Performance of a Mobile 3D Camera to Evaluate Simulated Pathological Gait in Practical Scenarios
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Instrumentation
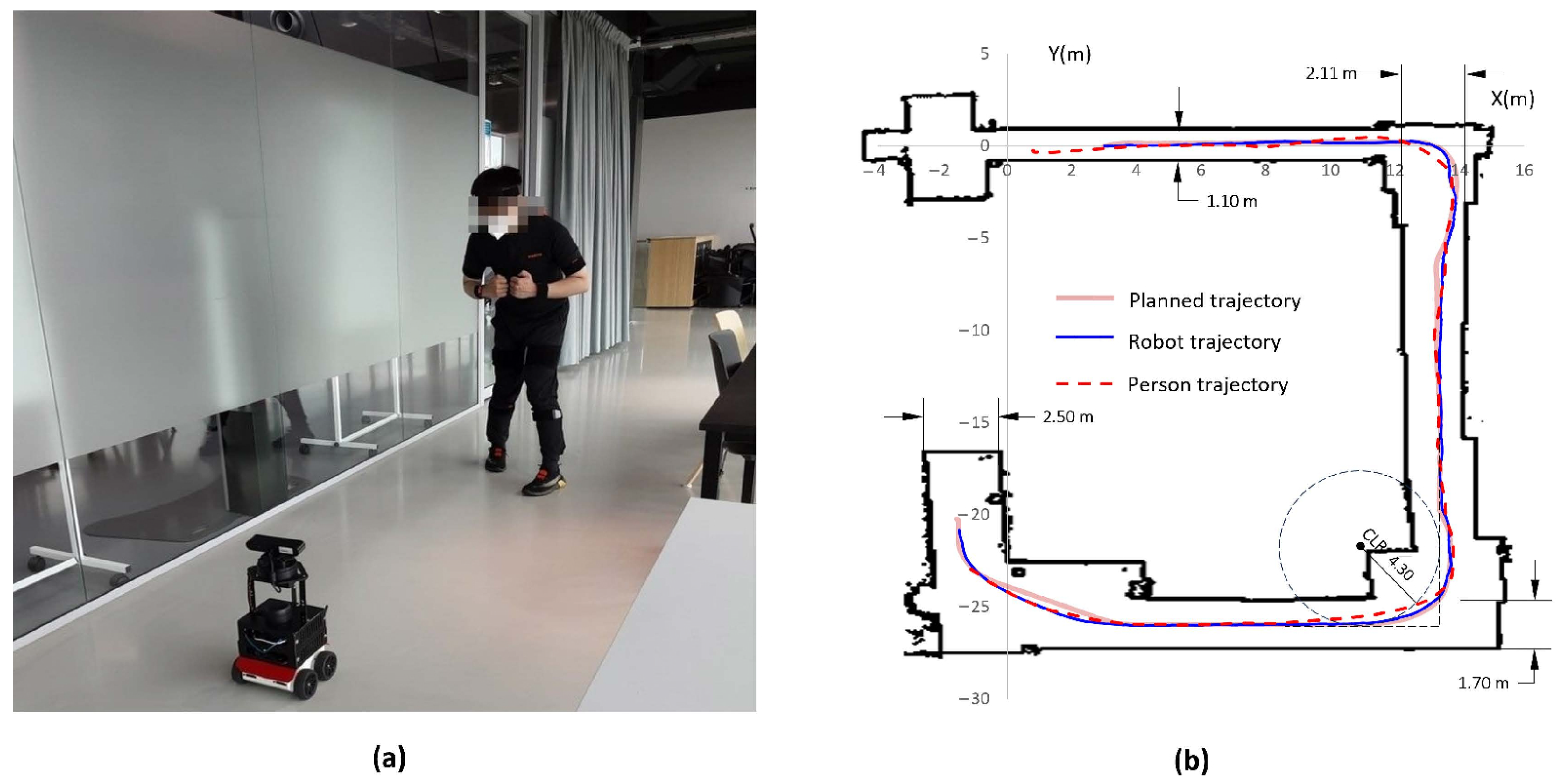
2.3. Conditions and Trajectory
2.4. Data Processing
2.5. Data Analysis and Statistics
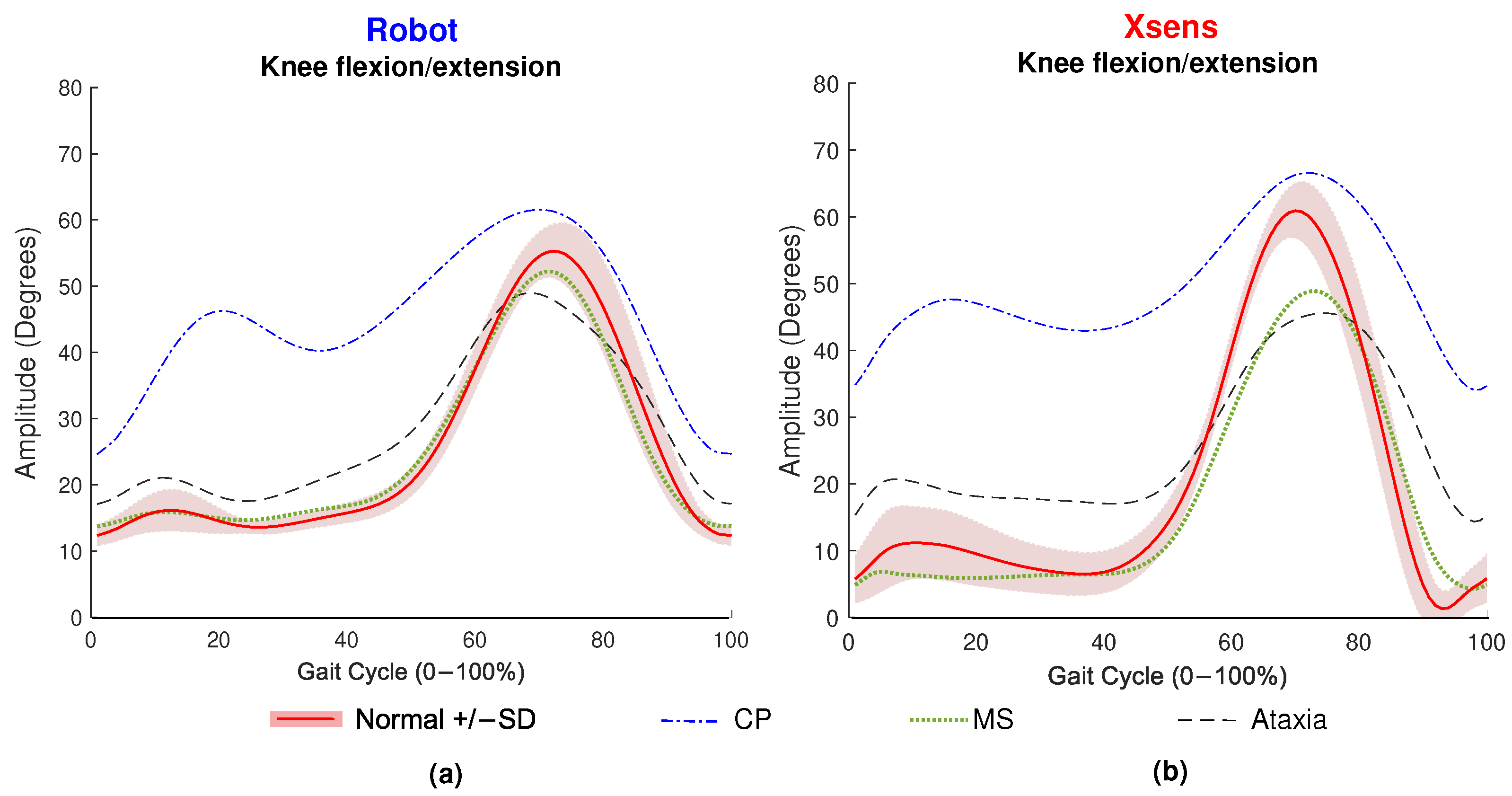
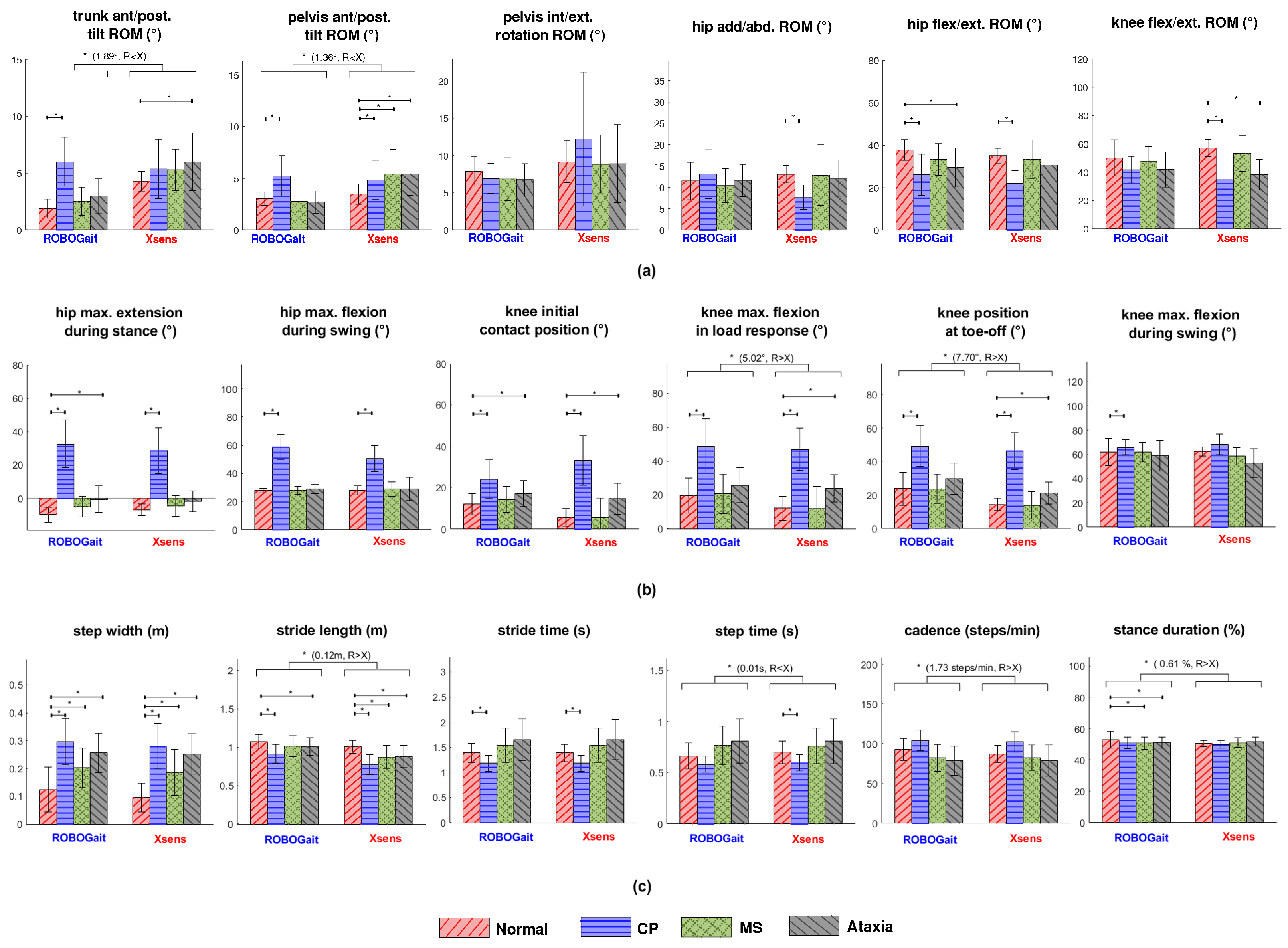
3. Results
4. Discussion
4.1. Interpretation of Results
4.2. Limitations and Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Springer, S.; Seligmann, G. Validity of the Kinect for Gait Assessment: A Focused Review. Sensors 2016, 16, 194. [Google Scholar] [CrossRef] [PubMed]
- Eltoukhy, M.; Oh, J.; Kuenze, C.; Signorile, J. Improved kinect-based spatiotemporal and kinematic treadmill gait assessment. Gait Posture 2017, 51, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Pfister, A.; West, A.M.; Bronner, S.; Noah, J.A. Comparative abilities of Microsoft Kinect and Vicon 3D motion capture for gait analysis. J. Med. Eng. Technol. 2014, 38, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Ilg, W.; Giese, M.A.; Ludolph, N. Validation of enhanced kinect sensor based motion capturing for gait assessment. PLoS ONE 2017, 12, e0175813. [Google Scholar] [CrossRef]
- Marques, N.R.; Spinoso, D.H.; Cardoso, B.C.; Moreno, V.C.; Kuroda, M.H.; Navega, M.T. Is it possible to predict falls in older adults using gait kinematics? Clin. Biomech. 2018, 59, 15–18. [Google Scholar] [CrossRef]
- Geerse, D.J.; Coolen, B.H.; Roerdink, M. Walking-adaptability assessments with the Interactive Walkway: Between-systems agreement and sensitivity to task and subject variations. Gait Posture 2017, 54, 194–201. [Google Scholar] [CrossRef]
- Guffanti, D.; Brunete, A.; Hernando, M.; Rueda, J.; Navarro, E. ROBOGait: A Mobile Robotic Platform for Human Gait Analysis in Clinical Environments. Sensors 2021, 21, 6786. [Google Scholar] [CrossRef]
- Cifuentes, C.A.; Frizera, A. Human-Robot Interaction Strategies for Walker-Assisted Locomotion, 1st ed.; Springer Tracts in Advanced Robotics, 115; Springer: Cham, Switzerland, 2016; p. 105. [Google Scholar]
- Guffanti, D.; Brunete, A.; Hernando, M. Development and validation of a ROS-based mobile robotic platform for human gait analysis applications. Robot. Auton. Syst. 2021, 145, 103869. [Google Scholar] [CrossRef]
- Novacheck, T.F.; Trost, J.P.; Sohrweide, S. Examination of the Child with Cerebral Palsy. Orthop. Clin. N. Am. 2010, 41, 469–488. [Google Scholar] [CrossRef] [PubMed]
- Stolze, H.; Klebe, S.; Petersen, G.; Raethjen, J.; Wenzelburger, R.; Witt, K.; Deuschl, G. Typical features of cerebellar ataxic gait. J. Neurol. Neurosurg. Psychiatry 2002, 73, 310–312. [Google Scholar] [CrossRef]
- Bulley, C.; Mercer, T.H.; Hooper, J.E.; Cowan, P.; Scott, S.; van der Linden, M.L. Experiences of functional electrical stimulation (FES) and ankle foot orthoses (AFOs) for foot-drop in people with multiple sclerosis. Disabil. Rehabil. Assist. Technol. 2015, 10, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Spomer, A.M.; Yan, R.Z.; Schwartz, M.H.; Steele, K.M. Synergies are minimally affected during emulation of cerebral palsy gait patterns. J. Biomech. 2022, 133, 110953. [Google Scholar] [CrossRef] [PubMed]
- Guffanti, D.; Brunete, A.; Hernando, M.; Gambao, E.; Álvarez, D. ANN-based Optimization of Human Gait Data Obtained from a Robot-Mounted 3D Camera: A Multiple Sclerosis Case Study. IEEE Robot. Autom. Lett. 2022, 7, 8901–8908. [Google Scholar] [CrossRef]
- Xsens Technologies B.V. MVN User Manual; Xsens Technologies B.V.: Enschede, The Netherlands, 2021. [Google Scholar]
- Schepers, M.; Giuberti, M.; Bellusci, G. Xsens mvn: Consistent tracking of human motion using inertial sensing. Xsens Technol. 2018, 1, 1–8. [Google Scholar]
- Wu, G.; van der Helm, F.C.T.; Veeger, H.E.J.; Makhsous, M.; Van Roy, P.; Anglin, C.; Nagels, J.; Karduna, A.R.; McQuade, K.; Wang, X.; et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—Part II: Shoulder, elbow, wrist and hand. J. Biomech. 2005, 38, 981–992. [Google Scholar] [CrossRef]
- Francisco, M.; Carratalá, M. La Marcha Humana: Biomecánica, Evaluación y Patología; Editorial Médica Panamericana: Madrid, Spain, 2020; p. 268. [Google Scholar]
- Zeni, J.A.; Richards, J.G.; Higginson, J.S. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 2007, 27, 710–714. [Google Scholar] [CrossRef]
- Kim, C.J.; Son, S.M. Comparison of Spatiotemporal Gait Parameters between Children with Normal Development and Children with Diplegic Cerebral Palsy. J. Phys. Ther. Sci. 2014, 26, 1317–1319. [Google Scholar] [CrossRef]
- Comber, L.; Galvin, R.; Coote, S. Gait deficits in people with multiple sclerosis: A systematic review and meta-analysis. Gait Posture 2017, 51, 25–35. [Google Scholar] [CrossRef]
- Buckley, E.; Mazzà, C.; McNeill, A. A systematic review of the gait characteristics associated with Cerebellar Ataxia. Gait Posture 2018, 60, 154–163. [Google Scholar] [CrossRef]
- Miller, F. Knee Flexion Deformity in Cerebral Palsy. In Cerebral Palsy; Miller, F., Bachrach, S., Lennon, N., O’Neil, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 2137–2158. [Google Scholar] [CrossRef]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Braga Rodrigues, T.; Ó Catháin, C.; Devine, D.; Moran, K.; O’Connor, N.; Murray, N. An evaluation of a 3D multimodal marker-less motion analysis system. In Proceedings of the 10th ACM Multimedia Systems Conference, Amherst, MA, USA, 18–21 June 2019; pp. 213–221. [Google Scholar] [CrossRef]
- Benjaminse, A.; Bolt, R.; Gokeler, A.; Otten, B. A validity study comparing xsens with vicon. In Proceedings of the 38th International Society of Biomechanics in Sport Conference, Online, 20–24 July 2020. [Google Scholar]
- Kalron, A.; Achiron, A.; Dvir, Z. Muscular and Gait Abnormalities in Persons With Early Onset Multiple Sclerosis. J. Neurol. Phys. Ther. 2011, 35, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Lamine, H.; Bennour, S.; Laribi, M.; Romdhane, L.; Zaghloul, S. Evaluation of Calibrated Kinect Gait Kinematics Using a Vicon Motion Capture System. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 111–112. [Google Scholar] [CrossRef] [PubMed]
| Body Segment | N. Trackers | Abbreviation | Position |
|---|---|---|---|
| Foot | 2 | FOOT | Middle of bridge of foot |
| Lower leg | 2 | LLEG | Flat on the shin bone |
| Upper leg | 2 | ULEG | Lateral side above knee |
| Pelvis | 1 | PELV | Flat on sacrum |
| Sternum | 1 | STERN | Flat, in the middle of the chest |
| Shoulder | 2 | SHOULD | Scapula (shoulder blades) |
| Upper arm | 2 | UARM | Lateral side above elbow |
| Fore arm | 2 | FARM | Lateral and flat side of the wrist |
| Head | 1 | HEAD | At the back of the head |
| Total of sensors | 15 |
| Measure | Value (%) | Formula | |||
|---|---|---|---|---|---|
| CP | MS | Ataxia | Average | ||
| Accuracy | 72.2 | 83.3 | 55.6 | 70.4 | (TP + TN)/(P + N) |
| Sensitivity | 76.9 | 33.3 | 37.5 | 49.3 | TP/(TP + FN) |
| Specificity | 60.0 | 93.3 | 70.0 | 74.4 | TN/(FP + TN) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guffanti, D.; Lemus, D.; Vallery, H.; Brunete, A.; Hernando, M.; Horemans, H. Performance of a Mobile 3D Camera to Evaluate Simulated Pathological Gait in Practical Scenarios. Sensors 2023, 23, 6944. https://doi.org/10.3390/s23156944
Guffanti D, Lemus D, Vallery H, Brunete A, Hernando M, Horemans H. Performance of a Mobile 3D Camera to Evaluate Simulated Pathological Gait in Practical Scenarios. Sensors. 2023; 23(15):6944. https://doi.org/10.3390/s23156944
Chicago/Turabian StyleGuffanti, Diego, Daniel Lemus, Heike Vallery, Alberto Brunete, Miguel Hernando, and Herwin Horemans. 2023. "Performance of a Mobile 3D Camera to Evaluate Simulated Pathological Gait in Practical Scenarios" Sensors 23, no. 15: 6944. https://doi.org/10.3390/s23156944
APA StyleGuffanti, D., Lemus, D., Vallery, H., Brunete, A., Hernando, M., & Horemans, H. (2023). Performance of a Mobile 3D Camera to Evaluate Simulated Pathological Gait in Practical Scenarios. Sensors, 23(15), 6944. https://doi.org/10.3390/s23156944








