]
- Gómez-Hens, A.; Fernández-Romero, J.M. Microfluidic Systems in Analytical Chemistry. Encycl. Anal. Chem. 2017, 1–20. [Google Scholar]
- Lafleur, J.P.; Senkbeil, S.; Jensen, T.G.; Kutter, J.P. Gold nanoparticle-based optical microfluidic sensors for analysis of environmental pollutants. Lab Chip 2012, 12, 4651–4656. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.D.; Gaualas, V.G.; Daunert, S.; Bachas, L.G. Microfluidic ion-sensing devices. Anal. Chim. Acta 2008, 613, 20–30. [Google Scholar] [CrossRef]
- Basabe-Desmonts, L.; Benito-Lopez, F.; Gardeniers, H.; Duwel, R.; van den Berg, A.; Reinhoudt, D.N.; Crego-Calama, M. Fluorescent sensor array in a microfluidic chip. Anal. Bioanal. Chem. 2008, 390, 307–315. [Google Scholar] [CrossRef]
- Kim, H.; Rao, B.A.; Jeong, J.W.; Mallick, S.; Kang, S.-M.; Choi, J.S.; Lee, C.-S.; Son, Y.-A. A highly selective dual-channel Cu2+ and Al3+ chemodosimeter in aqueous systems: Sensing in living cells and microfluidic flows. Sens. Actuators B 2015, 210, 173–182. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Wu, T.; Lefevre, J.P.; Leray, I.; Delaire, J.A. Fluorimetric lead detection in a microfluidic device. Lab. Chip. 2009, 9, 2818–2823. [Google Scholar] [CrossRef]
- Bell, J.; Climent, E.; Hecht, M.; Buurman, M.; Rurack, K. Combining a Droplet-Based Microfluidic Tubing System with Gated Indicator Releasing Nanoparticles for Mercury Trace Detection. ACS Sens. 2016, 1, 334–338. [Google Scholar] [CrossRef]
- Depauw, A.; Dossi, E.; Kumar, N.; Fiorini-Debuisschert, C.; Huberfeld, G.; Ha-Thi, M.H.; Rouach, N.; Leray, I. A Highly Selective Potassium Sensor for the Detection of Potassium in Living Tissues. Chem. Eur. J. 2016, 22, 14902–14911. [Google Scholar] [CrossRef]
- Demas, J.N.; Crosby, G.A. Measurement of Photoluminescence Quantum Yields-Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Loock, H.-P.; Wentzell, P.D. Detection limits of chemical sensors: Applications and misapplications. Sens. Actuators B 2012, 173, 157–163. [Google Scholar] [CrossRef]


| 1 | 2 | |
|---|---|---|
| Log K11 | 4.10 ± 0.04 | 4.22 ± 0.02 |
| Log K21 | 3.82 ± 0.05 | 3.91 ± 0.07 |
| K21/K11 | 0.5 | 0.49 |
| ε (M−1.cm−1) | 21,000 | 37,500 |
| ΦF | 0.39 | 0.43 |
| ΔIF (λexc) | 2.5 (365 nm) | 2 (405 nm) |
| LOD (µM) | 0.6 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Depauw, A.; Jonusauskaite, L.; Ghasemi, R.; Lefevre, J.-P.; Mongin, C.; Génot, V.; Delaire, J.; Leray, I. A Highly Sensitive and Selective Optical Sensor for the On-Line Detection of Cesium in Water. Sensors 2023, 23, 7826. https://doi.org/10.3390/s23187826
Depauw A, Jonusauskaite L, Ghasemi R, Lefevre J-P, Mongin C, Génot V, Delaire J, Leray I. A Highly Sensitive and Selective Optical Sensor for the On-Line Detection of Cesium in Water. Sensors. 2023; 23(18):7826. https://doi.org/10.3390/s23187826
Chicago/Turabian StyleDepauw, Alexis, Laura Jonusauskaite, Rasta Ghasemi, Jean-Pierre Lefevre, C. Mongin, Valérie Génot, Jacques Delaire, and Isabelle Leray. 2023. "A Highly Sensitive and Selective Optical Sensor for the On-Line Detection of Cesium in Water" Sensors 23, no. 18: 7826. https://doi.org/10.3390/s23187826
APA StyleDepauw, A., Jonusauskaite, L., Ghasemi, R., Lefevre, J.-P., Mongin, C., Génot, V., Delaire, J., & Leray, I. (2023). A Highly Sensitive and Selective Optical Sensor for the On-Line Detection of Cesium in Water. Sensors, 23(18), 7826. https://doi.org/10.3390/s23187826







