Laser Sensing and Chemometric Analysis for Rapid Detection of Oregano Fraud
Abstract
1. Introduction
- Cost: chromatography and mass spectrometry can be expensive to set up and maintain. Chromatography equipment, consumables, and solvents can add up to significant costs, especially for high-performance systems. Purchasing a mass spectrometer and ensuring its proper functioning may pose financial challenges for smaller research facilities or laboratories.
- Time-consuming: chromatographic separations and mass spectrometry sample preparation can be time-consuming, especially for complex mixtures. The process may take hours or even days to complete, depending on the complexity of the sample, which can introduce the potential for mistakes or loss of analytes during the process.
- Complexity: both chromatography and mass spectrometry are complex techniques that require skilled operators and a deep understanding of the principles behind the methods. Data analysis can be complicated, and it often requires specialised software and expert personnel to handle the measurements effectively.
- Sample preparation: both chromatography and mass spectrometry often require careful sample preparation to ensure accurate results. Preparing samples can be labour-intensive and may introduce errors if not conducted correctly.
- Sensitivity: both chromatography and mass spectrometry might lack the sensitivity needed to detect low-abundance compounds in a mixture. This limitation can be an issue when analysing trace amounts or minor components in a sample.
2. Materials and Methods
2.1. LPAS System
- It is mounted on a trolley and can therefore be used in the field (Figure 2);
- The cell has been redesigned to facilitate sample loading using a small drawer;
- Almost all subsystems have been replaced by new models with improved performance (Table 1);
- The wavelength range of the laser source has been extended (Table 2).
2.2. Measurement Run of July 2020 (“07-2020”)
- 0% (OR): Oregano leaflets with ICEA (https://icea.bio/en/ accessed on 4 July 2023) certificate of conformity were purchased from Bioagricola Bosco (Favara AG, Italy) and ground using a ball pestle impact grinder;
- 100% (OL): Ground olive leaves were purchased from Sigma–Aldrich (olive leaf dry extract 1478265);
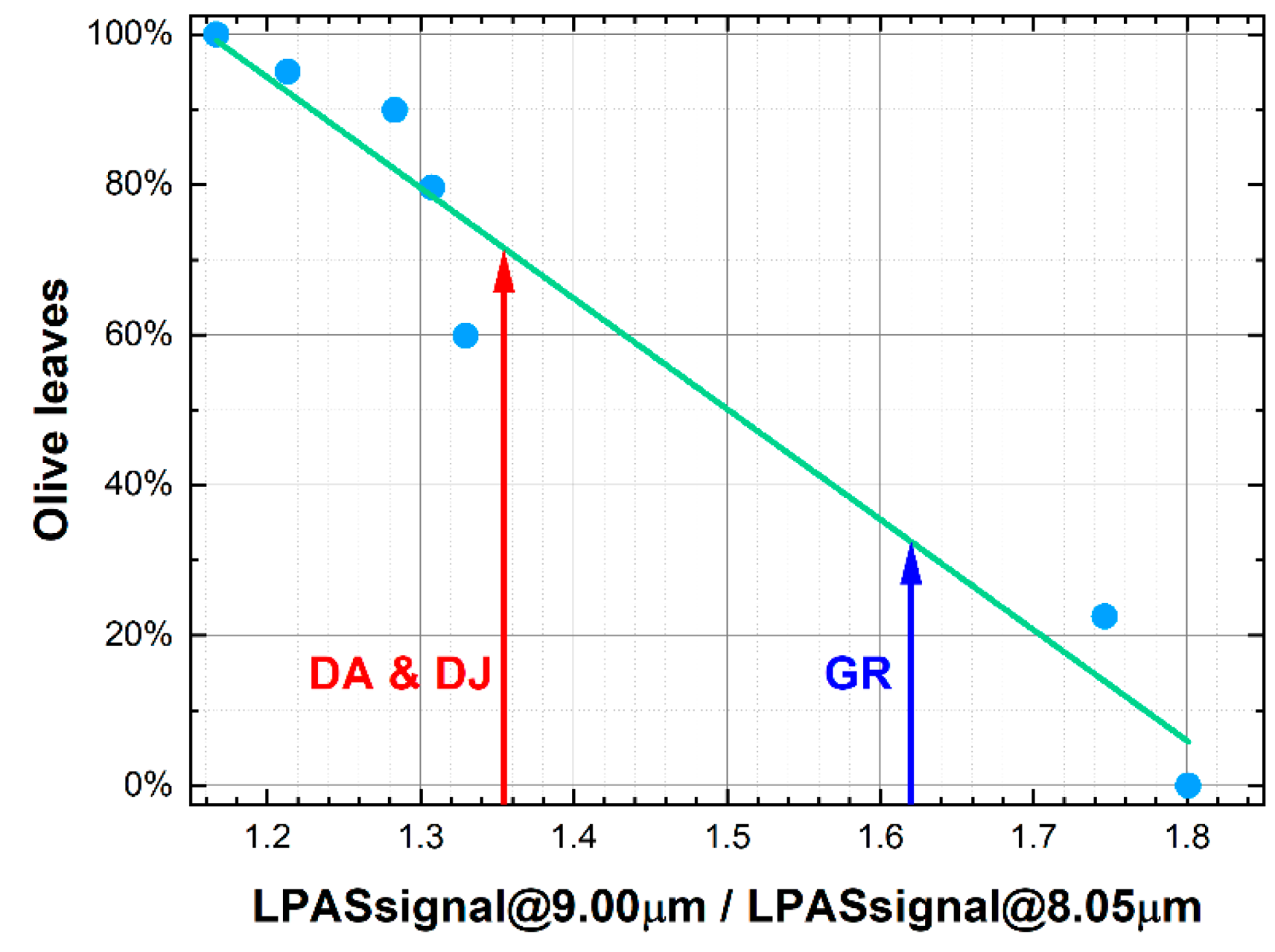
- 20%, 60%, 80%, 90% and 95%: Mixtures with OL/(OR + OL) mass ratios of 20%, 60%, 80%, 90% and 95%, respectively, were prepared using a high-precision analytical balance;
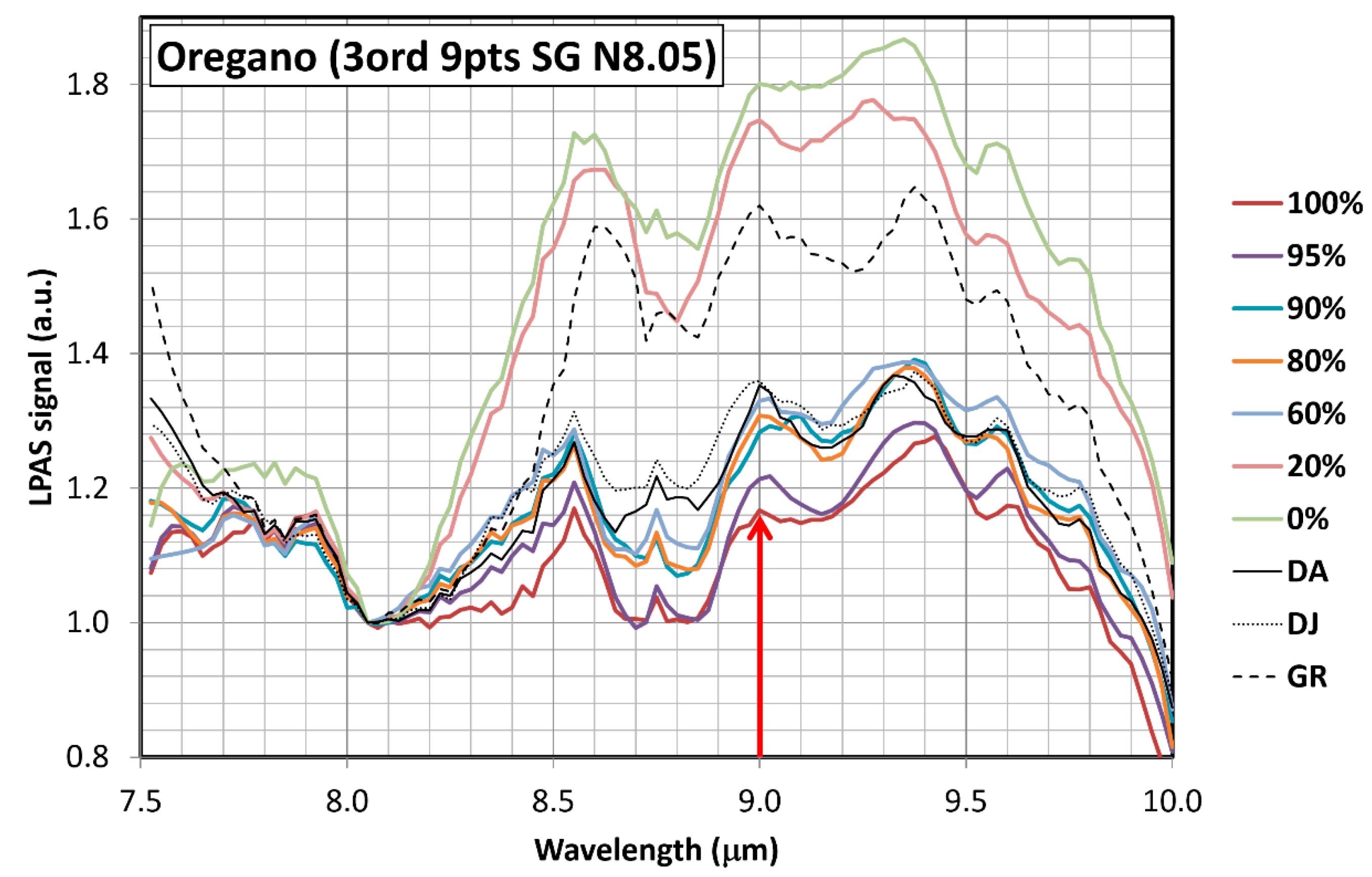
- DA, DJ and GR: commercial ground oregano sold by three different companies.
2.3. Measurement Run of February 2021 (“02-2021”)
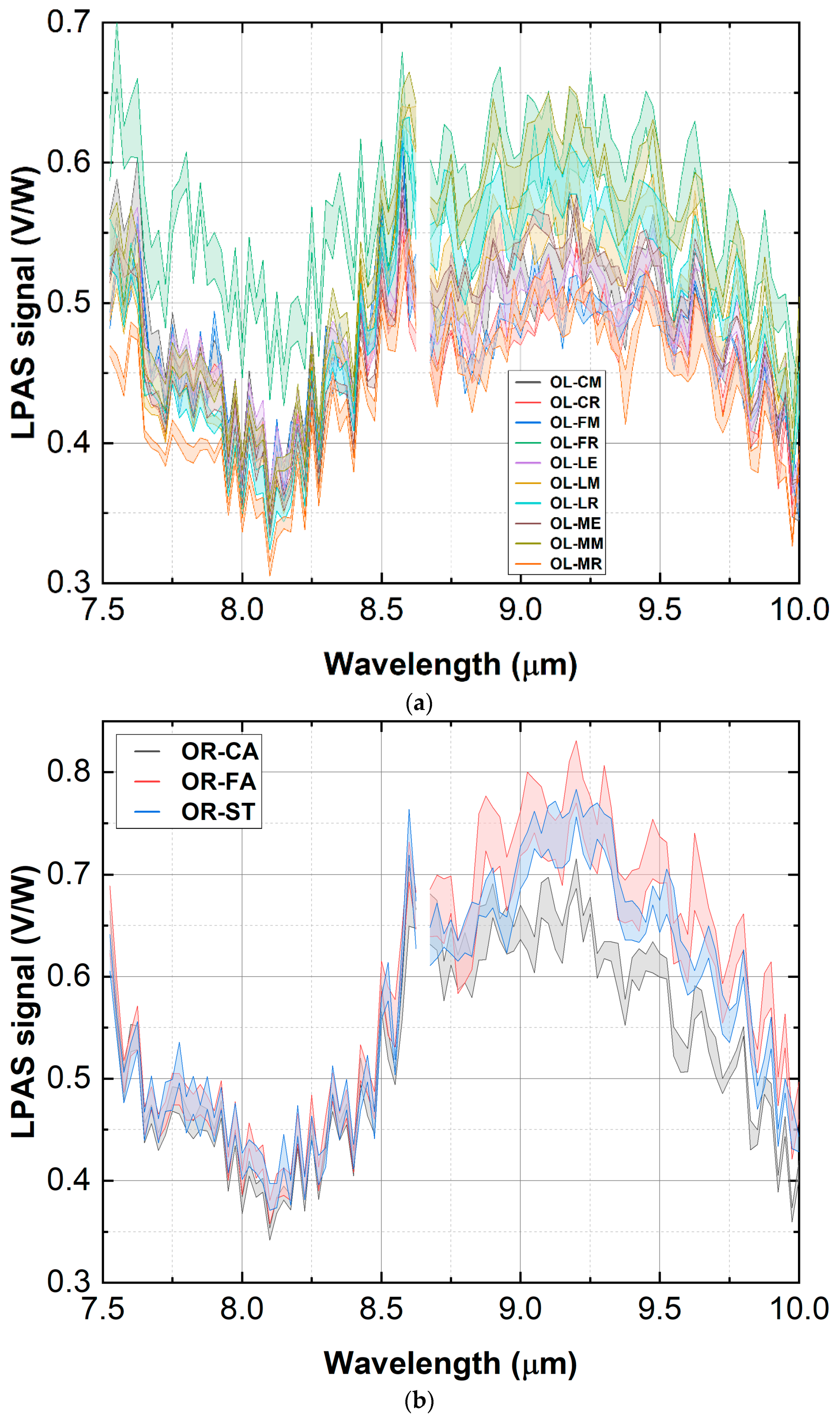
- Olive
- ○
- OL-CM: Canino cultivar from the Mario field;
- ○
- OL-CR: Canino cultivar from the Rolando field;
- ○
- OL-FM: Frantoio cultivar from the Mario field;
- ○
- OL-FR: Frantoio cultivar from the Rolando field;
- ○
- OL-LE: Leccino cultivar from the ENEA field;
- ○
- OL-LM: Leccino cultivar from the Mario field;
- ○
- OL-LR: Leccino cultivar from the Rolando field;
- ○
- OL-ME: Maurino cultivar from the ENEA field;
- ○
- OL-MM: Maurino cultivar from the Mario field;
- ○
- OL-MR: Maurino cultivar from the Rolando field.
- Oregano
- ○
- OR-CA: oregano twigs (impossible to adulterate) from Cosenza CS, Italy;
- ○
- OR-FA: oregano twigs from Favignana TP, Italy;
- ○
- OR-ST: oregano sample of 07-2020 from Favara AG, Italy.
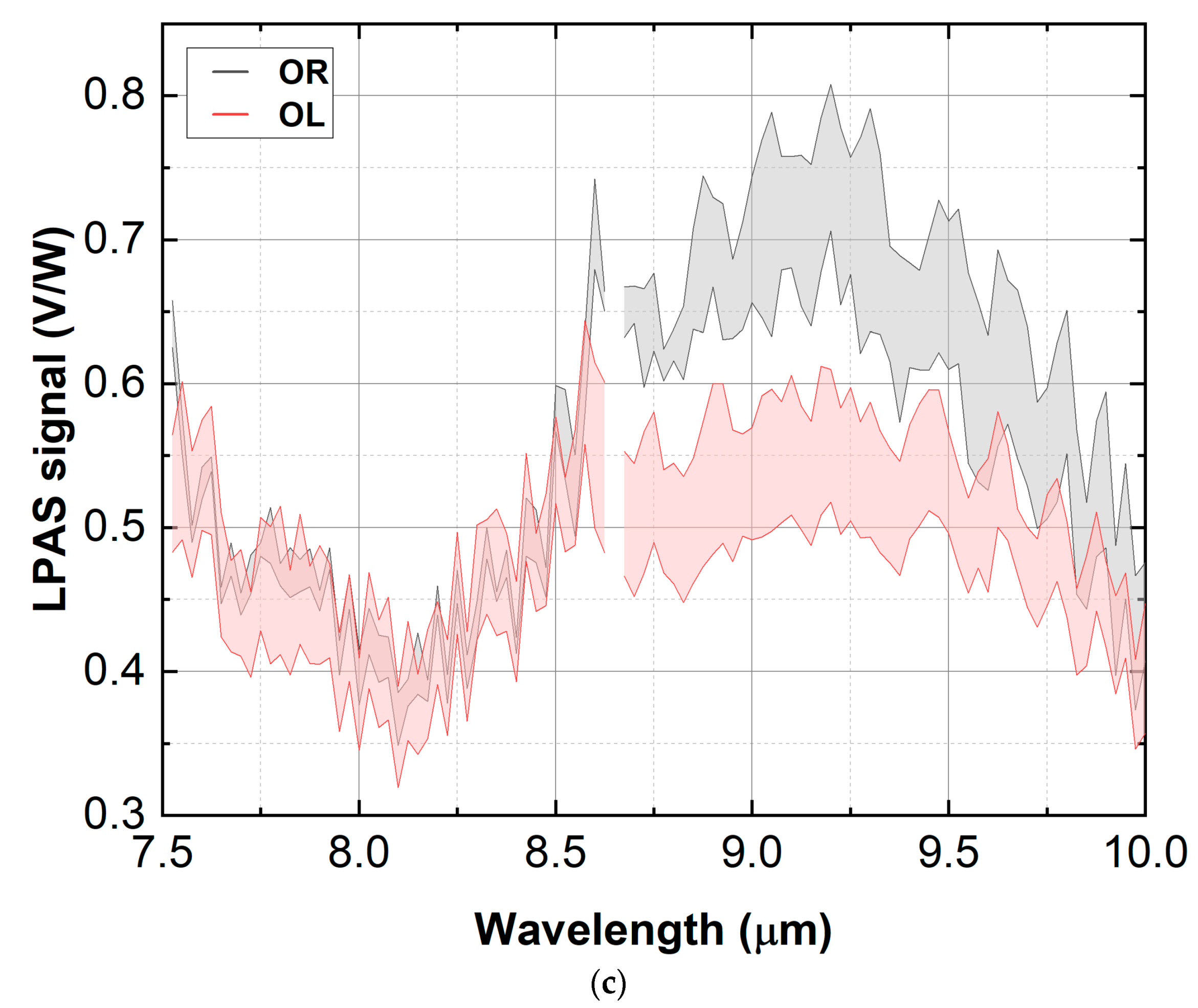
- OL: obtained by mixing cultivars in these proportions to reproduce an average composition of central Italy: Frantoio 40%, Leccino 30%, Maurino 20% and Canino 10%;
- OR: obtained by mixing varieties in these proportions to reproduce an average composition of southern Italy: Cosenza 34%, Favignana 33% and Favara 33%;
- OL20inOR, OL60inOR, OL80inOR, OL90inOR and OL95inOR: blends with OL/(OL + OR) mass ratios of 20%, 60%, 80%, 90% and 95%, respectively, obtained using a high-precision analytical balance.
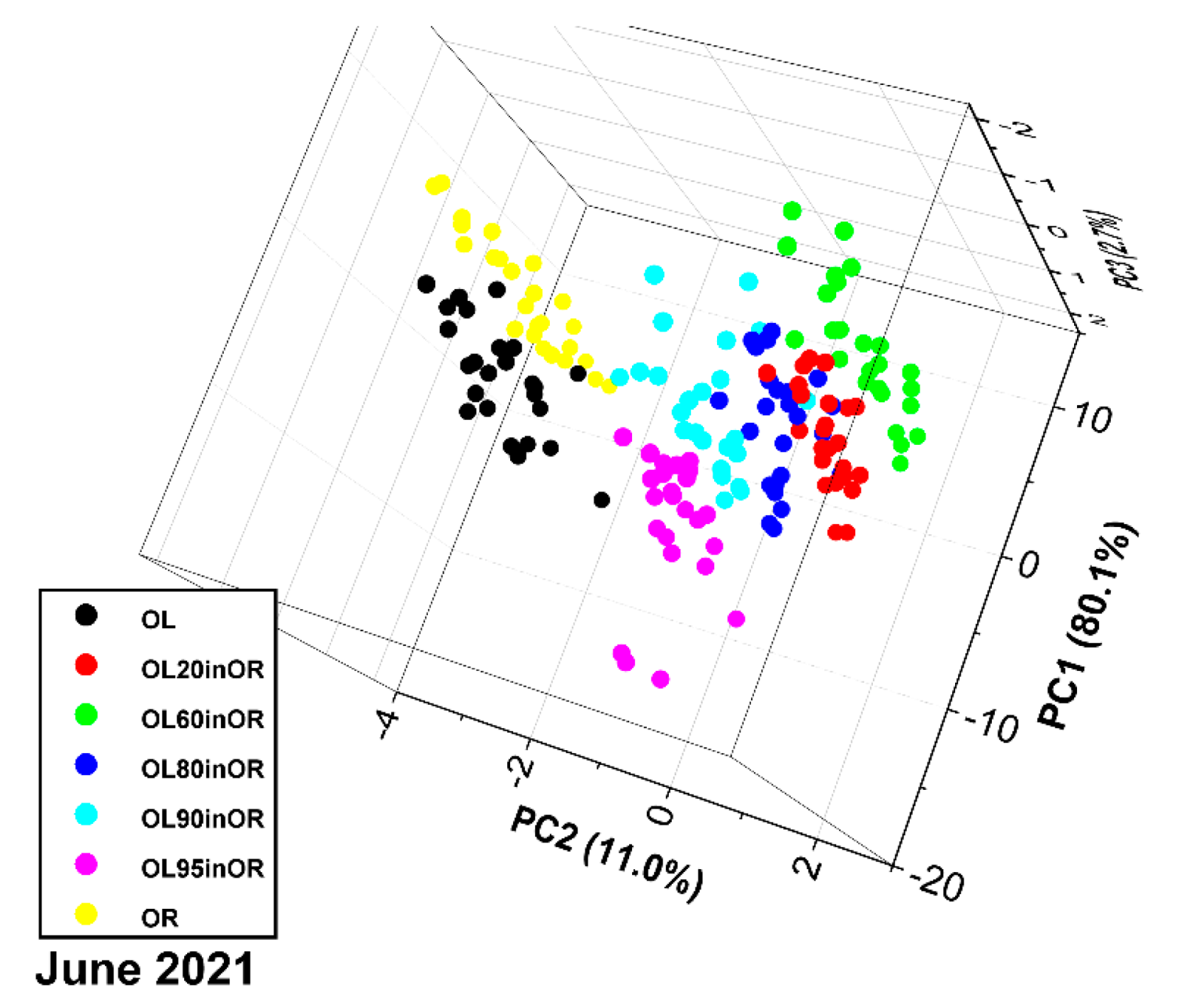
2.4. Measurement Run of June 2021 (“06-2021”)
- OL: obtained by mixing cultivars in these proportions to reproduce an average composition of central Italy: Pendolino 40%, Leccino 30%, Maurino 20% and Canino 10% (Pendolino, also coming from ENEA controlled fields, replaced Frantoio due to the lack of the latter cultivar);
- OR: obtained only from the Cosenza variety (this variety was chosen to test the sensor because its spectrum is closer to that of olive, see Section 3.2.1);
- OL20inOR, OL60inOR, OL80inOR, OL90inOR and OL95inOR: blends with OL/(OL + OR) mass ratios of 20%, 60%, 80%, 90% and 95%, respectively, obtained using a high-precision analytical balance.
3. Results
- The QCL scanned wavelengths from λ1 to λ2 with a step size of Δλ.
- The lock-in amplifier and the power meter measured the photoacoustic signal (V) and the laser power (W), respectively, at each wavelength. Each measurement took 1 s and was repeated N times, and these measurements were averaged.
- The LPAS signal (V/W) is given by the ratio of these averages (thus normalising the photoacoustic signal to the laser power).
3.1. Measurement Run 07-2020
- λ1 = 7.525 μm;
- λ2 = 10.000 μm;
- Δλ = 0.025 μm;
- N = 10;
- One spectrum per sample was measured, and each spectrum was acquired in less than twenty minutes.
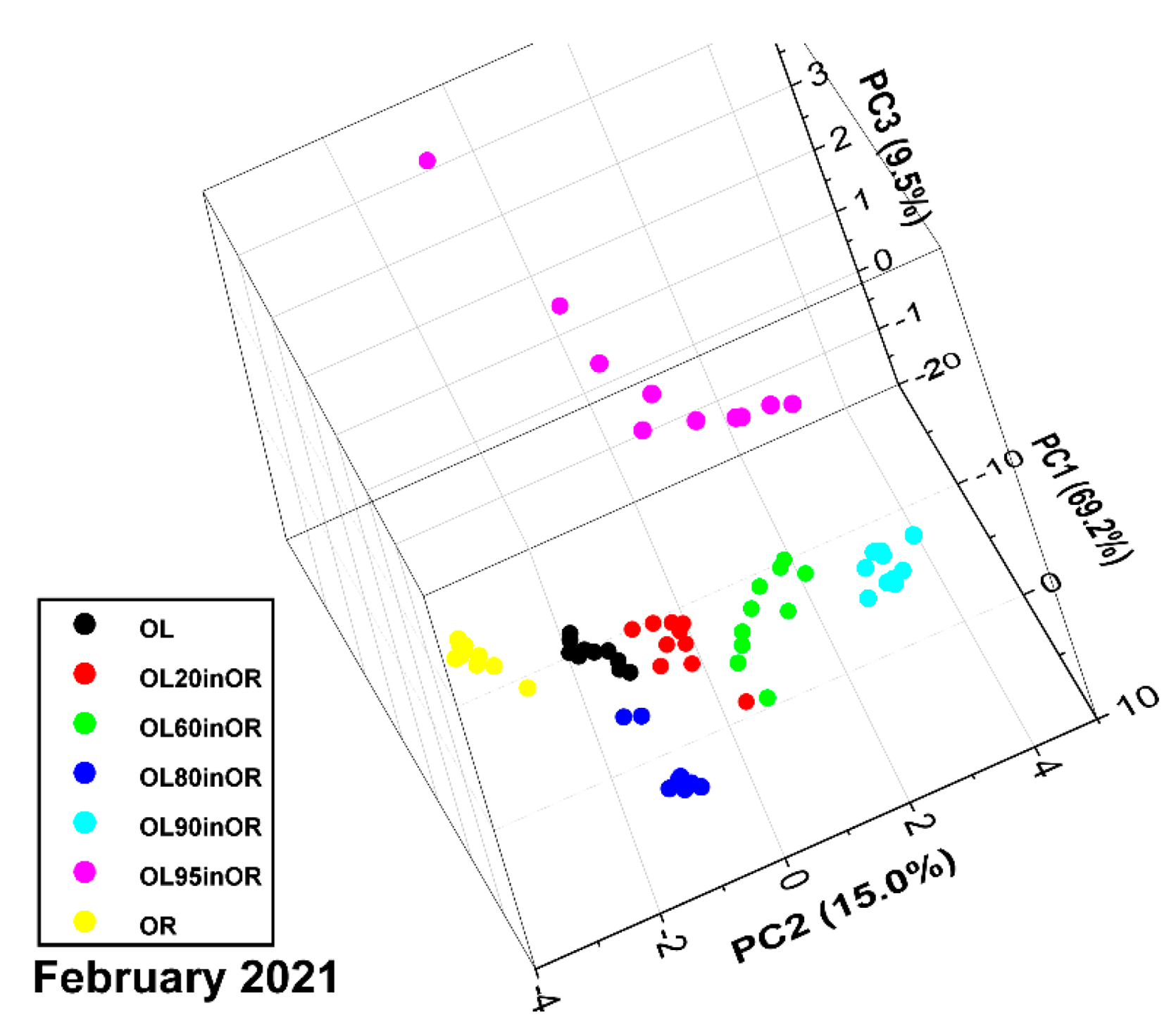
3.2. Measurement Run 02-2021
3.2.1. Consistency Check
- λ1 = 7.525 μm;
- λ2 = 10.000 μm;
- Δλ = 0.025 μm;
- N = 5;
- Three spectra per sample were measured, and each spectrum was acquired in less than ten minutes.
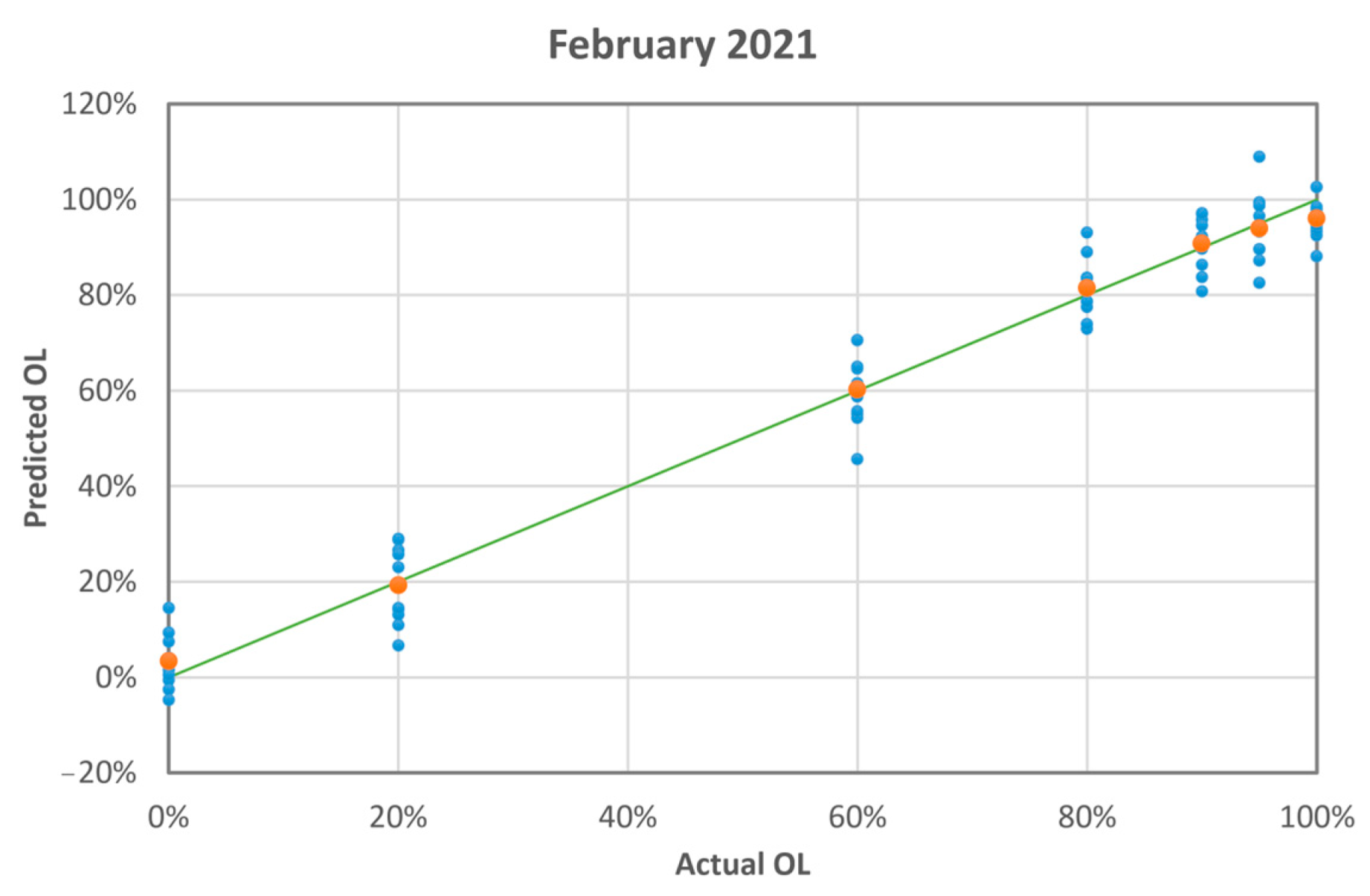
3.2.2. Chemometric Analysis
- λ1 = 7.6 μm;
- λ2 = 10.0 μm;
- Δλ = 0.1 μm;
- N = 10;
- Ten spectra per sample were measured, and each spectrum was acquired in less than five minutes).
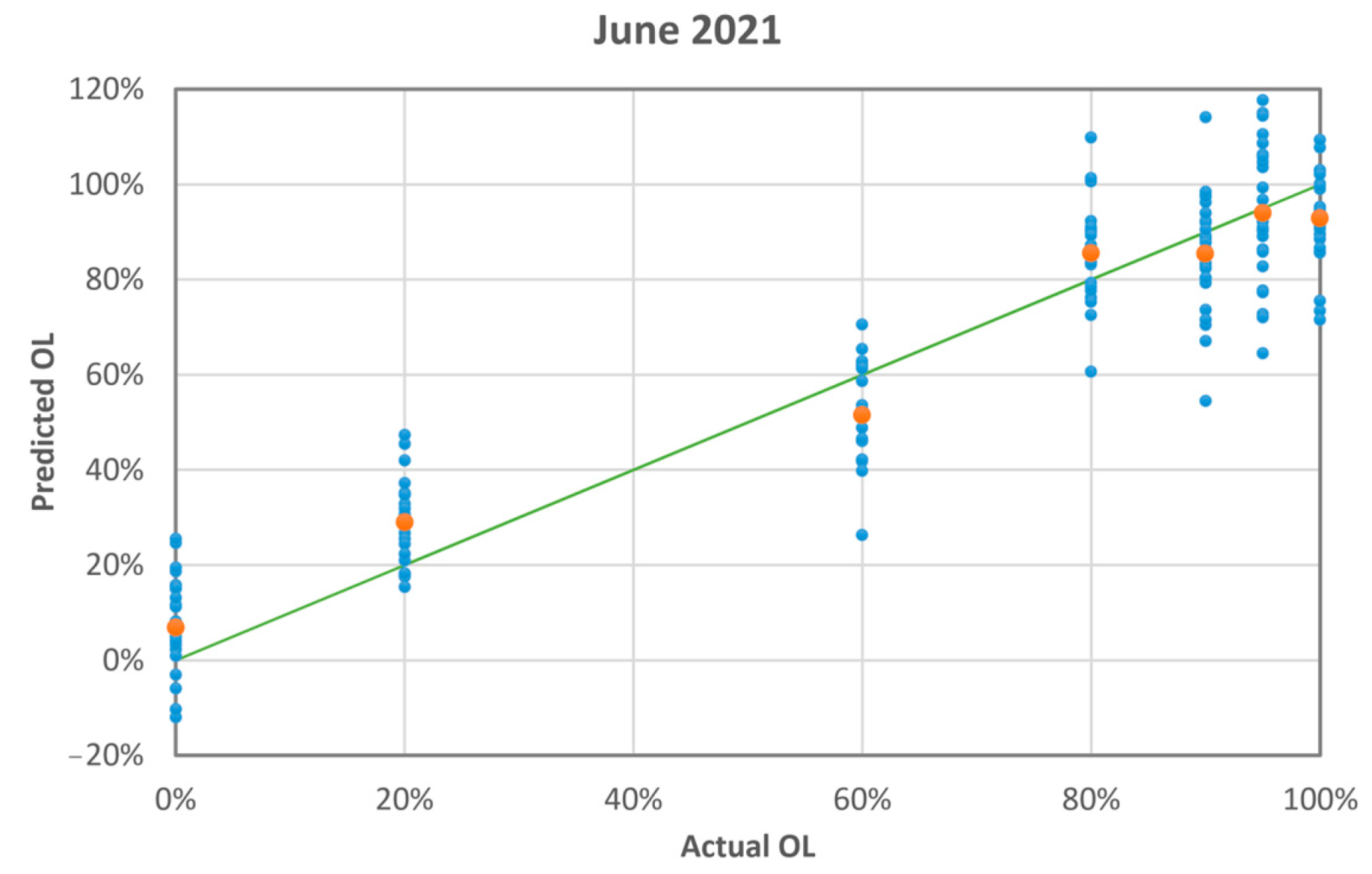
3.3. Measurement Run 06-2021
- λ1 = 7.5 μm;
- λ2 = 10.0 μm;
- Δλ = 0.1 μm;
- N = 3;
- Twenty-five spectra per sample were measured, and each spectrum was acquired in less than two minutes.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, L.; Duong, L.T.; Mentreddy, R.S. The U.S. import demand for spices and herbs by differentiated sources. J. Appl. Res. Med. Aromat. Plants 2019, 12, 13–20. [Google Scholar] [CrossRef]
- Galvin-King, P.; Haughey, S.A.; Elliott, C.T. Herb and spice fraud; the drivers, challenges and detection. Food Control 2018, 88, 85–97. [Google Scholar] [CrossRef]
- Moore, J.C.; Spink, J.; Lipp, M. Development and application of a database of food ingredient fraud and economically motivated adulteration from 1980 to 2010. J. Food Sci. 2012, 77, R118–R126. [Google Scholar] [CrossRef] [PubMed]
- Morin, J.F.; Lees, M. (Eds.) FoodIntegrity Handbook, a Guide to Food Authenticity Issues and Analytical Solutions; Eurofins: Nantes, France, 2018. [Google Scholar] [CrossRef]
- Moore, J.C. Food fraud: Public health threats and the need for new analytical detection approaches. In NABC Report 23: Food Security: The Intersection of Sustainability, Safety and Defense; Abel Ponce de León, F., Ed.; NABC: Ithaca, NY, USA, 2011; pp. 209–220. Available online: https://ecommons.cornell.edu/handle/1813/51364 (accessed on 28 June 2023).
- Fiorani, L.; Giubileo, G.; Mangione, L.; Puiu, A.; Saleh, W. Food Fraud Detection by Laser Photoacoustic Spectroscopy; RT/2017/41/ENEA; ENEA: Rome, Italy, 2017; Available online: https://iris.enea.it/retrieve/handle/20.500.12079/6809/558/RT-2017-41-ENEA.pdf (accessed on 28 June 2023).
- Haisch, C. Photoacoustic spectroscopy for analytical measurements. Meas. Sci. Technol. 2012, 23, 012001. [Google Scholar] [CrossRef]
- Hernández-Aguilar, C.; Domínguez-Pacheco, A.; Cruz-Orea, A.; Ivanov, R. Photoacoustic spectroscopy in the optical characterization of foodstuff: A review. J. Spectrosc. 2019, 2019, 5920948. [Google Scholar] [CrossRef]
- Fiorani, L.; Giubileo, G.; Mannori, S.; Puiu, A.; Saleh, W. QCL Based Photoacoustic Spectrometer for Food Safety; RT/2019/1/ENEA; ENEA: Rome, Italy, 2019; Available online: https://iris.enea.it/retrieve/handle/20.500.12079/6831/580/RT-2019-01-ENEA.pdf (accessed on 28 June 2023).
- Fiorani, L.; Artuso, F.; Giardina, I.; Lai, A.; Mannori, S.; Puiu, A. Photoacoustic laser system for food fraud detection. Sensors 2021, 21, 4178. [Google Scholar] [CrossRef] [PubMed]
- Pucci, E.; Palumbo, D.; Puiu, A.; Lai, A.; Fiorani, L.; Zoani, C. Characterization and discrimination of Italian olive (Olea europaea sativa) cultivars by production area using different analytical methods combined with chemometric analysis. Foods 2022, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Black, C.; Haughey, S.A.; Chevallier, O.P.; Galvin-King, P.; Elliott, C.T. A comprehensive strategy to detect the fraudulent adulteration of herbs: The oregano approach. Food Chem. 2016, 210, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Massaro, A.; Negro, A.; Bragolusi, M.; Miano, B.; Tata, A.; Suman, M.; Piro, R. Oregano authentication by mid-level data fusion of chemical fingerprint signatures acquired by ambient mass spectrometry. Food Control 2021, 126, 108058. [Google Scholar] [CrossRef]
- Van De Steene, J.; Ruyssinck, J.; Fernandez-Pierna, J.-A.; Vandermeersch, L.; Maes, A.; Van Langenhove, H.; Walgraeve, C.; Demeestere, K.; De Meulenaer, B.; Jacxsens, L.; et al. Authenticity analysis of oregano: Development, validation and fitness for use of several food fingerprinting techniques. Food Res. Int. 2022, 162, 111962. [Google Scholar] [CrossRef] [PubMed]
- Flügge, F.; Kerkow, T.; Kowalski, P.; Bornhöft, J.; Seemann, E.; Creydt, M.; Schütze, B.; Günther, U.L. Qualitative and quantitative food authentication of oregano using NGS and NMR with chemometrics. Food Control 2023, 145, 109497. [Google Scholar] [CrossRef]
- Hibbert, D.B. Experimental design in chromatography: A tutorial review. J. Chromatogr. B 2012, 910, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Chait, B.T. Mass spectrometry in the postgenomic era. Annu. Rev. Biochem. 2011, 80, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Draper, N.R.; Smith, H. Applied Regression Analysis, 3rd ed.; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- Kalivas, J.H.; Brown, S.D. Calibration methodologies. In Comprehensive Chemometrics, 2nd ed.; Brown, S.D., Tauler, R., Walczak, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 3, pp. 213–247. [Google Scholar] [CrossRef]
- OriginPro. Available online: https://www.originlab.com/ (accessed on 28 June 2023).
- ChemFlow. Available online: https://vm-chemflow-francegrille.eu/ (accessed on 28 June 2023).


| Element | Manufacturer | Model |
|---|---|---|
| BS | Thorlabs | WG71050 |
| C | ENEA 1 | N.A. |
| CH | Thorlabs | MC2000B-EC |
| F | Hewlett-Packard | 5489A |
| LIA | Zurich Instruments | MFLI |
| M | Thorlabs | PF10-03-M02 |
| MP | Knowles | EK23024000 |
| PC | AAEON | ACP-1106 |
| PM | Gentec-EO | UP12E-10S-H5-INT |
| QCL | DRS Daylight Solutions | MIRcat-1200 |
| W | Thorlabs | WG71050-E4 |
| Wavelength range | 6.0–11.1 µm |
| Linewidth | 100 MHz |
| Wavelength accuracy | 1 cm−1 |
| Average power | 60 mW |
| Power stability | 3% |
| Spatial mode | TEM00 |
| Beam divergence | 4 mrad |
| Beam pointing stability | 2 mrad |
| Spot size | 2.5 mm |
| Polarisation | Vertical 100:1 |
| Actual OL [%] | Predicted OL [%] (Average ± Standard Deviation) | Absolute Difference |
|---|---|---|
| 0 | 3 ± 4 | 3.3 |
| 20 | 19 ± 3 | 0.7 |
| 60 | 60 ± 4 | 0.2 |
| 80 | 82 ± 4 | 1.5 |
| 90 | 91 ± 3 | 0.8 |
| 95 | 94 ± 4 | 1.0 |
| 100 | 96 ± 3 | 4.0 |
| Actual OL [%] | Predicted OL [%] (Average ± Standard Deviation) | Absolute Difference |
|---|---|---|
| 0 | 7 ± 7 | 6.8 |
| 20 | 29 ± 7 | 9.0 |
| 60 | 52 ± 7 | 8.5 |
| 80 | 86 ± 7 | 5.5 |
| 90 | 86 ± 9 | 4.5 |
| 95 | 94 ± 8 | 1.0 |
| 100 | 93 ± 8 | 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorani, L.; Lai, A.; Puiu, A.; Artuso, F.; Ciceroni, C.; Giardina, I.; Pollastrone, F. Laser Sensing and Chemometric Analysis for Rapid Detection of Oregano Fraud. Sensors 2023, 23, 6800. https://doi.org/10.3390/s23156800
Fiorani L, Lai A, Puiu A, Artuso F, Ciceroni C, Giardina I, Pollastrone F. Laser Sensing and Chemometric Analysis for Rapid Detection of Oregano Fraud. Sensors. 2023; 23(15):6800. https://doi.org/10.3390/s23156800
Chicago/Turabian StyleFiorani, Luca, Antonia Lai, Adriana Puiu, Florinda Artuso, Claudio Ciceroni, Isabella Giardina, and Fabio Pollastrone. 2023. "Laser Sensing and Chemometric Analysis for Rapid Detection of Oregano Fraud" Sensors 23, no. 15: 6800. https://doi.org/10.3390/s23156800
APA StyleFiorani, L., Lai, A., Puiu, A., Artuso, F., Ciceroni, C., Giardina, I., & Pollastrone, F. (2023). Laser Sensing and Chemometric Analysis for Rapid Detection of Oregano Fraud. Sensors, 23(15), 6800. https://doi.org/10.3390/s23156800








