Abstract
A hybrid noble nanoparticle/DNAzyme electrochemical biosensor is proposed for the detection of Pb2+, Cd2+, and Cr3+. The sensor takes advantage of a well-studied material that is known for its selective interaction with heavy metal ions (i.e., DNAzymes), which is combined with metallic nanoparticles. The double-helix structure of DNAzymes is known to dissociate into smaller fragments in the presence of specific heavy metal ions; this results in a measurable change in device resistance due to the collapse of conductive inter-nanoparticle DNAzyme bridging. The paper discusses the effect of DNAzyme anchoring groups (i.e., thiol and amino functionalization groups) on device performance and reports on the successful detection of all three target ions in concentrations that are well below their maximum permitted levels in tap water. While the use of DNAzymes for the detection of lead in particular and, to some extent, cadmium has been studied extensively, this is one of the few reports on the successful detection of chromium (III) via a sensor incorporating DNAzymes. The sensor showed great potential for its future integration in autonomous and remote sensing systems due to its low power characteristics, simple and cost-effective fabrication, and easy automation and measurement.
1. Introduction
Given the current situation regarding the amount and diversity of environmental contaminants, there is an urgent need for efficient and dependable pollutant-detection solutions that can both report and quantify them in natural resources such as air or water. Heavy metal ions (HMIs), pesticides, metalloids, inorganic nutrients, pharmaceuticals, and antibiotics are among the most common analytes, while tap, lake, river, ground, and waste water are the most common environmental matrices. Pollutant detection has, in turn, proven to be a challenging task due to the great variability and complexity of the sample matrix, thus requiring highly sensitive detection methods that enable selective detection with sufficiently low limits [1].
HMIs, which can often be found in water, are one of the main hazardous byproducts of rapid industrialization, and pose one of the main threats to human and animal life alike. Water contamination is extremely critical as the contaminants entering drinking or irrigation water accumulate along the food chain. In addition, HMI contamination can also harm plants and soil; HMIs have severe adverse effects on plant fertility, and in some cases can even result in plant death. Cadmium (Cd2+) is one of the most common and dangerous trace metal ions that can permeate in the environment, particularly in the water [2,3]. Cadmium’s half-life is about 10–30 years [4], i.e., it does not naturally degrade, but instead accumulates in vital organs such as the intestine, liver, and kidneys. [5]. Cd2+ toxicity can be clearly seen in its interference with many of the necessary nutrients for proper cellular function; this inhibits the absorption of iron, calcium, or zinc in the intestines [6], resulting in slow growth, dysfunction of the liver and kidneys, anemia, and bone damage. Cadmium can also occupy protein-binding sites, interfere with the secretion and regulation of protease-related hormones, reduce the antioxidant capacity of the body, and damage organs and tissues. Lead (Pb2+) is also one of the most persistent and toxic pollutants. Lead pollution sources mainly include waste batteries, automobile exhausts, mining, and metal smelting [7,8]. This can cause Pb2+ to enter the food chain and pollute water resources [9,10]. Even low doses of Pb2+ can accumulate in the human body and are difficult to excrete; long-term exposure or excessive intake of Pb2+ can cause serious disease in organs or ecosystems [11]. In particular, Pb2+ can be more detrimental to children than adults by impeding brain and height development [12]. Chromium is commonly used in steel processing, coating materials, pigments, and catalysis; it is mutagenic and carcinogenic [13] and its toxicity has raised serious environmental concerns. Chromium has nine different oxidation states, with trivalent Cr3+ and hexavalent Cr6+ (present as Cr2O72− in acid and CrO42− in alkaline solutions) being the most common ones. The toxicity of chromium is strongly related to its oxidation state [14]. For example, a trace amount of Cr3+ is needed for glucose and fat metabolism and, thus, Cr3+ is considered to be essential for life [15]. However, Cr3+ is still toxic at high concentrations and is currently classified as a group 3 carcinogen [16].
Several analytical methods have been developed that can detect heavy metals in water; atomic fluorescence spectroscopy (AFS) [17], atomic absorption spectroscopy (AAS) [18], inductively coupled plasma atomic emission spectroscopy (ICP-AES) [19,20], and inductively coupled plasma-mass spectrometry are some of the most common methods for HMI determination. However, these methods are often time consuming, expensive, require sophisticated equipment and highly trained personnel, and are not practical for in situ detection [21]. Electrochemical methods have shown the potential to be just that—easy, cost effective, and precise methods that, because of their easy automation and integration with read-out circuits and electronics, can eventually be used for on-site target detection. Sensors in general and, most importantly, nanomaterial-based sensors, have the capacity to assist analytical techniques via on-site screening of water bodies and, eventually, replace many of the water-quality sensors that are used today for HMI detection [1]; at the same time, nanosensors offer low-cost and low-power consumption, as well as portability when integrated with point-of-care systems that can also be combined with emerging technologies such as the Internet of Things (IoT) [22]. Nanomaterial-enabled water-quality sensors rely on a number of transduction methods that can be applied for the detection of environmental contaminants and particularly HMIs, such as optical sensors (i.e., colorimetric and fluorescence) for the detection of Cd2+, Pb2+, Hg2+, and Cu2+ [23,24,25,26,27,28,29]; electrochemical sensors [30]; and field effect transistors [31]. The use of functional biomaterials such as DNAzymes is also common in the development of highly sensitive and selective biosensors for HMI detection. DNAzymes are enzymatic, single-stranded (ss), synthetic DNA sequences that present a high specificity towards metal ions; binding of metal ions to specific sites in the DNA sequence leads to DNA cleaving and to its eventual breakdown. There are a wide range of DNAzymes that have been reported to enable the bio-recognition of HMIs, such as Pb2+, while in the case of Cd2+, the development of suitable and selective DNAzymes is more challenging due to the thiophilicity of Cd2+ [32]. The main competing technologies for DNAzyme0based sensors are colorimetric or fluorescent sensors [33,34,35] and electrochemical sensors, i.e., amperometric [36,37] or impedimetric sensors [38,39]. Nanomaterials are also often combined with DNAzymes; among the most common scenarios is the use of nanomaterials as optical quenchers [40], as enhancers for the optical properties of respective sensing systems (e.g., the utilization of localized surface plasmon resonance (LSPR) characteristics of Au nanoparticles (NPs) [41], the use of quantum dots so as to achieve chemiluminescence resonance energy transfer (CRET) signals [42], or as appropriate platforms for DNA immobilization [43]. There are many articles and reviews [21,44] dedicated to the use of DNAzymes for Pb2+ detection; the detection principle is again either colorimetric, fluorimetric, or electrochemical, while typical limits of detection (LoD) range between 0.5–400 nM, 100 pM–500 nM, and 6.4–910 pM for colorimetric, fluorescence, and electrochemical sensors, respectively. Typical LoDs in the case of Cd2+ DNAzyme-based biosensors fall in the range of 1–11.3 nM [32,45]; in the case of a non-specific DNAzyme-based biosensor fabricated using a field-effect transistor (FET) and single-walled carbon nanotubes (SWNTs), the use of a mathematical model and data analysis results in a Cd2+ LoD of 34 pM [46]. It is also worth noting that in the case of Cr3+, there are very few publications related to DNAzyme-based sensors, focusing either on the use of solid state nanochannels in tandem with DNAzymes in order to modify the ionic current in the presence of Cr3+ ions within the nanochannel [47], the development of a molecular beacon for the detection of Cr3+ and Cr6+ [48], and on fluorescence [49] or colorimetric sensing concepts [50].
In the current paper, an electrochemical biosensor based on platinum (Pt) NPs and DNAzymes is proposed for the simultaneous and label-free detection of HMI targets, namely Pb2+, Cd2+, and Cr3+. The two-dimensional NP film is deposited in between interdigitated electrodes (IDE), serving as “expanded” nano-gapped electrodes. Target-appropriate DNAzymes were chosen for the selective detection of all of the target materials; apart from its catalytic activity in the presence of HMIs, the DNAzymes layer offers enhanced device conductivity, which is reduced in the presence of the target material due to the collapse of conductive DNAzyme inter-nanoparticle bridging. Two functional groups have been employed for the surface immobilization of DNAzymes (i.e., thiol and amino groups). The sensor was able to detect Pb2+, Cd2+, and Cr3+ concentrations that are well below their respective permitted levels in tap water. This group has previously reported on the combination of biomaterials such as DNAzymes, oligonucleotides, and aptamers with metallic NPs [51,52,53] for the development of bio-sensing devices. The current work expands on these results by further optimizing the device concept, resulting in the development of a highly sensitive and flexible sensing platform that is capable of detecting and quantifying three distinctive HMIs. The proposed hybrid nanomaterial/DNAzyme electrochemical biosensor serves as a flexible and modular biosensing platform that can be expanded so as to enable the detection of additional environmental contaminants by accommodating additional DNAzymes. It is also worth noting that this is one of the few reports discussing the successful detection of Cr3+ using DNAzymes, regardless of the sensing technique, while to the best of the authors’ knowledge, this is the sole report regarding electrochemical biosensors. In addition, the results discussed herein highlight the importance and impact of DNA functional groups (i.e., amino and thiol) on the biosensor’s performance, allowing for the optimization of the biosensor; optimization is achieved both in terms of improved biosensor performance as well as in terms of simpler and faster fabrication. Overall, the biosensor presented herein poses as a unique sensing solution within the field of electrochemical biosensors as it utilizes the NP layer in a radically different manner than most electrochemical-based sensing systems and by taking advantage of double stranded (ds) DNA’s electrical properties [54]. In addition, the biosensors stand out as an attractive and cost-effective solution by avoiding many of the common drawbacks encountered in most nanomaterial/DNAzyme electrochemical-sensing systems, namely arduous and expensive development that often involves the use and combination of numerous and wildly varying materials and/or complicated fabrication techniques. At the same time, it relies on a relatively simple and low-power characterization method (resistance measurement under a 1V bias), rendering it suitable for future integration to portable and remote environmental-monitoring systems, as well as to water treatment and remediation platforms.
2. Materials and Methods
2.1. Interdigitated Electrode & Pt Nanoparticle Fabrication
Silicon substrates with a thermal SiO2 oxide layer of 300 nm in thickness were used for the development of the biosensor. Gold IDEs with a 10 μm inter-finger distance were patterned on top of the oxidized substrates via optical lithography and an e-gun metallization step. Titanium (10 nm in thickness) was used as an intermediate adhesion layer between SiO2 and Au, resulting in a total IDE thickness of 40 nm. Naked Pt NPs were deposited using a physical vapor deposition technique, i.e., DC magnetron sputtering [51,52,53]; sputtering is a room-temperature technique that allows for the simultaneous control of both the nanoparticle size (controlled via the sputtering target to the deposition-substrate distance), as well as nanoparticle surface coverage and density (dependent on the overall deposition time) and, thus, the conductivity and resistance of the device [51,52]. Device resistance was monitored in situ during NP deposition and was interrupted as soon as the desired resistance was achieved. Devices used in the current paper were prepared with a NP surface coverage just below the percolation threshold for optimum device sensitivity and according to previous results reported by this group [51,52].
2.2. Materials and Reagents Used for Functionalization
All of the employed reagents were of analytical grade and were obtained from Merck (Merch SA). All of the buffers were prepared with deionized water (18.2 MΩ cm at 25 °C resistivity) from a Millipore MilliQ system. Oligo nucleotides employed for the formation of the ds DNAzymes Gr5, detection of Pb2+, Cd2+, and Cr3+ ions were purchased from Integrated DNA Technologies, BVBA (Leuven, Belgium). Their sequences were Gr5 catalytic strand: GTTCGCCATCTGAAGTAGCGCCGCCGTATAGTGACT; BN-Cd16 catalytic strand: GTTCGCCATCTTCCTTCGATAGTTAAAATAGTGACT; and Ce13d catalytic strand: GTTCGCCATAGGTCAAAGGTGGGTGCGAGTTTTTACTCGTTATAGTGACT.
Following hybridization with a common substrate strand, whose sequence was AG-TCACTATrAGGAAGATGGCGAAC, they were capable of recognizing Pb2+, Cd2+, and Cr3+ ions, respectively, and inducing self-catalysis at the ribonucleotide base. The substrate strand was modified at 5′ with either an amino C6 linker or a thiol C6 linker to allow for its immobilization onto the sensor surfaces. The following materials and reagents were used: materials (3-Aminopropyl)triethoxysilane (APTES) (an aminosilane with alkoxysilane molecules); glutaraldehyde and ethanol; Phosphate Buffered Saline (PBS) of pH = 7.4; phosphate buffer 1 M, pH = 8, 0.001% tween20; ethanolamine; MOPS buffer (3-(Nmorpholino)propanesulfonic acid) (50 mM MOPS/25 mM NaCl, pH = 7.5) and MES buffer (2-(N-morpholino)ethanesulfonic acid) (50 mM MES/25 mM NaCl/0.8 mM Phosphate Buffer, pH = 6); and 6-mercapto-1-hexanol (MCH).
2.3. Surface Functionalization and DNAzymes Immobilization
All of the preparation steps were conducted at room temperature, unless stated otherwise. Two different techniques were used for the functionalization of the surfaces. The first method used amino-modified DNA sequences, while in the second method, thiol-modified DNA sequences were employed. It must be emphasized that during every step of the following processes, only the active area of the sensors was processed, namely the IDEs surface area.
For the amino-modified DNA immobilization technique, the following steps were applied, as described in Figure 1. The first step involved the activation of the sample’s surface (SiO2) with oxygen plasma; in the second step, the samples were functionalized with APTES. This was essential for the next step of the process, where the covalent bond between glutaraldehyde with the alkoxysilane molecules was established. APTES was used in a 5% v/v solution of ethanol and DI water (solution 1). The chips were kept in dark room conditions for 2 h and were then rinsed with a DI water and ethanol solution (5% v/v) and baked at 110 °C for 1 h. Glutaraldehyde was dissolved in a 5% PBS 1× buffer solution; the samples remained in this solution for 1 h and were subsequently washed with the same buffer and DI water, and finally gently blow dried with N2. The surface functionalization step was followed by the probe immobilization and target hybridization steps, respectively. Initially, the ss DNA substrate probes (aminated oligonucleotides) were immobilized on the second aldehyde group of glutaraldehyde. DNA deposition was performed via drop-casting from 10 μΜ phosphate buffer. After covalent coupling, ethanolamine was used in a 10 μM solution in phosphate buffer to remove any non-specifically bound catalytic strands from the surface and to act as an interaction barrier between the immobilized single DNA strands. The final step of the process involved the hybridization of the DNAzyme sequences with the immobilized substrate strands, which was performed from either a 5 μM MOPS buffer in the case of Pb2+ and Cd2+ detection, or a 5 μM MES buffer in the case of Cr3+ detection. It should be noted that after every step, the samples were washed with the same buffer solutions the drop-casted substance was dissolved in, and the rinsed with DI water and blow-dried with N2, while in humid conditions.
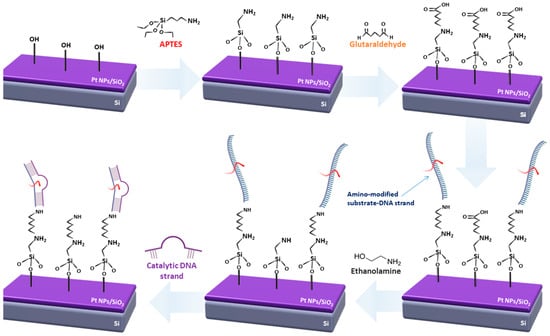
Figure 1.
Schematic representation of the immobilization process for amino-modified DNAzymes.
For the thiol-modified DNA immobilization technique, there was no need for surface modification prior to probe immobilization and target hybridization (Figure 2). A 5 μΜ phosphate buffer with the dissolved ssDNA substrate probes was heated at 95 °C for 5 min, and then deposited on the IDEs via drop casting. After 1 h in humid conditions, MCH was employed in the same phosphate buffer solution, in order to convey an identical blocking effect as ethanolamine on the amino-modified DNA sequences. Finally, the DNAzyme sequences were hybridized with the immobilized substrate strands using the same MOPS or MES buffer solutions as in the amino-modified DNA immobilization technique.

Figure 2.
Schematic representation of the immobilization process for thiol-modified DNAzymes.
After the successful formation of the ds DNAzymes film on the biosensor’s surface, the device was blow dried using gentle N2 flow and could be immediately used for heavy metal ion detection and recognition while exposed to room temperature and humidity and without any special requirements. The device could be also stored in humid conditions and in a temperature between 4 and 5 °C; the proposed biosensors could successfully detect all three heavy metal ion targets, even if stored in such conditions for over a month.
2.4. Surface Characterization and XPS Analysis
Scanning electron microscopy (SEM) analysis was conducted for sensors functionalized with both amino and thiol linkers and for every step of the immobilization process; SEM characterization showed that electron charging on the sensors’ surface increased with each additional layer, which prevented detailed SEM imaging. In addition, each of the DNAzymes functionalization steps (as described in Figure 1 and Figure 2) was verified using fluorescence microscopy. To that end, all of the DNA strands used in this work (substrate and enzymatic/catalytic) were labeled with fluorescent tags, as discussed in [51].
In order to confirm the immobilization and loading of the DNAzymes layer on the sensor’s surface, as well as to observe any possible differentiation between amino and thiol linkers, X-ray photoelectron spectroscopy (XPS) analysis was employed. XPS analysis of plain Si/SiO2 silicon slabs, Si/SiO2 slabs with Pt NPs, Si/SiO2 with thiol or amino-Dnazymes, and Si/SiO2 with Pt NPs and amino or thiol-DNAzymes was conducted using a MAX200 system. The XPS measurements took place in the analysis chamber of MAX200, at room temperature and ~4 × 10−8 mbar pressure, using conventional non-monochromatic MgKα X-rays and a Hemispherical Electron Energy Analyzer (SPECS EA200) with Multi-Channel Detection properly calibrated according to ISO15472 and ISO24237. The analyzer operated under conditions optimized for a better signal intensity (constant pass energy of 100 eV, maximum lens aperture). The measurements always took place along the specimen surface normal (0° take-off angle). The analyzed area was laterally defined by a ~4 × 7 mm2 rectangle, centrally placed over each specimen, so as to always take measurements within the specimen surface. The maximum analyzed depth, from the outermost surface inwards, was 15 nm, with the signal intensity decreasing roughly exponentially with increasing depth.
XPS analysis confirmed that without the presence of the Pt NP layer (i.e., for bare SiO2 substrates), there was no organic loading in the case of thiol-modified DNAzymes (distinct XPS peaks for N, C, and P) on the biosensor’s surface. On the contrary, amino-modified DNAzymes could successfully attach on both bare and Pt NP-modified surfaces. Quantitative analysis of the XPS results confirmed these observations, indicating that the number of deposited biomolecules was considerably less for thiol-modified DNAzymes compared with the amino-modified DNAzymes, which, again, is to be expected as the functionalized bio-molecules could attach on the entire surface of the biosensors.
2.5. Sensor Characterization
The finalized biosensors were characterized in a homemade electrochemical cell using a Keithley 2400 for resistance monitoring under a 1 V DC bias; the actual characterization process has been described in length in [51]. In Figure 3, the typical dynamic sensor-response can be seen for a sensor measured as described in [51]. The introduction of a buffer solution on the top of the sensor resulted in a drastic decrease in sensor resistance, while a new steady-state for sensor resistance was achieved in fewer than 10 s. The introduction of a buffer solution spiked with the given HMI concentration was detected as an increase in the sensor’s resistance.
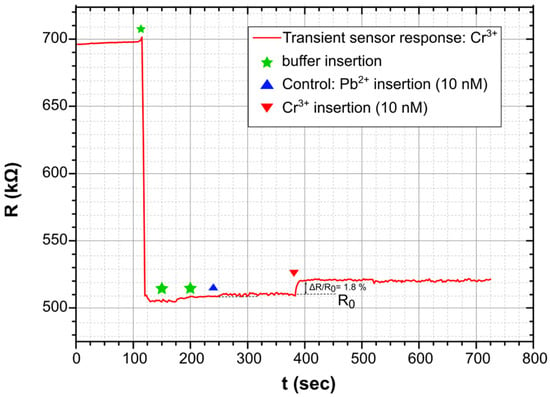
Figure 3.
Typical dynamic response of a biosensor when combining Pt nanoparticles and thiol modified DNAzymes. Detection of 10 nM of Cr3+. In the current study, sensors that incorporate thiol-modified DNAzymes for lead and cadmium, as well as sensors that incorporate amino-modified DNAzymes for all heavy metal ions, feature a similar dynamic response.
3. Results and Discussion
The charge-transport mechanism for NP films developed via sputtering and with a surface coverage just below the percolation threshold has been discussed in length in previous publications by this group [51,52]. In short, the charge-transport mechanism in this case is governed by quantum mechanical phenomena such as tunneling and/or variable range hopping, etc.; devices that fall in this regime are often associated with a thermally activated Arrhenius-type mechanism for conductivity that resembles that of a semi-conductor [51]. It is thus obvious that such devices are extremely sensitive to any variation in their environment, such as any decrease or increase in inter-nanoparticle distance, changes in the dielectric constant of the surrounding medium, and the introduction of functionalization materials between distinct NPs or between NP aggregates. On the other hand, dsDNA exhibits interesting electrical properties and is known to facilitate charge transport via tunneling over shorter oligonucleotides or via multi-step hopping over longer DNA paths [54]; this is also the case for ssDNA, albeit with conductivity that is order of magnitudes lower than that of dsDNA [55]. The electrical properties of dsDNA and its respective charge transport, using the HOMO/LUMO and the π-electronic system of stacked base pairs, have been studied extensively, yet many phenomena still need to be understood; more specifically, distinctive DNA base pairs have been found to promote charge transport, while others act as electric barriers [54]. Overall, dsDNA conductivity in aqueous environments relies both on base pair π-stacking as well as charge transport via the outer sphere of the sugar phosphodiester backbone of the DNA, including bound water molecules. As discussed in previous publications by this group, the stacking of conductive molecular bridges such as DNAzymes between the NP film leads to enhanced conductivity [51]; in that case and as a proof of concept, the NP film was functionalized with one specific DNAzyme that showed a high affinity towards Pb2+. As expected, the conductive bridging offered by the DNAzyme collapsed selectively in the presence of Pb2+ ions, translating to an increase in the as-measured resistance of the device.
Herein, our proposed multi-sensing platform was expanded towards the detection of Pb2+, Cd2+, and Cr3+ by using additional and highly specific DNAzymes; it is worth noting that despite the numerous reports in the literature regarding fluorimetric and colorimetric sensors for Cr3+ detection [56], or reports discussing the development of FET sensors [57], up until now, there has been a very limited number of publications related to the detection of Cr3+ ions using DNAzymes, regardless of the detection principle. In addition, and in order to investigate the role of different functionalization modifications, our ds catalytic strands were functionalized with either thiol or amino functional groups. In Figure 4, the response of the proposed biosensors towards Pb2+, Cd2+, and Cr3+ for thiol-modified catalytic strands can be seen. The results correspond to a relative change in resistance (ΔR/R0%); as a result of the cleavage of the substrate strand and its dissociation into two smaller fragments, there was an increase in the measured resistance of the device [51]. The mean base resistance or Rb (Rb: initial device resistance) of the sensors used in these experiments was in the range of 500–950 kΩ, while it had a standard deviation of 5.6%. For the detection of each distinctive HMI concentration, 10 different DNAzyme biosensors were used in total in order to calibrate the biosensors. The standard deviation of these precision measurements was in the range of 0.28% and 1.2%. The sensors had a limit of detection (LoD) of 0.8 nM, 1 nm, and 10 nm for Pb2+, Cd2+, and Cr3+ respectively. The response time of the sensors was between 7 and 19 s (response time is the time needed for the sensor to reach 70% of its steady state for a specific HMI concentration). The cross-sensitivity and selectivity of the biosensors was also examined by introducing a buffer solution containing an HMI that was non-specific to the respective DNAzyme; the introduction of the non-specific HMI was performed before the introduction of the target-specific HMI.
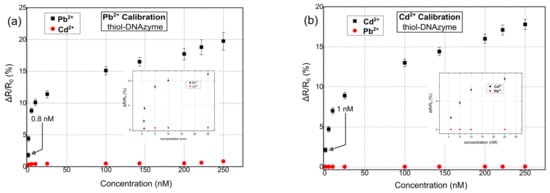
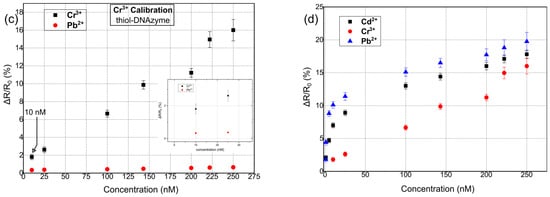
Figure 4.
Relative resistance response % for hybrid nanoparticle/DNAzyme biosensors, incorporating a thiol anchoring group. Sensor response is calculated as ΔR/R0, where R0 is the resistance of the biosensor after the introduction of the buffer solution and prior to the introduction of any heavy-metal ion solution. (a) Sensor calibration for varying concentrations of Pb2+. (b) Sensor calibration for varying concentrations of Cd2+. (c) Sensor calibration for varying concentrations of Cr3+. (d) Cumulative results for the detection of all three heavy metal ions: Pb2+, Cd2+, and Cr3+.
Figure 5 shows the response of the hybrid biosensors towards Pb2+, Cd2+, and Cr3+ for amino-modified catalytic strands. Again, the Rb of the biosensors was chosen to be in the range of 500–950 kΩ. As in the case of thiol-modified DNAzymes, 10 distinctive DNAzyme biosensors were used for calibrating the sensor response towards each HMI concentration. The standard deviation of the precision measurements was between 0.3% and 0.9%. In this case, the sensors were characterized by a LoD of 20 nM, 20 nm, and 44 nm for Pb2+, Cd2+, and Cr3+, respectively, and a response time between 6 and 20 s. Finally, their cross-sensitivity/selectivity was evaluated towards an HMI that was non-specific to the respective DNAzyme.
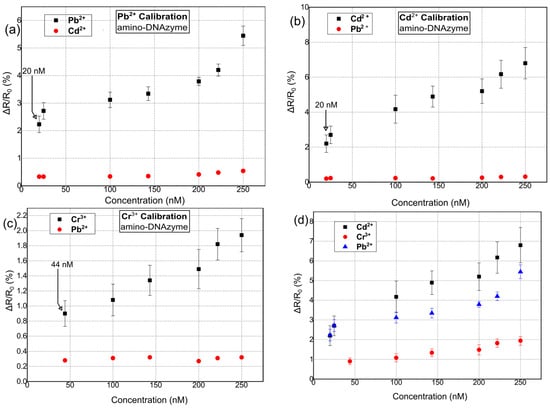
Figure 5.
Relative resistance response % for hybrid nanoparticle/DNAzyme biosensors, incorporating an amino-anchoring group. Sensor response is calculated as ΔR/R0, where R0 is the resistance of the biosensor after the introduction of the buffer solution and prior to the introduction of any heavy-metal ion solution. (a) Sensor calibration for varying concentrations of Pb2+. (b) Sensor calibration for varying concentrations of Cd2+. (c) Sensor calibration for varying concentrations of Cr3+. (d) Cumulative results for the detection of all three heavy metal ions: Pb2+, Cd2+, and Cr3+.
As can be seen in Figure 6, the DNAzyme modification had an effect on device performance and sensitivity. To be more specific, thiol-modified DNAzymes showed greater sensitivity when compared with their amino-modified counterparts. Platinum NPs have found wide use as promising nanomaterials for biomedical applications [58,59]; this would not be possible if not for their facile bio-conjugation with a wide range of bio-materials. There are many reports on the successful immobilization of both thiol [60] and amino [61]-modified biomolecules (directly on the surface of platinum nanoparticles, i.e., without the necessity for an intermediate functionalization layer such as APTES and GOPTS), intercalating aptamers, oligos, dsDNA, etc., in-between Pt NPs. Herein, we followed this approach for both thiol and amino modified DNAzymes.
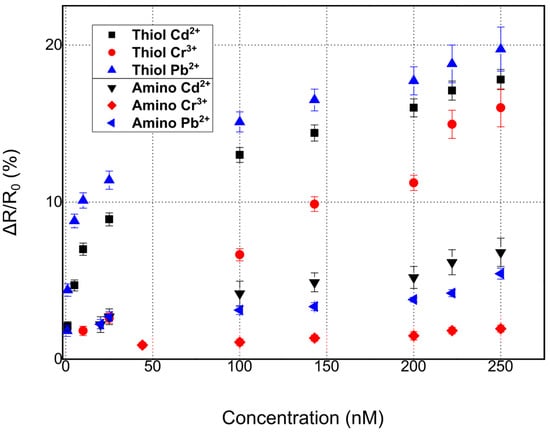
Figure 6.
Comparative chart for the detection of all three heavy metal ions, Pb2+, Cd2+, and Cr3+, using two distinctive anchoring groups: thiol and amino-modified DNAzymes.
The schematic in Figure 7 showcases the DNAzyme distribution on top of two-dimensional Pt NP films, as produced via the sputtering technique; the schematic aims to provide a realistic depiction of the device, in accordance with TEM characterization results [51,52,53]. As can be seen, there was a random distribution of inter-nanoparticle gaps (noted as “d”) that could be under 1 nm (where charge transport via tunneling can occur) or well over 2 nm (where charge transport could only occur via the hybridized DNAzymes-layer, and for appropriate distances that matched the length of the dsDNA [51]). The dissociation of the substrate strand after the successful recognition of target HMIs had a significant effect on conductivity; to be more specific, the collapse of conductive DNA bridging resulted in an increase in device resistance for inter-nanoparticle gaps over 2 nm [51]. For thiol-modified DNAzymes, it is clear that the sensing mechanism was dominated by conductive DNA bridging. In the case of amino DNAzymes without any functionalization on the sensor’s surface, our experiments did not result in the detection of any HMIs in sufficiently low concentrations (i.e., under 200 nM); hence, they were not included in this paper. At this point, it is worth noting that the quality of the anchoring group (i.e., amino or thiol) can play a crucial role in device conductivity with thiol modification groups, offering improved charge transport due to stronger bonds with the metallic NPs [54]. In our case, the low sensitivity observed in the case of amino DNAzymes led to the incorporation of a surface functionalization process (as described in the experimental section of the paper) in order to enhance the attachment of the amino groups on the Pt NP film and, hence, improve the device performance. The incorporation of surface functionalization layers, such as the aminosilane APTES followed by its covalent bonding to glutaraldehyde and ultimately to ssDNA strands through carbonyl groups, may have enhanced the amino to nanoparticles bonding strength, but at the same time it seemed to affect and disturb charge transport by forcing it to transit through additional functionalization layers and inter-layer chemical bonds. In addition, and as can be seen in Figure 7, the immobilization of DNAzymes on the SiO2 substrate physically obstructed the inter-nanoparticle conductive bridging, thus resulting in a lower sensitivity. In conclusion, apart from the improved sensing response in the case of thiol-modified DNAzymes, thiol-based devices offer a much simpler fabrication and significantly shorter preparation time and are hence extremely cost-effective when compared with their amino DNAzyme counterparts. It is worth noting that the proposed devices could be easily used in tandem with advanced functional materials intended for water treatment (e.g., carbon-based materials) [62,63,64] in order to offer a holistic solution for water monitoring and remediation. Finally, the sensors cοuld be reused, as discussed in [51]; however, the regeneration process has been proved to be cumbersome and unnecessary. That is because a small fragment of the substrate strand remained attached to the surface. Regeneration of the surface would require the complete removal of this fragment through the disruption of the covalent bond tethering to the sensor surface. This, in turn, would necessitate the functionalization of the sensor surface again, which is a costly and inefficient process.

Figure 7.
Schematic representation of the DNAzyme distribution on top of the two-dimensional platinum (Pt) nanoparticle (NP) film, for amino and thiol-modified DNAzymes. The enzyme strand (es), substrate strand (ss), and cleavage site can be seen in the respective DNAzymes schematic; upon recognition of a heavy metal ion (HMI) target, the substrate strand is cleaved. The Pt NP film offers a wide range of inter-nanoparticle gaps (noted as “d”) that can both be under 1 nm and well over 2 nm.
4. Conclusions
A hybrid electrochemical biosensor for the detection of three distinctive metal ions (i.e., Pb2+, Cd2+, and Cr3+) has been presented. This is one of the few DNAzyme-related works that discusses the successful detection of Cr3+ ions using sensors, regardless of the choice of detection principle, and the sole report on successful electrochemical detection. The sensor is based on the combination of noble metallic nanoparticles (i.e., platinum) and DNAzymes. The biosensor was functionalized with target-specific catalytic DNA strands that can bind on the sensor’s surface via two different anchoring groups that are often used in the literature, namely thiol and amino functional groups, in order to study their effect on sensor performance. The biosensor was ultimately able to detect all three heavy metal ions in sufficiently low concentrations that are well below their permitted levels in tap water, for both anchoring groups. However, our results show that the direct attachment of catalytic DNAs on the noble nanoparticle surface (i.e., in the case of thiol-modified DNAzymes), results in sensors with an improved performance and much simpler and faster fabrication. A surface-functionalization technique has been proposed in order to enhance the binding strength of amino-modified DNAzymes to the surface, which resulted in significant improvement in sensor performance; still, sensors utilizing thiol-modified DNAzymes continued to outperform their amino counterparts.
The sensors have been proven to be reliable with a good sensitivity, precision, and sufficient dynamic range that can be further expanded towards increased concentrations if needed. In addition, and as indicated by previous results [51], the sensors are re-usable, but do require additional re-hybridization steps after target detection between catalytic strands and substrate strands. At the same time, the biosensors offer simple instrumentation and measurement, i.e., device resistance under a low bias (1 V). This further highlights the simplicity and added value of the device, while its low-power properties along with its easy automation render it well suited for remote and autonomous environmental monitoring systems or water-treatment systems, going forward into the IoT era. It is also worth noting that the proposed biosensor can be developed as a multi-sensing array for the simultaneous detection and screening of additional HMIs, while the range of candidate environmental contaminants can be expanded. The eventual goal of the present work is the integration of the biosensors in a single, disposable, and low-cost platform, which will allow for the multiplexed detection of all three HMIs in a single measurement. Future work entails revisiting the biomaterial protocols so as to further facilitate the reusability of the device, as well as revisiting the direct attachment of amino-modified catalytic strands on the nanoparticle’s surface in order to investigate any underlying phenomena that contribute to the sensor’s sensitivity.
Author Contributions
Conceptualization, E.S., D.T., G.T. (Georgios Tsekenis) and E.A.; methodology, E.S., D.T., G.T. (Georgios Tsekenis) and E.A.; validation, E.S., E.A. and C.P.; formal analysis, E.S., E.A. and C.P.; investigation, E.S., E.A., C.P., G.T. (Georgia Tzourmana), A.Z., S.K., S.L. and A.R.; resources, D.T.; data curation, E.S., E.A. and C.P.; writing—original draft preparation, E.S.; writing—review and editing, E.S. and D.T.; visualization, E.S. and C.P.; supervision, D.T., E.A., E.S. and G.T. (Georgios Tsekenis); project administration, D.T.; funding acquisition, E.S and D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the MICSYS (a microfluidic-chip-based system for the detection of contaminants in water) project, co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship, and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: Τ2ΕΔΚ-02144).
Data Availability Statement
Data supporting reported results can be found here: https://figshare.com/articles/dataset/Hybrid_nanoparticle-DNAzyme_electrochemical_biosensor_for_the_detection_of_divalent_heavy_metal_ions_and_Cr3_/23790675, accessed on 1 August 2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vikesland, P.J. Nanosensors for water quality monitoring. Nat. Nanotechnol. 2018, 13, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Bruning-Fann, C.S.; Kaneene, J.B. The effects of nitrate, nitrite and N-nitroso compounds on human health: A review. Vet. Hum. Toxicol. 1993, 35, 521–538. [Google Scholar]
- Davis, T.W.; Harke, M.J.; Marcoval, M.A.; Goleski, J.; Orano-Dawson, C.; Berry, D.L.; Gobler, C.J. Effects of nitrogenous compounds and phosphorus on the growth of toxic and non-toxic strains of Microcystis during cyanobacterial blooms. Aquat. Microb. Ecol. 2010, 61, 149–162. [Google Scholar] [CrossRef]
- Wang, H.; Engstrom, A.K.; Xia, Z. Cadmium impairs the survival and proliferation of cultured adult subventricular neural stem cells through activation of the JNK and p38 MAP kinases. Toxicology 2017, 380, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Liu, F.; Liang, X.; Yin, Y.; Liu, G. The role of zinc chelate of hydroxy analogue of methionine in cadmium toxicity: Effects on cadmium absorption on intestinal health in piglets. Animal 2020, 14, 1382–1391. [Google Scholar] [CrossRef]
- Ma, H.; Gao, F.; Zhang, X.; Cui, B.; Liu, Y.; Li, Z. Formation of iron plaque on roots of Iris pseudacorus and its consequence for cadmium immobilization is impacted by zinc concentration. Ecotoxicol. Environ. Saf. 2020, 193, 110306. [Google Scholar] [CrossRef]
- Verstraeten, S.V.; Aimo, L.; Oteiza, P.I. Aluminium and lead: Molecular mechanisms of brain toxicity. Arch. Toxicol. 2008, 82, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Pei, X.; Rusinek, C.A.; Bange, A.; Haynes, E.N.; Heineman, W.R.; Papautsky, I. Determination of lead with a copper-based electrochemical sensor. Anal. Chem. 2017, 89, 3345–3352. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Ju, H. Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens. Bioelectron. 2015, 63, 276–286. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Mousavi, H.Z.; Sajjadi, S.M. A novel magnetic ion imprinted polymer as a selective magnetic solid phase for separation of trace lead(II) ions from agricultural products, and optimization using a Box-Behnken design. Food Chem. 2017, 237, 275–281. [Google Scholar] [CrossRef]
- Xie, X.; Chai, Y.; Yuan, Y.; Yuan, R. Dual triggers induced disassembly of DNA polymer decorated silver nanoparticle for ultrasensitive electrochemical Pb2+ detection. Anal. Chim. Acta 2018, 1034, 56–62. [Google Scholar] [CrossRef]
- Lin, Y.W.; Liu, C.W.; Chang, H.T. Fluorescence detection of mercury(II) and lead(II) ions using aptamer/reporter conjugates. Talanta 2011, 84, 324–329. [Google Scholar] [CrossRef]
- Standeven, A.M.; Wetterhahn, K.E. Chromium(VI) Toxicity: Uptake, Reduction, and DNA Damage. J. Am. Coll. Toxicol. 1989, 8, 1275–1283. [Google Scholar] [CrossRef]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef]
- Anderson, R.A. Chromium as an Essential Nutrient for Humans. Regul. Toxicol. Pharmacol. 1997, 26, S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Xu, Z.; Wang, J. Flow injection on-line solid phase extraction for ultra-trace lead screening with hydride generation atomic fluorescence spectrometry. Analyst 2006, 131, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Calevro, F.; Campani, S.; Ragghianti, M.; Bucci, S.; Mancino, G. Tests of toxicity and teratogenicity in biphasic vertebrates treated with heavy metals (Cr3+, A13+, Cd2+). Chemosphere 1998, 37, 3011–3017. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Van Mol, W.; Adams, F. Determination of cadmium, copper and lead in environmental samples. An evaluation of flow injection on-line sorbent extraction for flame atomic absorption spectrometry. Anal. Chim. Acta 1994, 285, 33–43. [Google Scholar] [CrossRef]
- Elfering, H.; Andersson, J.; Poll, K. Determination of organic lead in soils and waters by hydride generation inductively coupled plasma atomic emission spectrometry. Analyst 1998, 123, 669–674. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropoulou, M.; Ochsenkühn, K.M. Comparison of inductively coupled plasma–atomic emission spectrometry, anodic stripping voltammetry and instrumental neutron-activation analysis for the determination of heavy metals in airborne particulate matter. Fresenius J. Anal. Chem. 2001, 369, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Man, Y.; Li, A.; Jin, X.; Liu, X.; Pan, L. DNAzyme-based biosensor for detection of lead ion: A review. Microchem. J. 2017, 131, 145–153. [Google Scholar] [CrossRef]
- Zulkifli, C.Z.; Garfan, S.; Talal, M.; Harun, N.H.; Chiang, H.H. IoT-Based Water Monitoring Systems: A Systematic Review. Water 2022, 14, 3621. [Google Scholar] [CrossRef]
- Beqa, L.; Kumar, A.; Khan, S.S.A.; Senapati, D.; Arumugam, S.R.; Ray, P.C. Gold nanoparticle-based simple colorimetric and ultrasensitive dynamic light scattering assay for the selective detection of Pb(II) from paints, plastics, and water samples. ACS Appl. Mater. Interfaces 2011, 3, 668–673. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Qu, C.; Chen, L. Highly sensitive visual detection of copper ions based on the shape-dependent LSPR spectroscopy of gold nanorods. Langmuir 2014, 30, 3625–3630. [Google Scholar] [CrossRef]
- Algarra, M.; Campos, B.B.; Alonso, B.; Miranda, M.S.; Martínez, A.M.; Casado, C.M.; Esteves da Silva, J.C.G. Thiolated DAB dendrimers and CdSe quantum dot nanocomposites for Cd(II) or Pb(II) sensing. Talanta 2012, 88, 403–407. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.; Guo, S.; Wu, N. Detection of lead(II) with a “turn-on” fluorescent biosensor based on energy transfer from CdS/ZnS quantum dots to graphene oxide. Biosens. Bioelectron. 2013, 43, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sung, T.W.; Lo, Y.L. Highly sensitive and selective sensor based on silica-coated CdSe/ZnS nanoparticles for Cu2+ ion detection. Sens. Actuators B 2012, 165, 119–125. [Google Scholar] [CrossRef]
- Chao, M.R.; Chang, Y.Z.; Chen, J.L. Hydrophilic ionic liquid-passivated CdTe quantum dots for mercury ion detection. Biosens. Bioelectron. 2013, 42, 397–402. [Google Scholar] [CrossRef]
- Gui, R.; An, X.; Su, H.; Shen, W.; Chen, Z.; Wang, X. A near-infrared-emitting CdTe/CdS core/shell quantum dots-based off-on fluorescence sensor for highly selective and sensitive detection of Cd2+. Talanta 2012, 94, 257–262. [Google Scholar] [CrossRef]
- Li, X.G.; Zhang, J.L.; Huang, M.R. Chemical response of nanocomposite membranes of electroactive polydiaminonaphthalene nanoparticles to heavy metal ions. J. Phys. Chem. C 2014, 118, 11990–11999. [Google Scholar] [CrossRef]
- Jiang, S.; Cheng, R.; Ng, R.; Huang, Y.; Duan, X.F. Highly sensitive detection of mercury(II) ions with few-layer molybdenum disulfide. Nano Res. 2015, 8, 257–262. [Google Scholar] [CrossRef]
- Wang, G.; Wu, M.; Chu, L.T.; Chen, T.H. Portable microfluidic device with thermometer-like display for real-time visual quantitation of Cadmium(II) contamination in drinking water. Anal. Chim. Acta 2021, 1160, 338444. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Y.S.; Xue, J.H.; Zhou, B.; Cao, J.X.; Chen, S.H.; Li, M.H.; Wang, X.F.; Zhu, Y.F.; Huang, Y.Q. A novel strategy for dual-channel detection of metallothioneins and mercury based on the conformational switching of functional chimera aptamer. J. Pharm. Biomed. 2015, 107, 258–264. [Google Scholar] [CrossRef]
- Deng, P.; Zheng, S.; Yun, W.; Zhang, W.; Yang, L. A visual and sensitive Hg2+ detection strategy based on split DNAzyme amplification and peroxidase-like activity of hemin-graphene composites. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 210, 335–340. [Google Scholar] [CrossRef]
- Li, C.; Dai, P.; Rao, X.; Shao, L.; Cheng, G.; He, P.; Fang, Y. An ultra-sensitive colorimetric Hg2+-sensing assay based on DNAzyme-modified Au NP aggregation, MNPs and an endonuclease. Talanta 2015, 132, 463–468. [Google Scholar] [CrossRef]
- Wang, G.; Huang, H.; Zhang, X.; Wang, L. Electrically contacted enzyme based on dual hairpin DNA structure and its application for amplified detection of Hg2+. Biosens. Bioelectron. 2012, 35, 108–114. [Google Scholar] [CrossRef]
- Chang, Y.; Tang, X.; Huang, J.; Chai, Y.; Zhuo, Y.; Li, H.; Yuan, R. Programming a “crab claw”-like DNA nanomachine as a super signal amplifier for ultrasensitive electrochemical assay of Hg2+. Anal. Chem. 2021, 93, 12075–12080. [Google Scholar] [CrossRef]
- Wang, M.H.; Zhang, S.; Ye, Z.H.; Peng, D.L.; He, L.H.; Yan, F.F.; Yang, Y.Q.; Zhang, H.Z.; Zhang, Z.H. A gold electrode modified with amino-modified reduced graphene oxide, ion specific DNA and DNAzyme for dual electrochemical determination of Pb(II) and Hg(II). Microchim. Acta 2015, 182, 2251–2258. [Google Scholar] [CrossRef]
- Xie, S.; Tang, Y.; Tang, D.; Cai, Y. Highly sensitive impedimetric biosensor for Hg2+ detection based on manganese porphyrin-decorated DNA network for precipitation polymerization. Anal. Chim. Acta 2018, 1023, 22–28. [Google Scholar] [CrossRef]
- Pavadai, R.; Amalraj, A.; Subramanian, S.; Perumal, P. High catalytic activity of fluorophore-labeled Y-Shaped DNAzyme/3D MOF-MoS2NBs as a versatile biosensing platform for the simultaneous detection of Hg2+, Ni2+, and Ag+ Ions. ACS Appl. Mater. Interfaces 2021, 13, 31710–31724. [Google Scholar] [CrossRef]
- Chen, J.H.; Pan, J.F.; Chen, S. A naked-eye colorimetric sensor for Hg2+ monitoring with cascade signal amplification based on target-induced conjunction of split DNAzyme fragments. Chem. Commun. 2017, 53, 10224–10227. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Liu, X.L.; Willner, I. Chemiluminescent and chemiluminescence resonance energy transfer (CRET) detection of DNA, metal ions, and aptamersubstrate complexes using hemin/G-quadruplexes and CdSe/ZnS quantum dots. J. Am. Chem. Soc. 2011, 133, 11597–11604. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Wang, J.; Xiong, B.; Hou, X. Electrochemical impedance biosensor array based on DNAzyme-functionalized single-walled carbon nanotubes using Gaussian process regression for Cu(II) and Hg(II) determination. Microchim. Acta 2020, 187, 207. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Shi, L.; Ma, X.Y.; Fu, Y.; Zhang, X.F. Advances in the Functional Nucleic Acid Biosensors for Detection of Lead Ions. Crit. Rev. Anal. Chem. 2023, 53, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.J.J.; Liu, J. Rational evolution of Cd2+-specific DNAzymes with phosphorothioate modified cleavage junction and Cd2+ sensing. Nucleic Acids Res. 2015, 43, 6125–6133. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, S.; Nan, X.; Lixing, X.; Xiong, B. Non-specific DNAzyme-based biosensor with interfering ions for the Cd2+ determination in feed. Sens. Actuators B Chem. 2021, 329, 129139. [Google Scholar] [CrossRef]
- Zhu, L.; Miao, M.; Shao, X.; Luo, Y.; Xu, W. A Universal Electrochemical Biosensor Using Nick-HCR Nanostructure as Molecular Gate of Nanochannel for Detecting Chromium(III) Ions and MicroRNA. Anal. Chem. 2019, 91, 14992–14999. [Google Scholar] [CrossRef]
- Zhou, W.; Vazin, M.; Yu, T.; Ding, J.; Liu, J. In Vitro Selection of Chromium-Dependent DNAzymes for Sensing Chromium(III) and Chromium(VI). Eur. J. Chem. 2016, 22, 9835–9840. [Google Scholar] [CrossRef]
- Wu, X.; Tan, L.; Li, Y.; Zhang, L.; Liang, Y. Novel sensor array distinguishes heavy metal ions based on multiple fluorescence channels from dendritic mesoporous silica nanoparticles. Anal. Chim. Acta 2023, 1240, 340749. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, T.; Cheng, L.; Shen, R.; Zhang, J. Smartphone-assisted colorimetric biosensor for on-site detection of Cr3+ ion analysis. Anal. Chim. Acta 2022, 1199, 339603. [Google Scholar] [CrossRef]
- Skotadis, E.; Tsekenis, G.; Chatzipetrou, M.; Patsiouras, L.; Madianos, L.; Bousoulas, P.; Zergioti, I.; Tsoukalas, D. Heavy metal ion detection using DNAzyme-modified platinum nanoparticle networks. Sens. Actuators B Chem. 2017, 239, 962–969. [Google Scholar] [CrossRef]
- Skotadis, E.; Voutyras, K.; Chatzipetrou, M.; Tsekenis, G.; Patsiouras, L.; Madianos, L.; Chatzandroulis, S.; Zergioti, I.; Tsoukalas, D. Label-free DNA biosensor based on resistance change of platinum nanoparticles assemblies. Biosens. Bioelectron. 2016, 81, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Madianos, L.; Tsekenis, G.; Skotadis, E.; Patsiouras, L.; Tsoukalas, D. A highly sensitive impedimetric aptasensor for the selective detection of acetamiprid and atrazine based on microwires formed by platinum nanoparticles. Biosens. Bioelectron. 2018, 101, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Sebechlebská, T.; Kolivoška, V.; Šebera, J.; Fukal, J.; Řeha, D.; Buděšínský, M.; Rosenberg, I.; Bednárová, L.; Gasior, J.; Mészáros, G.; et al. Additive transport in DNA molecular circuits. J. Mater. Chem. C 2022, 10, 12022–12031. [Google Scholar] [CrossRef]
- Wang, K. DNA-based single-molecule electronics: From concept to function. J. Funct. Biomater. 2018, 9, 8. [Google Scholar] [CrossRef]
- Algethami, J.S. A Review on Recent Progress in Organic Fluorimetric and Colorimetric Chemosensors for the Detection of Cr3+/6+ Ions. Crit. Rev. Anal. Chem. 2022, 1–21. [Google Scholar] [CrossRef]
- Kim, E.B.; Imran, M.; Lee, E.H.; Akhtar, M.S.; Ameen, S. Multiple ions detection by field-effect transistor sensors based on ZnO@GO and ZnO@rGO nanomaterials: Application to trace detection of Cr (III) and Cu (II). Chemosphere 2022, 286, 131695. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- Gutiérrez de la Rosa, S.Y.; Muñiz Diaz, R.; Villalobos Gutiérrez, P.T.; Patakfalvi, R.; Gutiérrez Coronado, O. Functionalized Platinum Nanoparticles with Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 9404. [Google Scholar] [CrossRef]
- Kwon, D.; Lee, W.; Kim, W.; Shin, H.C.; Jeon, S. Colorimetric detection of penicillin antibiotic residues in pork using hybrid magnetic nanoparticles and penicillin class-selective, antibody-functionalized platinum nanoparticles. Anal. Methods 2015, 7, 7639–7645. [Google Scholar] [CrossRef]
- Ramakrishnan, S.K.; Martin, M.; Cloitre, T.; Firlej, L.; Gergely, C. Design rules for metal binding biomolecules: Understanding of amino acid adsorption on platinum crystallographic facets from density functional calculations. Phys. Chem. Chem. Phys. 2015, 17, 4193–4198. [Google Scholar] [CrossRef] [PubMed]
- Obey, G.; Adelaide, M.; Ramara, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, B.; Feng, L.; Zheng, J.; You, B.; Chen, J.; Zhao, X.; Zhang, C.; Jiang, S.; He, S. Progress in the use of organic potassium salts for the synthesis of porous carbon nanomaterials: Microstructure engineering for advanced supercapacitors. Nanoscale 2022, 14, 8216–8244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).