Use of Virtual Reality-Based Games to Improve Balance and Gait of Children and Adolescents with Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Selection of Tests
2.2. Evaluation of the Characteristics of the Trials
Quality of Evidence
2.3. Participants
2.4. Interventions
2.5. Evaluated Outcomes
2.6. Data Extraction and Analysis
3. Results
3.1. Flow of Trials through the Review
3.2. Characteristics of the Included Trials
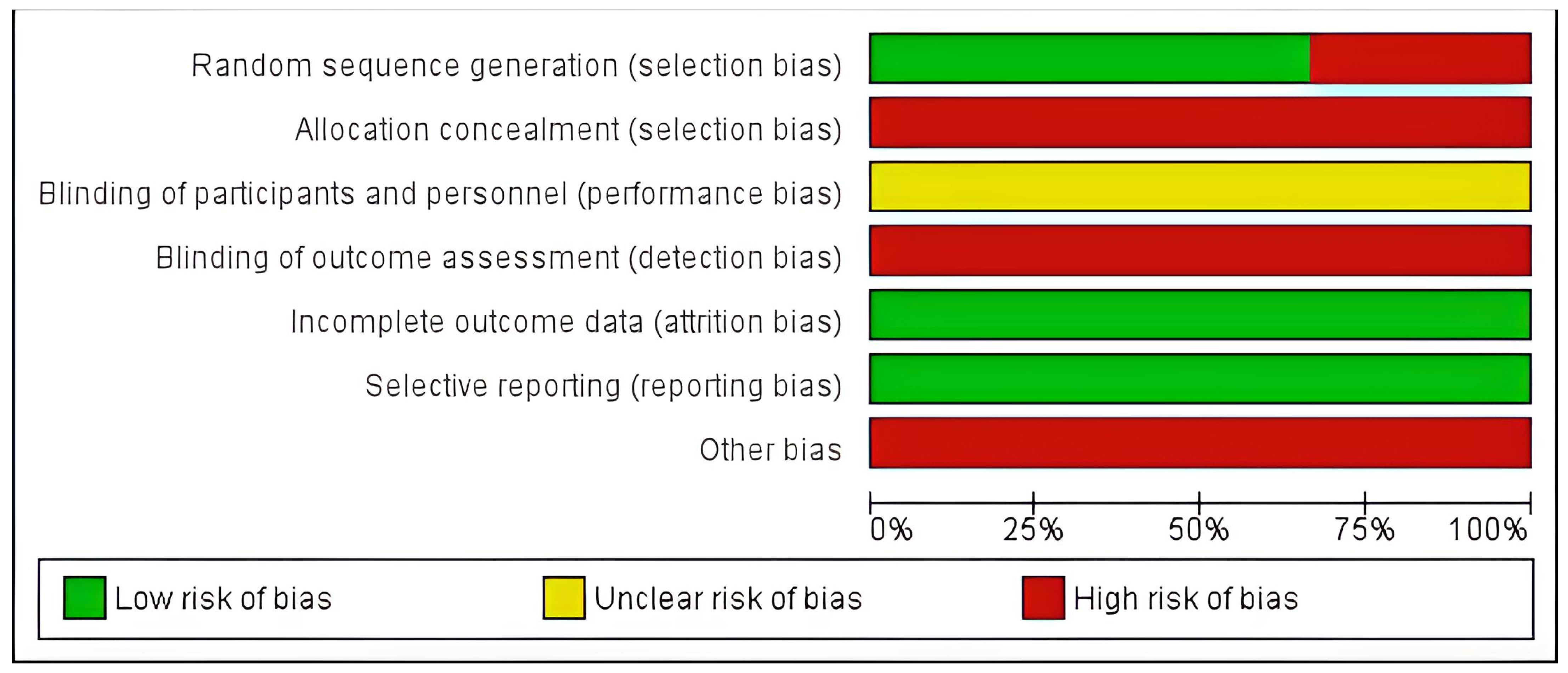
3.2.1. Risk of Bias
3.2.2. Participants
3.2.3. Intervention
3.2.4. Outcome Measures
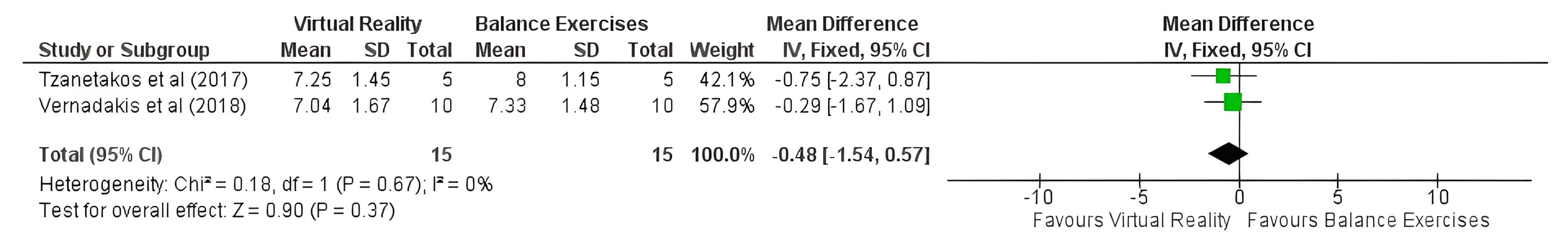
3.3. Meta-Analysis
4. Discussion
4.1. Biases Related to Sample Selection
4.2. Biases Related to the Methodological Aspects of the Trials
4.3. Bias Related to Sample Characteristics
Assessment of the Function of the Vestibular System
4.4. Other Limitations of the Trials
4.5. Considerations for Future Trials on the Topic
5. Conclusions and Implications for Future Trials on the Topic
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, A. Preventing deafness—An achievable challenge. The WHO perspective. Int. Congr. Ser. 2003, 1240, 183–191. [Google Scholar] [CrossRef]
- Olusanya, B.; Luxon, L.; Wirz, S. Benefits and challenges of newborn hearing screening for developing countries. Int. J. Pediatr. Otorhinolaryngol. 2003, 68, 287–305. [Google Scholar] [CrossRef]
- Olusanya, B.; Swanepoel, D.W.; ChapChap, M.J.; Castillo, S.; Habib, H.; Mukari, S.Z.; Martinez, N.V.; Lin, H.-C.; McPherson, B. Progress towards early detection services for infants with hearing loss in developing countries. BMC Health Serv. Res. 2007, 7, 14. [Google Scholar] [CrossRef]
- Mehra, S.; Eavey, R.D.; Keamy, D.G. The epidemiology of hearing impairment in the United States: Newborns, children, and adolescents. Otolaryngol. Neck Surg. 2009, 140, 461–472. [Google Scholar] [CrossRef]
- De Oliveira, J.S.; Rodrigues, L.B.; Aurélio, F.S.; Da Silva, V.B. Risk factors and prevalence of newborn hearing loss in a private health care system of Porto Velho, Northern Brazil. Rev. Paul. Pediatr. 2013, 31, 299–305. [Google Scholar] [CrossRef]
- Parving, A.; Hauch, A.-M. The causes of profound hearing impairment in a school for the deaf—A longitudinal study. Br. J. Audiol. 1994, 28, 63–69. [Google Scholar] [CrossRef]
- Morton, N.E. Genetic Epidemiology of Hearing Impairment. Ann. N. Y. Acad. Sci. 1991, 630, 16–31. [Google Scholar] [CrossRef]
- Cianfrone, G.; Pentangelo, D.; Cianfrone, F.; Mazzei, F.; Turchetta, R.; Orlando, M.P.; Altissimi, G. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: A reasoned and updated guide. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 601–636. [Google Scholar] [PubMed]
- Kaga, K. Vestibular compensation in infants and children with congenital and acquired vestibular loss in both ears. Int. J. Pediatr. Otorhinolaryngol. 1999, 49, 215–224. [Google Scholar] [CrossRef]
- Suarez, H.; Angeli, S.; Suarez, A.; Rosales, B.; Carrera, X.; Alonso, R. Balance sensory organization in children with profound hearing loss and cochlear implants. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 629–637. [Google Scholar] [CrossRef]
- Jacot, E.; Van Den Abbeele, T.; Debre, H.R.; Wiener-Vacher, S.R. Vestibular impairments pre- and post-cochlear implant in children. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 209–217. [Google Scholar] [CrossRef]
- Schwab, B.; Kontorinis, G. Influencing Factors on the Vestibular Function of Deaf Children and Adolescents—Evaluation by Means of Dynamic Posturography. Open Otorhinolaryngol. J. 2011, 5, 1–9. [Google Scholar] [CrossRef]
- Maes, L.; De Kegel, A.; Van Waelvelde, H.; Dhooge, I. Rotatory and Collic Vestibular Evoked Myogenic Potential Testing in Normal-Hearing and Hearing-Impaired Children. Ear Hear. 2014, 35, e21–e32. [Google Scholar] [CrossRef]
- Said, E.A.-F. Vestibular assessment in children with sensorineural hearing loss using both electronystagmography and vestibular-evoked myogenic potential. Egypt. J. Otolaryngol. 2014, 30, 43–52. [Google Scholar] [CrossRef]
- Kotait, M.A.; Moaty, A.S.; Gabr, T.A. Vestibular testing in children with severe-to-profound hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2019, 125, 201–205. [Google Scholar] [CrossRef]
- Melo, R.D.S.; Marinho, S.E.D.S.; Freire, M.E.A.; Souza, R.A.; Damasceno, H.A.M.; Raposo, M.C.F. Static and dynamic balance of children and adolescents with sensorineural hearing loss. Einstein 2017, 15, 262–268. [Google Scholar] [CrossRef]
- Jafari, Z.; Malayeri, S.A. The effect of saccular function on static balance ability of profound hearing-impaired children. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 919–924. [Google Scholar] [CrossRef]
- Melo, R.D.S.; Lemos, A.; Raposo, M.C.F.; Ferraz, K.M. Desempenho do equilíbrio dinâmico de escolares ouvintes e com perda auditiva sensorioneural. Rev. Bras. Med. Esporte 2014, 20, 442–446. [Google Scholar] [CrossRef]
- Melo, R.d.S.; da Silva, P.W.A.; Tassitano, R.M.; Macky, C.F.S.T.; da Silva, L.V.C. Avaliação do equilíbrio corporal e da marcha: Estudo comparativo entre surdos e ouvintes em idade escolar. Rev. Paul. Pediatr. 2012, 30, 385–391. [Google Scholar] [CrossRef]
- Majlesi, M.; Azadian, E.; Farahpour, N.; Jafarnezhad, A.A.; Rashedi, H. Lower limb muscle activity during gait in individuals with hearing loss. Australas. Phys. Eng. Sci. Med. 2017, 40, 659–665. [Google Scholar] [CrossRef]
- Jafarnezhadgero, A.A.; Majlesi, M.; Azadian, E. Gait ground reaction force characteristics in deaf and hearing children. Gait Posture 2017, 53, 236–240. [Google Scholar] [CrossRef]
- Melo, R.D.S. Gait performance of children and adolescents with sensorineural hearing loss. Gait Posture 2017, 57, 109–114. [Google Scholar] [CrossRef]
- Azadian, E.; Majlesi, M.; Jafarnezhadgero, A.A.; Granacher, U. The impact of hearing loss on three-dimensional lower limb joint torques during walking in prepubertal boys. J. Bodyw. Mov. Ther. 2020, 24, 123–129. [Google Scholar] [CrossRef]
- Chilosi, A.M.; Comparini, A.; Scusa, M.F.; Berrettini, S.; Forli, F.; Battini, R.; Cipriani, P.; Cioni, G. Neurodevelopmental disorders in children with severe to profound sensorineural hearing loss: A clinical study. Dev. Med. Child Neurol. 2010, 52, 856–862. [Google Scholar] [CrossRef]
- Livingstone, N.; Mcphillips, M. Motor skill deficits in children with partial hearing. Dev. Med. Child Neurol. 2011, 53, 836–842. [Google Scholar] [CrossRef]
- Martin, W.; Jelsma, J.; Rogers, C. Motor proficiency and dynamic visual acuity in children with bilateral sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1520–1525. [Google Scholar] [CrossRef]
- Fellinger, M.J.; Holzinger, D.; Aigner, M.; Beitel, C.; Fellinger, J. Motor performance and correlates of mental health in children who are deaf or hard of hearing. Dev. Med. Child Neurol. 2015, 57, 942–947. [Google Scholar] [CrossRef]
- Melo, R.S.; Lemos, A.; Paiva, G.S.; Ithamar, L.; Lima, M.C.; Eickmann, S.H.; Ferraz, K.M.; Belian, R.B. Vestibular rehabilitation exercises programs to improve the postural control, balance and gait of children with sensorineural hearing loss: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2019, 127, 109650. [Google Scholar] [CrossRef]
- Theunissen, S.C.; Rieffe, C.; Kouwenberg, M.; Soede, W.; Briaire, J.J.; Frijns, J.H. Depression in hearing-impaired children. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1313–1317. [Google Scholar] [CrossRef]
- Yigider, A.P.; Yilmaz, S.; Ulusoy, H.; Kara, T.; Kufeciler, L.; Kaya, K.H. Emotional and behavioral problems in children and adolescents with hearing loss and their effects on quality of life. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110245. [Google Scholar] [CrossRef]
- Hartman, E.; Houwen, S.; Visscher, C. Motor Skill Performance and Sports Participation in Deaf Elementary School Children. Adapt. Phys. Act. Q. 2011, 28, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Engel-Yeger, B.; Hamed-Daher, S. Comparing participation in out of school activities between children with visual impairments, children with hearing impairments and typical peers. Res. Dev. Disabil. 2013, 34, 3124–3132. [Google Scholar] [CrossRef]
- Wiegersma, P.H.; Van der Velde, A. Motor development of deaf children. J. Child Psychol. Psychiatry 1983, 24, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, F.; Loots, G.; Van Waelvelde, H. Motor Development of Deaf Children with and without Cochlear Implants. J. Deaf. Stud. Deaf. Educ. 2008, 13, 215–224. [Google Scholar] [CrossRef]
- Effgen, S.K. Effect of an Exercise Program on the Static Balance of Deaf Children. Phys. Ther. 1981, 61, 873–877. [Google Scholar] [CrossRef]
- Rine, R.M.; Braswell, J.; Fisher, D.; Joyce, K.; Kalar, K.; Shaffer, M. Improvement of motor development and postural control following intervention in children with sensorineural hearing loss and vestibular impairment. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 1141–1148. [Google Scholar] [CrossRef]
- Fotiadou, E.G.; Tsimaras, V.K.; Giagazoglou, P.F.; Sidiropoulou, M.P.; Karamouzi, A.M.; Angelopoulou, N.A. Effect of Rhythmic Gymnastics on the Rhythm Perception of Children with Deafness. J. Strength Cond. Res. 2006, 20, 298–303. [Google Scholar] [CrossRef]
- Melo, R.S.; Tavares-Netto, A.R.; Delgado, A.; Wiesiolek, C.C.; Ferraz, K.M.; Belian, R.B. Does the practice of sports or recreational activities improve the balance and gait of children and adolescents with sensorineural hearing loss? A systematic review. Gait Posture 2020, 77, 144–155. [Google Scholar] [CrossRef]
- Wolter, N.E.; Gordon, K.A.; Campos, J.L.; Madrigal, L.D.V.; Pothier, D.D.; Hughes, C.O.; Papsin, B.C.; Cushing, S.L. BalanCI: Head-Referenced Cochlear Implant Stimulation Improves Balance in Children with Bilateral Cochleovestibular Loss. Audiol. Neurotol. 2019, 25, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Peñeñory, V.M.; Manresa-Yee, C.; Riquelme, I.; Collazos, C.A.; Fardoun, H.M.; Alghazzawi, D.M. Interactive systems proposal for psychomotor rehabilitation in hearing impaired children. Commun. Comput Inform Sci. 2019, 1002, 58–67. [Google Scholar]
- Lansink, I.O.; van Kouwenhove, L.; Dijkstra, P.; Postema, K.; Hijmans, J. Effects of interventions on normalizing step width during self-paced dual-belt treadmill walking with virtual reality, a randomised controlled trial. Gait Posture 2017, 58, 121–125. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.W.; Faber, G.; Jonkers, I.; Van Dieen, J.H.; Verschueren, S.M. Virtual reality balance training for elderly: Similar skiing games elicit different challenges in balance training. Gait Posture 2018, 59, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Barr, C.; Gough, C.; van den Berg, M. How Commercially Available Virtual Reality–Based Interventions Are Delivered and Reported in Gait, Posture, and Balance Rehabilitation: A Systematic Review. Phys. Ther. 2020, 100, 1805–1815. [Google Scholar] [CrossRef]
- Raffegeau, T.E.; Fawver, B.; Clark, M.; Engel, B.T.; Young, W.R.; Williams, A.M.; Lohse, K.R.; Fino, P.C. The feasibility of using virtual reality to induce mobility-related anxiety during turning. Gait Posture 2020, 77, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Q.; He, C.-Q.; Bian, R. Effect of Virtual Reality on Balance in Individuals with Parkinson Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phys. Ther. 2020, 100, 933–945. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.M.; Munoz, R.; Ribeiro, S.; Wu, W.; de Albuquerque, V.H.C. REHAB FUN: An assistive technology in neurological motor disorders rehabilitation of children with cerebral palsy. Neural Comput. Appl. 2020, 32, 10957–10970. [Google Scholar] [CrossRef]
- Jurdi, S.; Montaner, J.; Garcia-Sanjuan, F.; Jaen, J.; Nacher, V. A systematic review of game technologies for pediatric patients. Comput. Biol. Med. 2018, 97, 89–112. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Cherng, R.-J.; Lin, C.-H. Development of a virtual reality environment for somatosensory and perceptual stimulation in the balance assessment of children. Comput. Biol. Med. 2004, 34, 719–733. [Google Scholar] [CrossRef]
- Liao, Y.; Vakanski, A.; Xian, M.; Paul, D.; Baker, R. A review of computational approaches for evaluation of rehabilitation exercises. Comput. Biol. Med. 2020, 119, 103687. [Google Scholar] [CrossRef]
- Arnoni, J.L.; Pavao, S.L.; dos Santos Silva, F.P.; Rocha, N.A. Effects of virtual reality in body oscillation and motor performance of children with cerebral palsy: A preliminary randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2019, 35, 189–194. [Google Scholar] [CrossRef]
- Jung, S.; Song, S.; Lee, D.; Lee, K.; Lee, G. Effects of Kinect Video Game Training on Lower Extremity Motor Function, Balance, and Gait in Adolescents with Spastic Diplegia Cerebral Palsy: A Pilot Randomized Controlled Trial. Dev. Neurorehabilit. 2021, 24, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ravi, D.; Kumar, N.; Singhi, P. Effectiveness of virtual reality rehabilitation for children and adolescents with cerebral palsy: An updated evidence-based systematic review. Physiotherapy 2017, 103, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fanchiang, H.D.; Howard, A. Effectiveness of Virtual Reality in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phys. Ther. 2018, 98, 63–77. [Google Scholar] [CrossRef]
- Pin, T.W. Effectiveness of interactive computer play on balance and postural control for children with cerebral palsy: A systematic review. Gait Posture 2019, 73, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I. Virtual Reality Enhances Gait in Cerebral Palsy: A Training Dose-Response Meta-Analysis. Front. Neurol. 2019, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Melo, R.S.; Ferraz, K.M.; Belian, R.B. Do Sports and Recreational Practices Improve Balance Performance, Gait and Running of Children with Sensorineural Hearing Loss? A Systematic Review. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=96309 (accessed on 7 September 2022).
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Kaya, M.; Sarıtaş, N. A Comparison of the Effects of Balance Training and Technological Games on Balance in Hearing-Impaired Individuals. J. Educ. Train. Stud. 2019, 7, 48–53. [Google Scholar] [CrossRef]
- Tzanetakos, N.; Papastergiou, M.; Vernadakis, N.; Antoniou, P. Utilizing physically interactive videogames for the balance training of adolescents with deafness within a physical education course. J. Phys. Educ. Sport 2017, 17, 614–623. [Google Scholar] [CrossRef]
- Vernadakis, N.; Papastergiou, M.; Giannousi, M.; Panagiotis, A. The effect of an exergame-based intervention on balance ability on deaf adolescents. Sport Sci. 2018, 11, 36–41. [Google Scholar]
- Korkmaz, C.; Akin, M. Effect of Nintendo-wii balance board training on dynamic balance in between 9-14 age hearing impaired sedentaries. Spor Eğitim Dergisi 2019, 3, 119–127. [Google Scholar]
- Irawan, B.; Sumaryanti, S.; Ichsan, M. Effect of water game model on gross motor improvement of deaf children in SLB N Mesuji Lampung. Int. J. Phys. Educ. Sports Health 2023, 10, 211–214. [Google Scholar]
- Korkmaz, C.; Akin, M. The effect of bosu, kangoo jump, Nintendo-wii balance board trainings on agility in hearing impaired sedentary. J. Sport Perform. Res. 2021, 12, 91–104. [Google Scholar] [CrossRef]
- Nadertabar, M.; Daramadi, P.S.; Pezeshk, S.; Farrokhi, N. The influence of computer games on visual-motor skills in deaf students. Middle East. J. Disabil. Studies. 2017, 7, 101. [Google Scholar]
- Asogwa, U.D.; Ofoegbu, T.O.; Ogbonna, C.S.; Eskay, M.; Obiyo, N.O.; Nji, G.C.; Ngwoke, O.R.; Eseadi, C.; Agboti, C.I.; Uwakwe, C.; et al. Effect of video-guided educational intervention on school engagement of adolescent students with hearing impairment. Medicine 2020, 99, e20643. [Google Scholar] [CrossRef]
- Schulz, K.F.; Chalmers, I.; Hayes, R.J.; Altman, D.G. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995, 273, 408–412. [Google Scholar] [CrossRef]
- Karbunarova, J.; Lvov University of Physical Culture; Kapбунарoва. Influence author methodic teaching swimming on coordination quality of children 6–10 years old with hearing disabilities. Slobozhanskyi Her. Sci. Sport 2016, 53, 35–38. [Google Scholar] [CrossRef]
- Ilkım, M.; Akyol, B. The Comparison of Some Motoric Characteristics of Hearing Impaired Individuals Sports Athletic and Gymnastic. Univers. J. Educ. Res. 2018, 6, 2148–2152. [Google Scholar] [CrossRef]
- Aref, N.; Boroujeni, S.T.; Ameri, E.A. The effect of swim training intervention on balance and systems involved in balance in adolescents with hearing impairment and vestibular disorder. J. Res. Sport Rehab. 2018, 6, 53–61. [Google Scholar] [CrossRef]
- De Kegel, A.; Maes, L.; Baetens, T.; Dhooge, I.; Van Waelvelde, H. The influence of a vestibular dysfunction on the motor development of hearing-impaired children. Laryngoscope 2012, 122, 2837–2843. [Google Scholar] [CrossRef]
- Maes, L.; De Kegel, A.; Van Waelvelde, H.; Dhooge, I. Association Between Vestibular Function and Motor Performance in Hearing-impaired Children. Otol. Neurotol. 2014, 35, e343–e347. [Google Scholar] [CrossRef]
- Melo, R.S.; Lemos, A.; Raposo, M.C.F.; Monteiro, M.G.; Lambertz, D.; Ferraz, K.M. Repercussions of the Degrees of Hearing Loss and Vestibular Dysfunction on the Static Balance of Children with Sensorineural Hearing Loss. Phys. Ther. 2021, 101, pzab177. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, M.; Gordon, K.A.; Polonenko, M.; Blaser, S.I.; Papsin, B.C.; Cushing, S.L. Vestibular and balance function is often impaired in children with profound unilateral sensorineural hearing loss. Hear. Res. 2019, 372, 52–61. [Google Scholar] [CrossRef]
- Ionescu, E.; Reynard, P.; Goulème, N.; Becaud, C.; Spruyt, K.; Ortega-Solis, J.; Thai-Van, H. How sacculo-collic function assessed by cervical vestibular evoked myogenic Potentials correlates with the quality of postural control in hearing impaired children? Int. J. Pediatr. Otorhinolaryngol. 2020, 130, 109840. [Google Scholar] [CrossRef]
- Potter, C.N.; Silverman, L.N. Characteristics of Vestibular Function and Static Balance Skills in Deaf Children. Phys. Ther. 1984, 64, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Kenna, M.A.; Stevens, K.; Licameli, G. Assessment of Saccular Function in Children with Sensorineural Hearing Loss. Arch. Otolaryngol. Neck Surg. 2009, 135, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, R.K.; Kumar, P. Vestibular evoked myogenic potentials in children with sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1308–1311. [Google Scholar] [CrossRef]
- Xu, X.-D.; Zhang, Q.; Hu, J.; Zhang, Y.; Chen, Y.-F.; Zhang, X.-T.; Xu, M. The hidden loss of otolithic function in children with profound sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 852–857. [Google Scholar] [CrossRef]
- Martens, S.; Dhooge, I.; Dhondt, C.; Vanaudenaerde, S.; Sucaet, M.; Rombaut, L.; Boudewyns, A.; Desloovere, C.; de Varebeke, S.J.; Vinck, A.-S.; et al. Vestibular Infant Screening (VIS)–Flanders: Results after 1.5 years of vestibular screening in hearing-impaired children. Sci. Rep. 2020, 10, 21011. [Google Scholar] [CrossRef]
- Gadsbøll, E.; Erbs, A.W.; Hougaard, D.D. Prevalence of abnormal vestibular responses in children with sensorineural hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 4695–4707. [Google Scholar] [CrossRef]
- Tarakci, D.; Huseyinsinoglu, B.E.; Tarakci, E.; Ozdincler, A.R. Effects of Nintendo Wii-Fit® video games on balance in children with mild cerebral palsy. Pediatr. Int. 2016, 58, 1042–1050. [Google Scholar] [CrossRef]
- Cooper, T.; Williams, J.M. Does an exercise programme integrating the Nintendo Wii-Fit Balance Board improve balance in ambulatory children with cerebral palsy? Phys. Ther. Rev. 2017, 22, 229–237. [Google Scholar] [CrossRef]
- Chesser, B.T.; Blythe, S.A.; Ridge, L.D.; Tomaszewski, R.E.R.; Kinne, B.L. Effectiveness of the Wii for pediatric rehabilitation in individuals with cerebral palsy: A systematic review. Phys. Ther. Rev. 2020, 25, 106–117. [Google Scholar] [CrossRef]
- Taracki, D.; Ozdincler, A.R.; Taracki, E.; Tutuncuoglu, F.; Ozmed, M. Wii-based balance therapy to improve balance function of children with cerebral palsy: A pilot study. J. Phys. Ther. Sci. 2013, 25, 1123–1127. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Ada, L.; Lee, S.-D. Balance and mobility training at home using Wii Fitin children with cerebral palsy: A feasibility study. BMJ Open 2018, 8, e019624. [Google Scholar] [CrossRef]
- Pavão, S.L.; Arnoni, J.L.B.; Oliveira, A.K.C.; Rocha, N.A.C.F. Impact of a virtual reality-based intervention on motor performance and balance of a child with cerebral palsy: A case study. Rev. Paul Pediatr. 2014, 32, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Arnoni, J.L.B.; Verdério, B.N.; Pinto, A.M.A.; Rocha, N.A.C.F. Effects of active videogame-based intervention on self-concept, balance, motor performance and adaptive success of children with cerebral palsy: Preliminary study. Fisioter. Pesqui. 2018, 25, 294–302. [Google Scholar] [CrossRef]
- Lotfi, Y.; Rezazadeh, N.; Moossavi, A.; Haghgoo, H.A.; Moghadam, S.F.; Pishyareh, E.; Bakhshi, E.; Rostami, R.; Sadeghi-Firoozabadi, V.; Khodabandelou, Y. Review Paper: Introduction of Pediatric Balance Therapy in Children with Vestibular Dysfunction: Review of Indications, Mechanisms, and Key Exercises. Iran. Rehabil. J. 2016, 14, 5–14. [Google Scholar] [CrossRef]
- Melo, R.D.S. Ampleness of head movements of children and adolescents with sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2017, 93, 133–140. [Google Scholar] [CrossRef]
- da Silva, P.W.A.; Souza, R.A.; Raposo, M.C.F.; Ferraz, K.M.; Melo, R.d.S. Head Position Comparison between Students with Normal Hearing and Students with Sensorineural Hearing Loss. Int. Arch. Otorhinolaryngol. 2013, 17, 363–369. [Google Scholar] [CrossRef]
- Daneshmandi, H.; Majalan, A.S.; Babakhani, M.; Karanian, F. The comparison of head and neck alignment in children with visual and hearing impairments and its relation with anthropometrical dimensions. Phys. Treat. J. 2014, 4, 69–76. [Google Scholar]
- Negahban, H.; Ali, M.B.C.; Nassadj, G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture 2017, 58, 126–129. [Google Scholar] [CrossRef]
- Weaver, T.S.; Shayman, C.S.; Hullar, T.E. The Effect of Hearing Aids and Cochlear Implants on Balance During Gait. Otol. Neurotol. 2017, 38, 1327–1332. [Google Scholar] [CrossRef]
- Stevens, M.N.; Barbour, D.L.; Gronski, M.P.; Hullar, T.E. Auditory contributions to maintaining balance. J. Vestib. Res. 2016, 26, 433–438. [Google Scholar] [CrossRef]
- Oikawa, K.; Kobayashi, Y.; Hiraumi, H.; Yonemoto, K.; Sato, H. Body balance function of cochlear implant patients with and without sound conditions. Clin. Neurophysiol. 2018, 129, 2112–2117. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, I.; Jonen, J.; Rahne, T.; Schwesig, R.; Lauenroth, A.; Hullar, T.E.; Plontke, S.K. Influence of hearing on vestibulospinal control in healthy subjects. HNO 2018, 66, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Berge, J.E.; Nordahl, S.H.G.; Aarstad, H.J.; Goplen, F.K. Hearing as an Independent Predictor of Postural Balance in 1075 Patients Evaluated for Dizziness. Otolaryngol. Neck Surg. 2019, 161, 478–484. [Google Scholar] [CrossRef]
- Seiwerth, I.; Jonen, J.; Rahne, T.; Lauenroth, A.; Hullar, T.E.; Plontke, S.K.; Schwesig, R. Postural regulation and stability with acoustic input in normal-hearing subjects. HNO 2020, 68, 100–105. [Google Scholar] [CrossRef]
- Cushing, S.L.; Chia, R.; James, A.L.; Papsin, B.C.; Gordon, K.A. A Test of Static and Dynamic Balance Function in Children with Cochlear Implants: The vestibular olympics. Arch. Otolaryngol. Neck Surg. 2008, 134, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.L.; Pothier, D.; Hughes, C.; Hubbard, B.J.; Gordon, K.A.; Papsin, B.C. Providing auditory cues to improve stability in children who are deaf. Laryngoscope 2012, 122, S101–S102. [Google Scholar] [CrossRef] [PubMed]
- Mazaheryazdi, M.; Moossavi, A.; Sarrafzadah, J.; Talebian, S.; Jalaie, S. Study of the effects of hearing on static and dynamic postural function in children using cochlear implants. Int. J. Pediatr. Otorhinolaryngol. 2017, 100, 18–22. [Google Scholar] [CrossRef]
- Suarez, H.; Ferreira, E.; Arocena, S.; Pintos, B.G.; Quinteros, M.; Suarez, S.; Gonzalez, M.P. Motor and cognitive performances in pre-lingual cochlear implant adolescents, related with vestibular function and auditory input. Acta Oto-Laryngol. 2019, 139, 367–372. [Google Scholar] [CrossRef]
- Bayat, A.; Farhadi, M.; Emamdjomeh, H.; Nadimi, Z.; Mirmomeni, G.; Saki, N. Influence of Cochlear Implantation on Balance Function in Pediatrics. Int. Tinnitus J. 2020, 24, 31–35. [Google Scholar]
- Wolter, N.E.; Gordon, K.A.; Campos, J.; Madrigal, L.D.V.; Papsin, B.C.; Cushing, S.L. Impact of the sensory environment on balance in children with bilateral cochleovestibular loss. Hear. Res. 2020, 400, 108134. [Google Scholar] [CrossRef]
- Suarez, H.; Alonso, R.; Arocena, S.; Ferreira, E.; Roman, C.S.; Suarez, A.; Lapilover, V. Sensorimotor interaction in deaf children. Relationship between gait performance and hearing input during childhood assessed in pre-lingual cochlear implant users. Acta Oto-Laryngol. 2017, 137, 346–351. [Google Scholar] [CrossRef]
- Sioud, R.; Khalifa, R.; Houel, N. Auditory cues behind congenitally blind subjects improve their balance control in bipedal upright posture. Gait Posture 2019, 70, 175–178. [Google Scholar] [CrossRef]
- Maheu, M.; Behtani, L.; Nooristani, M.; Houde, M.S.; Delcenserie, A.; Leroux, T.; Champoux, F. Vestibular Function Modulates the Benefit of Hearing Aids in People with Hearing Loss During Static Postural Control. Ear Hear. 2019, 40, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Szeto, B.; Zanotto, D.; Lopez, E.M.; Stafford, J.A.; Nemer, J.S.; Chambers, A.R.; Agrawal, S.K.; Lalwani, A.K. Hearing Loss Is Associated with Increased Variability in Double Support Period in the Elderly. Sensors 2021, 21, 278. [Google Scholar] [CrossRef]
- Morris, B.; Cosetti, M.; Kelly, J.; Yang, J.; Harel, D.; Medlin, A.; Lubetzky, A.V. Differing postural control patterns in individuals with bilateral and unilateral hearing loss. Am. J. Otolaryngol. 2023, 44, 103866. [Google Scholar] [CrossRef]
- Easton, R.D.; Greene, A.J.; DiZio, P.; Lackner, J.R. Auditory cues for orientation and postural control in sighted and congenitally blind people. Exp. Brain Res. 1998, 118, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Yost, W.A. Relationship between Postural Stability and Spatial Hearing. J. Am. Acad. Audiol. 2013, 24, 782–788. [Google Scholar] [CrossRef]
- Gandemer, L.; Parseihian, G.; Kronland-Martinet, R.; Bourdin, C. Spatial Cues Provided by Sound Improve Postural Stabilization: Evidence of a Spatial Auditory Map? Front. Neurosci. 2017, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.M.; Rumalla, K.; King, L.A.; Hullar, T.E. The effect of spatial auditory landmarks on ambulation. Gait Posture 2018, 60, 171–174. [Google Scholar] [CrossRef]
- Forli, F.; Giuntini, G.; Ciabotti, A.; Bruschini, L.; Löfkvist, U.; Berrettini, S. How does a bilingual environment affect the results in children with cochlear implants compared to monolingual-matched children? An Italian follow-up study. Int. J. Pediatr. Otorhinolaryngol. 2018, 105, 56–62. [Google Scholar] [CrossRef]
- Ehrmann-Müller, D.; Kühn, H.; Matthies, C.; Hagen, R.; Shehata-Dieler, W. Outcomes after cochlear implant provision in children with cochlear nerve hypoplasia or aplasia. Int. J. Pediatr. Otorhinolaryngol. 2018, 112, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, D.M.; Sladen, D.P.; DeJong, M.D.; Torres, J.H.; Dorman, M.F.; Carlson, M.L. Cochlear implantation for single-sided deafness in children and adolescents. Int. J. Pediatr. Otorhinolaryngol. 2019, 118, 128–133. [Google Scholar] [CrossRef]
- Lee, Y.; Sim, H. Bilateral cochlear implantation versus unilateral cochlear implantation in deaf children: Effects of sentence context and listening conditions on recognition of spoken words in sentences. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110237. [Google Scholar] [CrossRef]
- Sharma, S.D.; Cushing, S.L.; Papsin, B.C.; Gordon, K.A. Hearing and speech benefits of cochlear implantation in children: A review of the literature. Int. J. Pediatr. Otorhinolaryngol. 2020, 133, 109984. [Google Scholar] [CrossRef]
- Thierry, B.; Blanchard, M.; Leboulanger, N.; Parodi, M.; Wiener-Vacher, S.R.; Garabedian, E.-N.; Loundon, N. Cochlear implantation and vestibular function in children. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-W.; Hsu, C.-J.; Kuan, C.-C.; Chang, W.-H. Static balance function in children with cochlear implants. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Janky, K.L.; Givens, D. Vestibular, Visual Acuity, and Balance Outcomes in Children with Cochlear Implants: A preliminar report. Ear Hear. 2015, 36, e364–e372. [Google Scholar] [CrossRef]
- Kelly, A.; Liu, Z.; Leonard, S.; Toner, F.; Adams, M.; Toner, J. Balance in children following cochlear implantation. Cochlea- Implant. Int. 2018, 19, 22–25. [Google Scholar] [CrossRef]
- Ganc, M.; Kobosko, J.; Jedrzejczak, W.W.; Kochański, B.; Skarzynski, H. Psychomotor development of 4-year-old deaf children with cochlear implants: Three case studies. Int. J. Pediatr. Otorhinolaryngol. 2021, 141, 110570. [Google Scholar] [CrossRef]
- Reynard, P.; Ionescu, E.; Joly, C.; Ltaief-Boudrigua, A.; Coudert, A.; Thai-Van, H. Vestibular impairment in cochlear implanted children presenting enlarged vestibular aqueduct and enlarged endolymphatic sac. Int. J. Pediatr. Otorhinolaryngol. 2021, 141, 110557. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Raynor, E.M.; Lee, J.W.; Smith, S.L.; Heet, H.; Garrison, D.; Wrigley, J.; Kaylie, D.M.; Riska, K.M. Vestibular Dysfunction and Gross Motor Milestone Acquisition in Children with Hearing Loss: A Systematic Review. Otolaryngol. Neck Surg. 2021, 165, 493–506. [Google Scholar] [CrossRef]
- Tsuzuku, T.; Kaga, K. Delayed motor function and results of vestibular function tests in children with inner ear anomalies. Int. J. Pediatr. Otorhinolaryngol. 1992, 23, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Iwasaki, S.; Ushio, M.; Chihara, Y.; Fujimoto, C.; Egami, N.; Yamasoba, T. Effect of Vestibular Dysfunction on the Development of Gross Motor Function in Children with Profound Hearing Loss. Audiol. Neurotol. 2013, 18, 143–151. [Google Scholar] [CrossRef]
- Masuda, T.; Kaga, K. Relationship between acquisition of motor function and vestibular function in children with bilateral severe hearing loss. Acta Oto-Laryngol. 2014, 134, 672–678. [Google Scholar] [CrossRef]
- Kimura, Y.; Masuda, T.; Kaga, K. Vestibular Function and Gross Motor Development in 195 Children with Congenital Hearing Loss—Assessment of Inner Ear Malformations. Otol. Neurotol. 2018, 39, 196–205. [Google Scholar] [CrossRef]
- Janky, K.L.; Thomas, M.L.A.; High, R.R.; Schmid, K.K.; Ogun, O.A. Predictive Factors for Vestibular Loss in Children with Hearing Loss. Am. J. Audiol. 2018, 27, 137–146. [Google Scholar] [CrossRef]
- Melo, R.D.S.; Lemos, A.; Macky, C.F.D.S.T.; Raposo, M.C.F.; Ferraz, K.M. Postural control assessment in students with normal hearing and sensorineural hearing loss. Braz. J. Otorhinolaryngol. 2015, 81, 431–438. [Google Scholar] [CrossRef]
- An, M.-H.; Yi, C.-H.; Jeon, H.-S.; Park, S.-Y. Age-related changes of single-limb standing balance in children with and without deafness. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1539–1544. [Google Scholar] [CrossRef]
- Melo, R.D.S.; Lemos, A.; Raposo, M.C.F.; Belian, R.B.; Ferraz, K.M. Balance performance of children and adolescents with sensorineural hearing loss: Repercussions of hearing loss degrees and etiological factors. Int. J. Pediatr. Otorhinolaryngol. 2018, 110, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Soylemez, E.; Ertugrul, S.; Dogan, E. Assessment of balance skills and falling risk in children with congenital bilateral profound sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2019, 116, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Karakoc, K.; Mujdeci, B. Evaluation of balance in children with sensorineural hearing loss according to age. Am. J. Otolaryngol. 2021, 42, 102830. [Google Scholar] [CrossRef]
- Peñeñory, V.M.; Manresa-Yee, C.; Riquelme, I.; Collazos, C.A.; Fardoun, H.M. Scoping Review of Systems to Train Psychomotor Skills in Hearing Impaired Children. Sensors 2018, 18, 2546. [Google Scholar] [CrossRef]
- Martens, S.; Dhooge, I.; Dhondt, C.; Leyssens, L.; Sucaet, M.; Vanaudenaerde, S.; Rombaut, L.; Maes, L. Vestibular Infant Screening—Flanders: The implementation of a standard vestibular screening protocol for hearing-impaired children in Flanders. Int. J. Pediatr. Otorhinolaryngol. 2019, 120, 196–201. [Google Scholar] [CrossRef] [PubMed]


| Author | Country | Motor Skill | Design | Characteristics of the Volunteers | Number of Volunteers | Characteristics of the Interventions | ||
|---|---|---|---|---|---|---|---|---|
| CG | IG | CG | IG | |||||
| Kaya et al. [61] | Turkey | Balance | RCT | Adolescents with SNHL, male sex, and age range between 18–22 years old. | 24 | 12 | CG1: 30 min sessions, three times/week of balance training, for 8 weeks. CG2: Control group was not given any balance training. | IG: 30 min sessions, three times/week of practice with active videogames, for 8 weeks. |
| Tzanetakos et al. [62] | Greece | Balance | Quasi-randomized trial | Adolescents with profound SNHL (hearing loss degrees > 70 dB), both sexes and age range between 17–19 years old. | 05 | 05 | 15 min sessions, two times/weeks of balance training, for 5 weeks. | 15 min sessions, two times/week of practice with active videogames, for 5 weeks. |
| Vernadakis et al. [63] | Greece | Balance | RCT | Adolescents with profound SNHL (hearing loss degrees > 70 dB), both sexes and age range between 17–19 years old. | 10 | 10 | 15 min sessions, two times/weeks of balance training, for 8 weeks. | 15 min sessions, two times/week of practice with active videogames, for 8 weeks. |
| Author | Motor Skill | Outcome Measures | Instruments Used for the Assessment | Control Group | Intervention Group | Conclusions | ||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||||
| Kaya et al. [61] | Balance | Static Balance | Force Platform | CG1: 0.68 ± 0.18 a CG2: 0.82 ± 0.10 | CG1: 0.60 ± 0.13 a CG2: 0.81 ± 0.07 | IG: 0.79 ± 0.17 b | IG: 0.67 ± 0.11 b | Virtual reality-based games were as effective as traditional balance exercises to improve balance of adolescents with SNHL. However, the use of virtual reality-based games is more effective than not exercising to improve the balance of adolescents with SNHL. |
| Dynamic Balance | CG1: 1.65 ± 0.26 a CG2: 1.87 ± 0.28 | CG1: 1.46 ± 0.22 a CG2: 1.78 ± 0.23 | IG: 1.78 ± 0.36 b | IG: 1.48 ± 0.17 b | ||||
| Tzanetakos et al. [62] | Balance | Static Balance | Flamingo Balance Test | 9.00 ± 0.58 | 8.00 ± 1.15 | 7.75 ± 1.51 | 7.25 ± 1.45 | Balance exergames constitute a feasible, well-accepted and motivational balance training mode for adolescents with deafness, the effectiveness of which should be further researched. |
| Vernadakis et al. [63] | Balance | Static Balance | Flamingo Balance Test | 9.09 ± 0.74 | 7.04 ± 1.67 | 9.21 ± 0.71 | 7.33 ± 1.48 | Findings support the effectiveness of using the Nintendo Wii gaming console as an intervention for adolescent students with deafness, and specifically, its effects on physical function related to balance competence. |
| Quality Assessment | № of Patients | Effect | Quality of the Evidence | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Virtual Reality | Balance Exercises | Relative (95% CI) | Absolute (95% CI) | ||
| Balance: (follow up: mean 8 weeks; assessed with Force Platform) | ||||||||||||
| 01 [61] | RCT | very serious b,c,d,e | serious f | not serious | not serious | none | 12 | 12 | - | - | ⨁◯◯◯ VERY LOW | CRITICAL |
| Balance: (follow up: mean 7 weeks; assessed with Flamingo Balance Test) | ||||||||||||
| 02 [62,63] | QRT | very serious a,b,c,d,e | very serious g | not serious | not serious | none | 15 | 15 | - | −0.48 [−1.54 to 0.57] | ⨁◯◯◯ VERY LOW | CRITICAL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, R.S.; Lemos, A.; Delgado, A.; Raposo, M.C.F.; Ferraz, K.M.; Belian, R.B. Use of Virtual Reality-Based Games to Improve Balance and Gait of Children and Adolescents with Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis. Sensors 2023, 23, 6601. https://doi.org/10.3390/s23146601
Melo RS, Lemos A, Delgado A, Raposo MCF, Ferraz KM, Belian RB. Use of Virtual Reality-Based Games to Improve Balance and Gait of Children and Adolescents with Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis. Sensors. 2023; 23(14):6601. https://doi.org/10.3390/s23146601
Chicago/Turabian StyleMelo, Renato S., Andrea Lemos, Alexandre Delgado, Maria Cristina Falcão Raposo, Karla Mônica Ferraz, and Rosalie Barreto Belian. 2023. "Use of Virtual Reality-Based Games to Improve Balance and Gait of Children and Adolescents with Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis" Sensors 23, no. 14: 6601. https://doi.org/10.3390/s23146601
APA StyleMelo, R. S., Lemos, A., Delgado, A., Raposo, M. C. F., Ferraz, K. M., & Belian, R. B. (2023). Use of Virtual Reality-Based Games to Improve Balance and Gait of Children and Adolescents with Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis. Sensors, 23(14), 6601. https://doi.org/10.3390/s23146601








