Determining the Nutrient Content of Hydroponically-Cultivated Microgreens with Immersible Silicon Photonic Sensors: A Preliminary Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
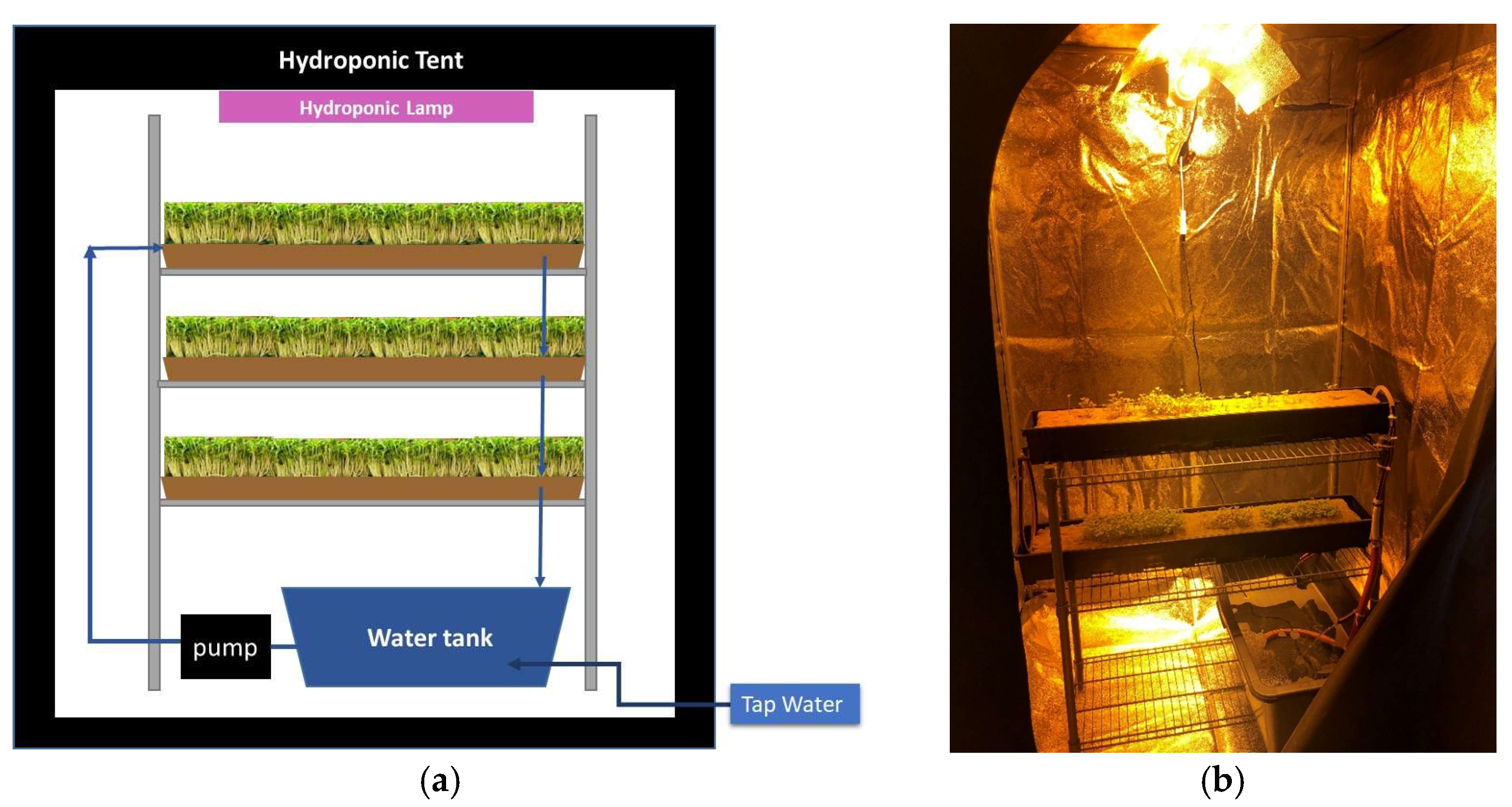
2.1. Hydroponic Installation
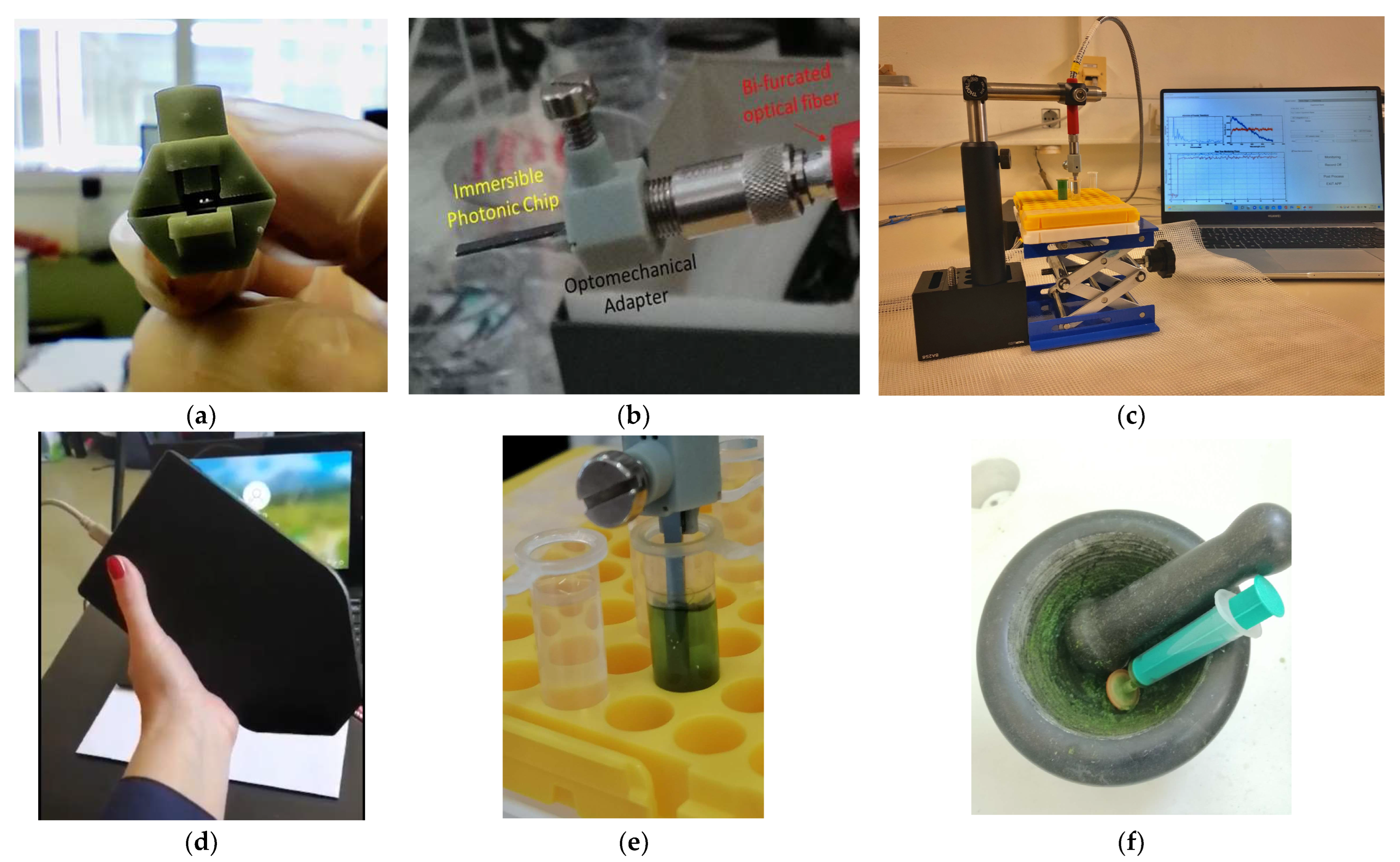
2.2. Immersible Photonic Sensors and Reader
2.3. Microgreen Culitvation
2.4. Determination of Total Phenolic Content and Antioxidant Activity
2.4.1. Folin–Cioacalteu Assay
2.4.2. DPPH Assay
2.4.3. ABTS Assay
3. Results and Discussion
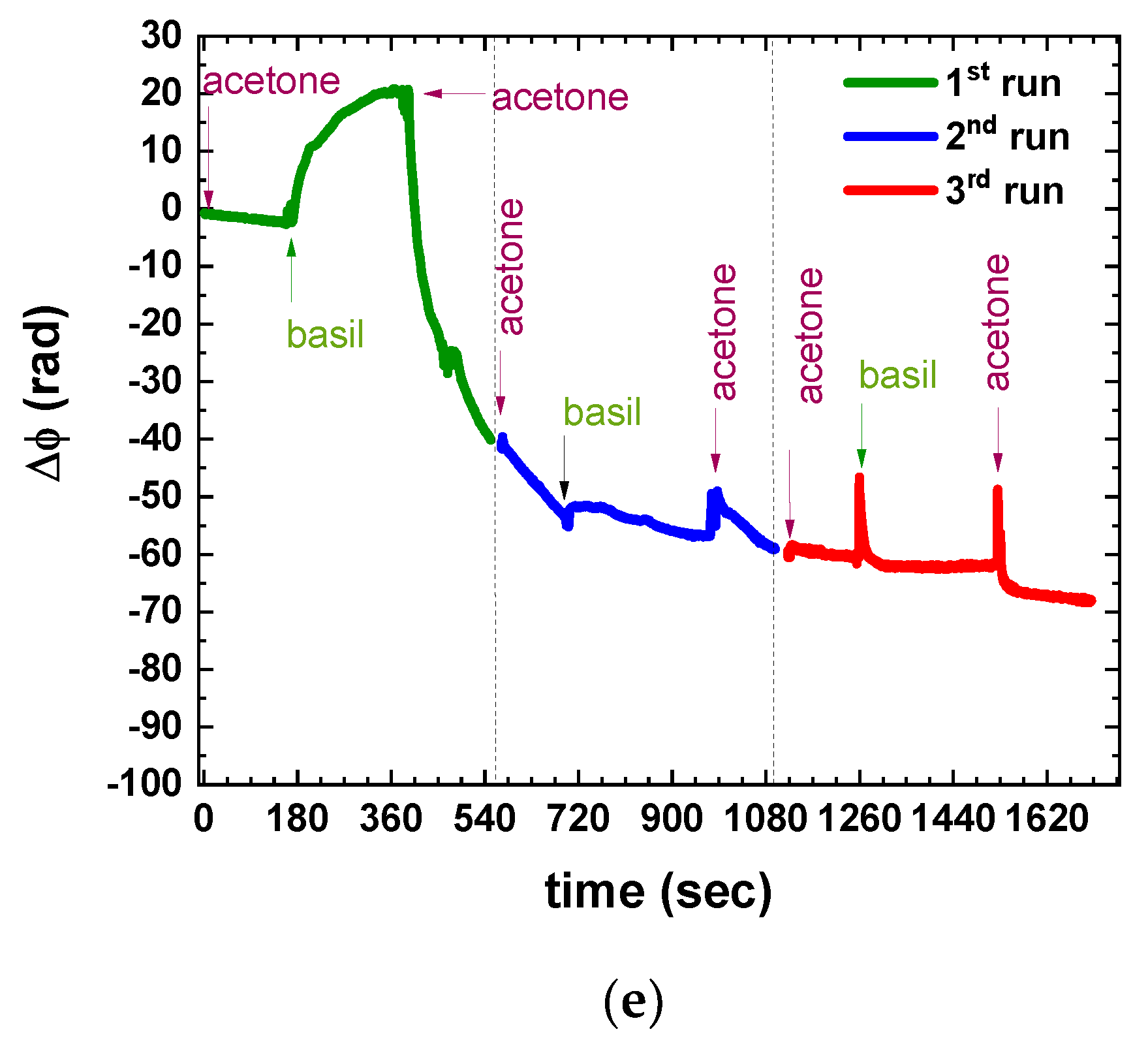
3.1. Defining the Measuring Protocol
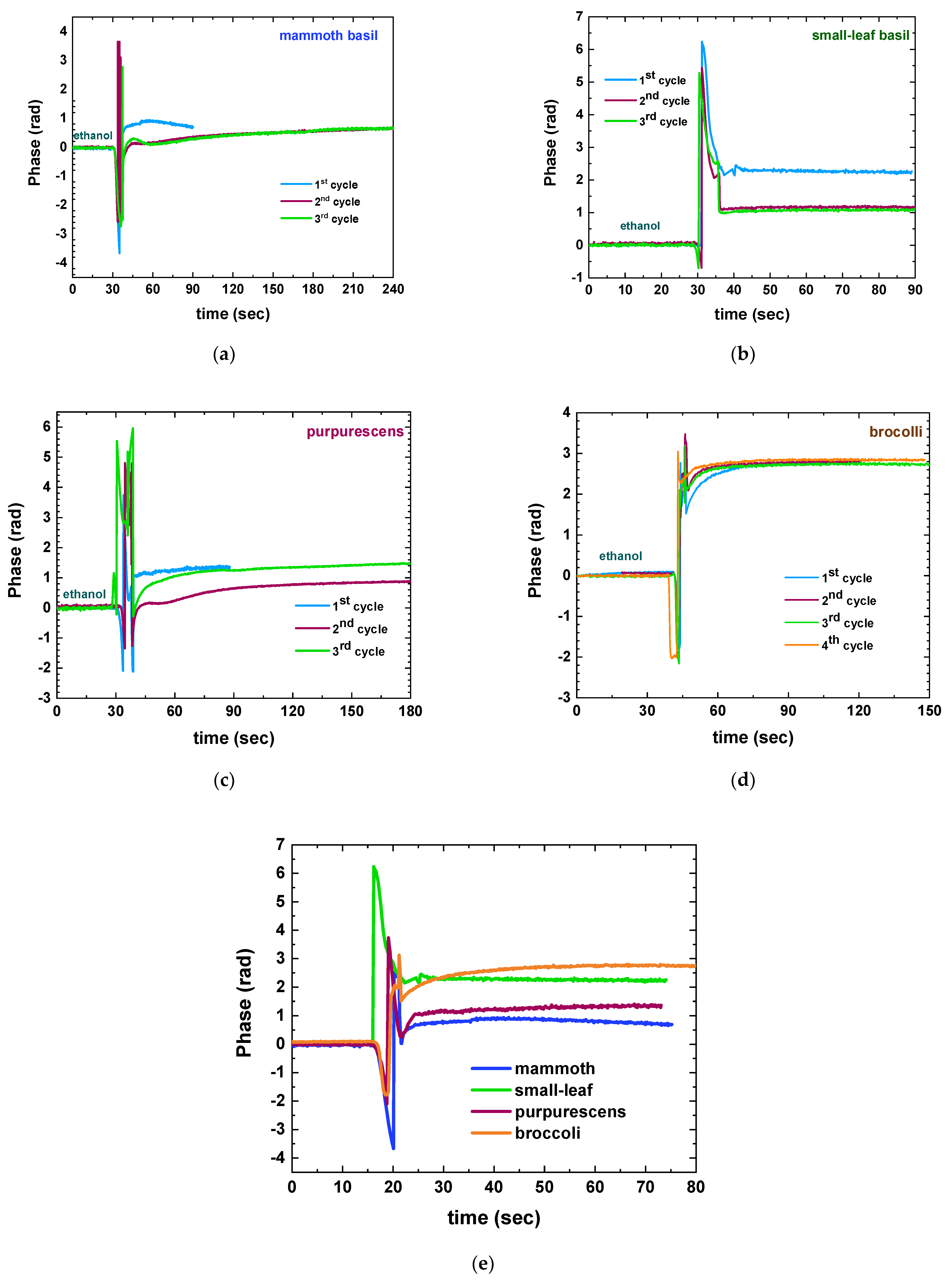
3.2. Correlation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Population Division of the United Nations Department of Economic and Social Affairs (UN DESA). World Population Prospects 2019: Highlights. (ST/ESA/SER.A/423). 2019. Available online: https://population.un.org/wpp/publications/files/wpp2019_highlights.pdf (accessed on 1 March 2023).
- Population Division of the United Nations Department of Economic and Social Affairs (UN DESA). World Population Prospects 2022: Summary of Results Ten Key Messages. 2022, pp. 2–3. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 1 March 2023).
- Renard, N. Urban Agriculture: Another Way to Feed Cities. Veolia Inst. Rev. 2019, 20, 66. [Google Scholar]
- Game, B.I.; Primus, R. Brief for GSDR 2015—Urban Agriculture. Available online: https://sdgs.un.org/documents/brief-gsdr-2015-urban-agriculture-20589 (accessed on 20 April 2023).
- Population Division of the United Nations Department of Economic and Social Affairs (UN DESA). The World’s Cities in 2018—Data Booklet. (ST/ESA/SER.A/417). 2018. Available online: https://www.un.org/en/development/desa/population/publications/pdf/urbanization/the_worlds_cities_in_2018_data_booklet.pdf (accessed on 1 March 2023).
- Ayuk, E.T.; Ramaswami, A.; Teixeira, I.; Akpalu, W.; Eckart, E.; Ferreira, J.; Kirti, D.; de Souza Leao, V. Urban Agriculture’s Potential to Advance Multiple Sustainability Goals: An International Resource Panel Think Piece; A Think Piece of the International Resource Panel; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Armanda, D.T.; Guinée, J.B.; Tukker, A. The second green revolution: Innovative urban agriculture’s contribution to food security and sustainability—A review. Glob. Food Sec. 2019, 22, 13–24. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.; Pearlstein, D.J.; Wheeler, R.M.; Johnson, C.M.; Wang, Q.; Fonseca, J.M. Microgreens for Home, Commercial, and Space Farming: A Comprehensive Update of the Most Recent Developments. Annu. Rev. Food Sci. Technol. 2023, 14, 539–562. [Google Scholar] [CrossRef]
- Mishra, G.P.; Dikshit, H.; Aski, M.; Sangwan, S.; Stobdan, T.; Dhaka, A.; Kumar, R.; Praveen, S. Microgreens: A Novel Food for Nutritional Security. In Conceptualizing Plant-Based Nutrition, 1st ed.; Ramesh, S.V., Praveen, S., Eds.; Springer: Singapore, 2022; pp. 123–156. [Google Scholar]
- Teng, J.; Liao, P.; Wang, M. The role of emerging micro-scale vegetables in human diet and health benefits—An updated review based on microgreens. Food Funct. 2021, 12, 1914–1932. [Google Scholar] [CrossRef] [PubMed]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Yu, L.L.; Wang, T.T.Y. The science behind microgreens as an exciting new food for the 21st century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Sharma, S.; Shree, B.; Sharma, D.; Kumar, S.; Kumar, V. Vegetable microgreens: The gleam of next generation super foods, their genetic enhancement, health benefits and processing approaches. Food Res. Int. 2022, 155, 111038. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional quality and health benefits of microgreens, a crop of modern agriculture. J. Futur. Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Moraru, P.I.; Rusu, T.; Mintas, O.S. Trial Protocol for Evaluating Platforms for Growing Microgreens in Hydroponic Conditions. Foods 2022, 11, 1327. [Google Scholar] [CrossRef]
- Rusu, T.; Cowden, R.J.; Moraru, P.I.; Maxim, M.A.; Ghaley, B.B. Overview of Multiple Applications of Basil Species and Cultivars and the Effects of Production Environmental Parameters on Yields and Secondary Metabolites in Hydroponic Systems. Sustainability 2021, 13, 11332. [Google Scholar] [CrossRef]
- Lanoue, J.; Louis, S.S.; Little, C.; Hao, X. Continuous lighting can improve yield and reduce energy costs while increasing or maintaining nutritional contents of microgreens. Front. Plant Sci. 2022, 13, 983222. [Google Scholar] [CrossRef]
- Othman, A.J.; Vodorezova, E.S.; Mardini, M.; Hanana, M.B. Dataset for the content of bioactive components and phytonutrients of (Ocimum basilicum and Brassica rapa) microgreens. Data Brief 2022, 40, 107737. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Luo, X.; Wang, Y.; Xie, X.; Lan, Y.; Li, L.; Zhu, T.; Ren, M. Combined metabolomics and transcriptomics analysis reveals the mechanism underlying blue light-mediated promotion of flavones and flavonols accumulation in Ligusticum chuanxiong Hort. Microgreens. J. Photochem. Photobiol. B Biol. 2023, 242, 112692. [Google Scholar] [CrossRef] [PubMed]
- Tavan, M.; Wee, B.; Brodie, G.; Fuentes, S.; Pang, A.; Gupta, D. Optimizing Sensor-Based Irrigation Management in a Soilless Vertical Farm for Growing Microgreens. Front. Sustain. Food Syst. 2021, 4, 622720. [Google Scholar] [CrossRef]
- Tatas, K.; Al-Zoubi, A.; Christofides, N.; Zannettis, C.; Chrysostomou, M.; Panteli, S.; Antoniou, A. Reliable IoT-Based Monitoring and Control of Hydroponic Systems. Technologies 2022, 10, 26. [Google Scholar] [CrossRef]
- Bhargava, Y.V.; Chittoor, P.K.; Bharatiraja, C.; Verma, R.; Sathiyasekar, K. Sensor Fusion Based Intelligent Hydroponic Farming and Nursing System. IEEE Sens. J. 2022, 22, 14584–14591. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, T.; Liu, J.; Lin, Z.; Li, S.; Wang, R.; Ge, Y. Soil pH value, organic matter and macronutrients contents prediction using optical diffuse reflectance spectroscopy. Comput. Electron. Agric. 2015, 111, 69–77. [Google Scholar] [CrossRef]
- Sekerli, Y.E.; Keskin, M.; Soysal, Y. Comparison of a Low-cost Prototype Optical Sensor with Three Commercial Systems in Predicting Water and Nutrient Contents of Turfgrass. Commun. Soil Sci. Plant Anal. 2021, 52, 586–600. [Google Scholar] [CrossRef]
- Mlinarić, S.; Gvozdić, V.; Vuković, A.; Varga, M.; Vlašiček, I.; Cesar, V.; Begović, L. The Effect of Light on Antioxidant Properties and Metabolic Profile of Chia Microgreens. Appl. Sci. 2020, 10, 5731. [Google Scholar] [CrossRef]
- Butkutė, B.; Taujenis, L.; Norkevičienė, E. Small-Seeded Legumes as a Novel Food Source. Variation of Nutritional, Mineral and Phytochemical Profiles in the Chain: Raw Seeds-Sprouted Seeds-Microgreens. Molecules 2019, 24, 133. [Google Scholar] [CrossRef]
- Allegretta, I.; Gattullo, C.E.; Renna, M.; Paradiso, V.M.; Terzano, R. Rapid multi-element characterization of microgreens via total-reflection X-ray fluorescence (TXRF) spectrometry. Food Chem. 2019, 296, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Leoni, B.; Caponio, F.; Santamaria, P. Simple tools for monitoring chlorophyll in broccoli raab and radish microgreens on their growing medium during cold storage. Prog. Nutr. 2018, 20, 415–422. [Google Scholar]
- Brambilla, M.; Romano, E.; Buccheri, M.; Cutini, M.; Toscano, P.; Cacini, S.; Massa, D.; Ferri, S.; Monarca, D.; Fedrizzi, M.; et al. Application of a low-cost RGB sensor to detect basil (Ocimum basilicum L.) nutritional status at pilot scale level. Precis. Agric. 2021, 22, 734–753. [Google Scholar] [CrossRef]
- Tran, T.N.; Keller, R.; Trinh, V.Q.; Tran, K.Q.; Kaldenhoff, R. Multi-Channel Spectral Sensors as Plant Reflectance Measuring Devices—Toward the Usability of Spectral Sensors for Phenotyping of Sweet Basil (Ocimum basilicum). Agronomy 2022, 12, 1174. [Google Scholar] [CrossRef]
- Misiakos, K.; Raptis, I.; Makarona, E.; Botsialas, A.; Salapatas, A.; Oikonomou, P.; Psarouli, A.; Petrou, P.S.; Kakabakos, S.E.; Tukkiniemi, K.; et al. All-silicon monolithic Mach-Zehnder interferometer as a refractive index and bio-chemical sensor. Opt. Express 2014, 22, 26803–26813. [Google Scholar] [CrossRef]
- Misiakos, K.; Raptis, I.; Salapatas, A.; Makarona, E.; Botsialas, A.; Hoekman, M.; Stoffer, R.; Jobst, G. Broad-band Mach-Zehnder interferometers as high performance refractive index sensors: Theory and monolithic implementation. Opt. Express 2014, 22, 8856–8870. [Google Scholar] [CrossRef]
- Psarouli, A.; Salapatas, A.; Botsialas, A.; Petrou, P.S.; Raptis, I.; Makarona, E.; Jobst, G.; Tukkiniemi, K.; Sopanen, M.; Stoffer, R.; et al. Monolithically integrated broad-band Mach-Zehnder interferometers for highly sensitive label-free detection of biomolecules through dual polarization optics. Sci. Rep. 2015, 5, 17600. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Makarona, E.; Salapatas, A.; Misiakos, K.; Synolaki, E.; Ioannidis, A.; Chatzipanagiotou, S.; Ritvos, M.A.; Pasternack, A.; Ritvos, O.; et al. Directly immersible silicon photonic probes: Application to rapid SARS-CoV-2 serological testing. Biosens. Bioelectron. 2022, 215, 114570. [Google Scholar] [CrossRef]
- Kourti, D.; Angelopoulou, M.; Misiakos, K.; Makarona, E.; Economou, A.; Petrou, P.; Kakabakos, S. Detection of Adulteration of Milk from Other Species with Cow Milk through an Immersible Photonic Immunosensor. Eng. Proc. 2023, 35, 582. [Google Scholar]
- Kaparakou, E.H.; Kanakis, C.D.; Gerogianni, M.; Maniati, M.; Vekrellis, K.; Skotti, E.; Tarantilis, P.A. Quantitative determination of aloin, antioxidant activity, and toxicity of Aloe vera leaf gel products from Greece. J. Sci. Food Agric. 2021, 101, 414–423. [Google Scholar] [CrossRef]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Swieca, M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Edelenbos, M.; Christensen, L.P.; Grevsen, K. HPLC Determination of Chlorophyll and Carotenoid Pigments in Processed Green Pea Cultivars (Pisum sativum L.). J. Agric. Food Chem. 2001, 49, 4768–4774. [Google Scholar] [CrossRef] [PubMed]
- Kidmose, U.; Knuthsen, P.; Edelenbos, M.; Justesen, U.; Hegelund, E. Carotenoids and flavonoids in organically grown spinach (Spinacia oleracea L.) genotypes after deep frozen storage. J. Sci. Food Agric. 2001, 81, 918–923. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef] [PubMed]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Gaspari, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Nutrient Supplementation Configures the Bioactive Profile and Production Characteristics of Three Brassica L. Microgreens Species Grown in Peat-Based Media. Agronomy 2021, 11, 346. [Google Scholar] [CrossRef]

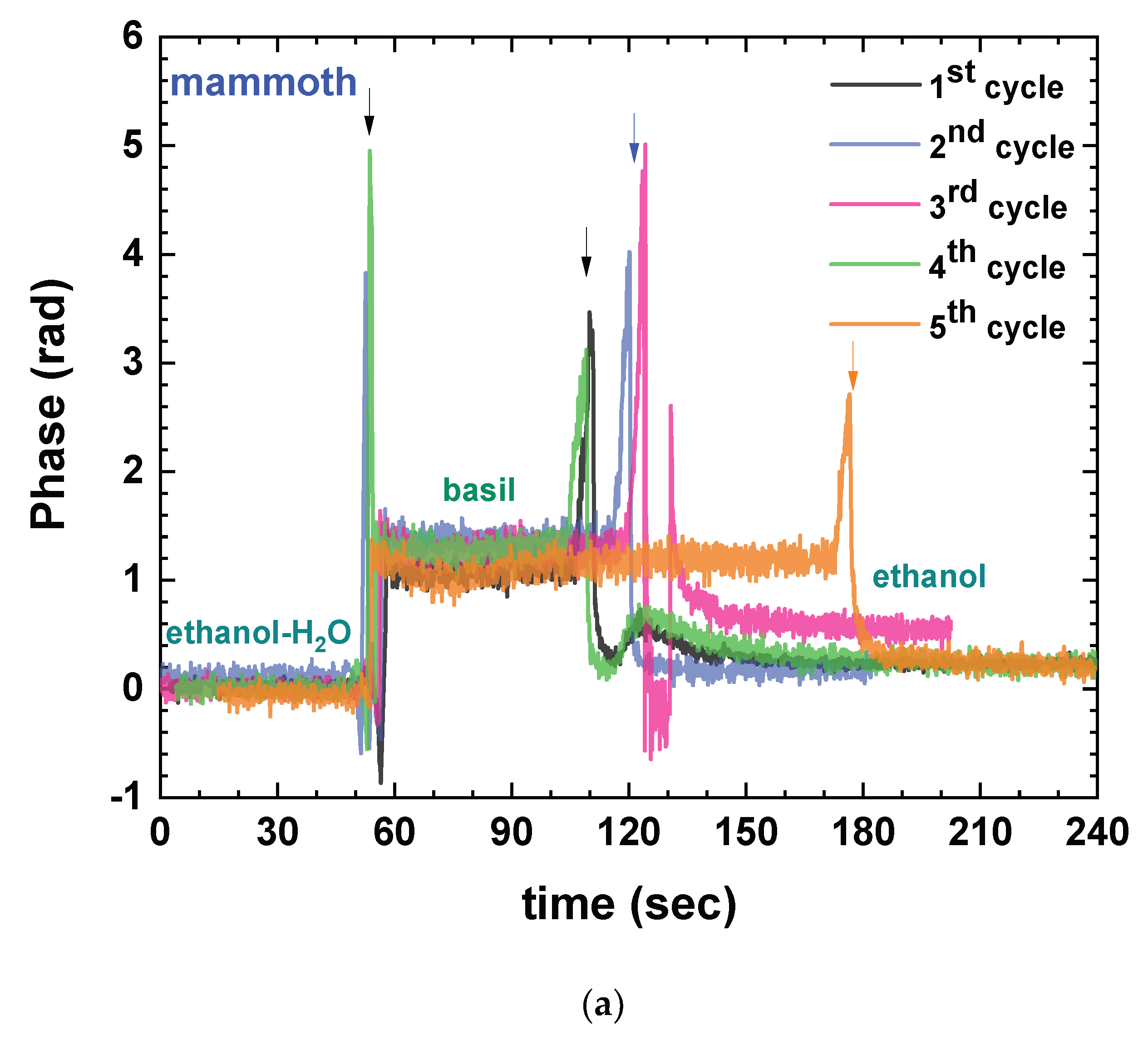
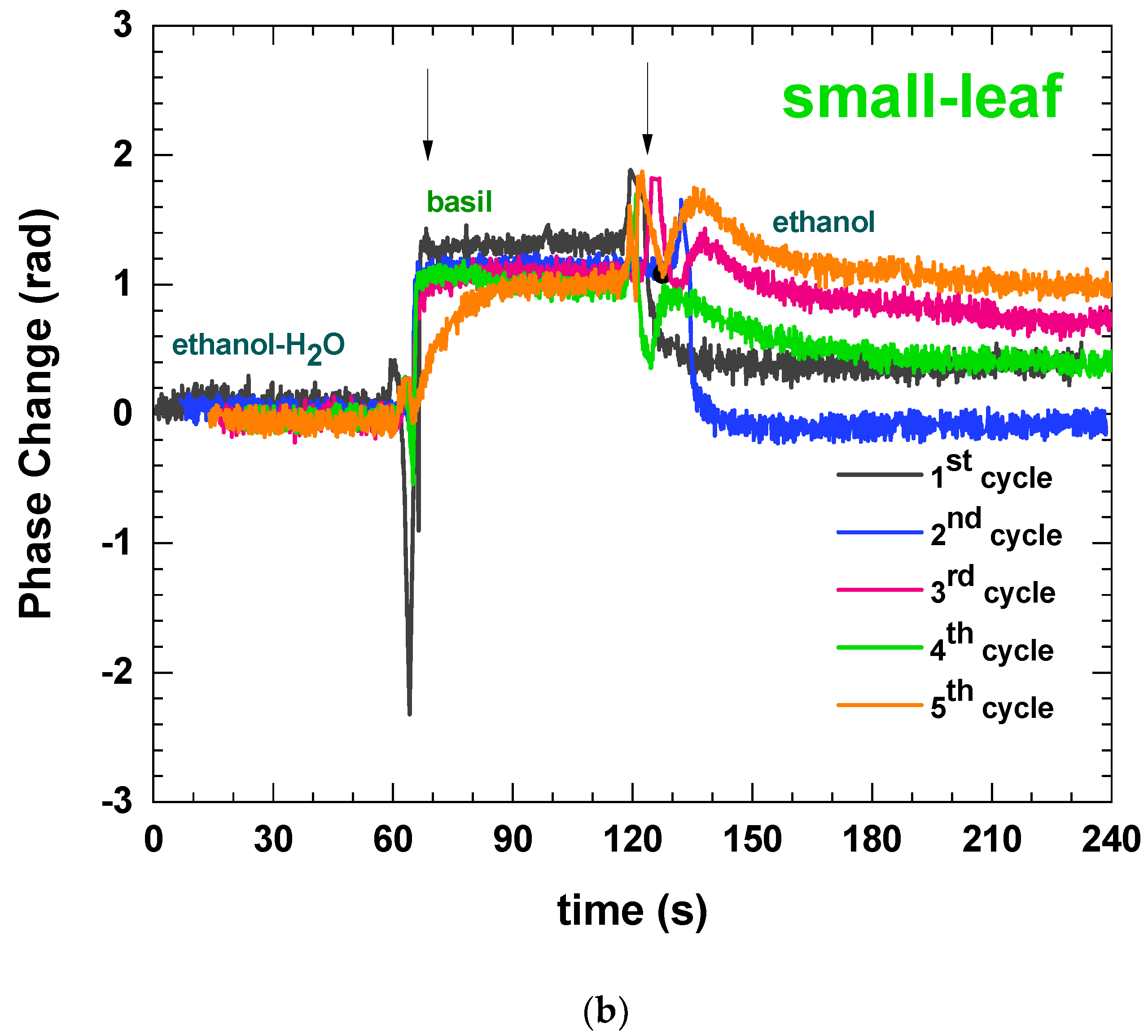

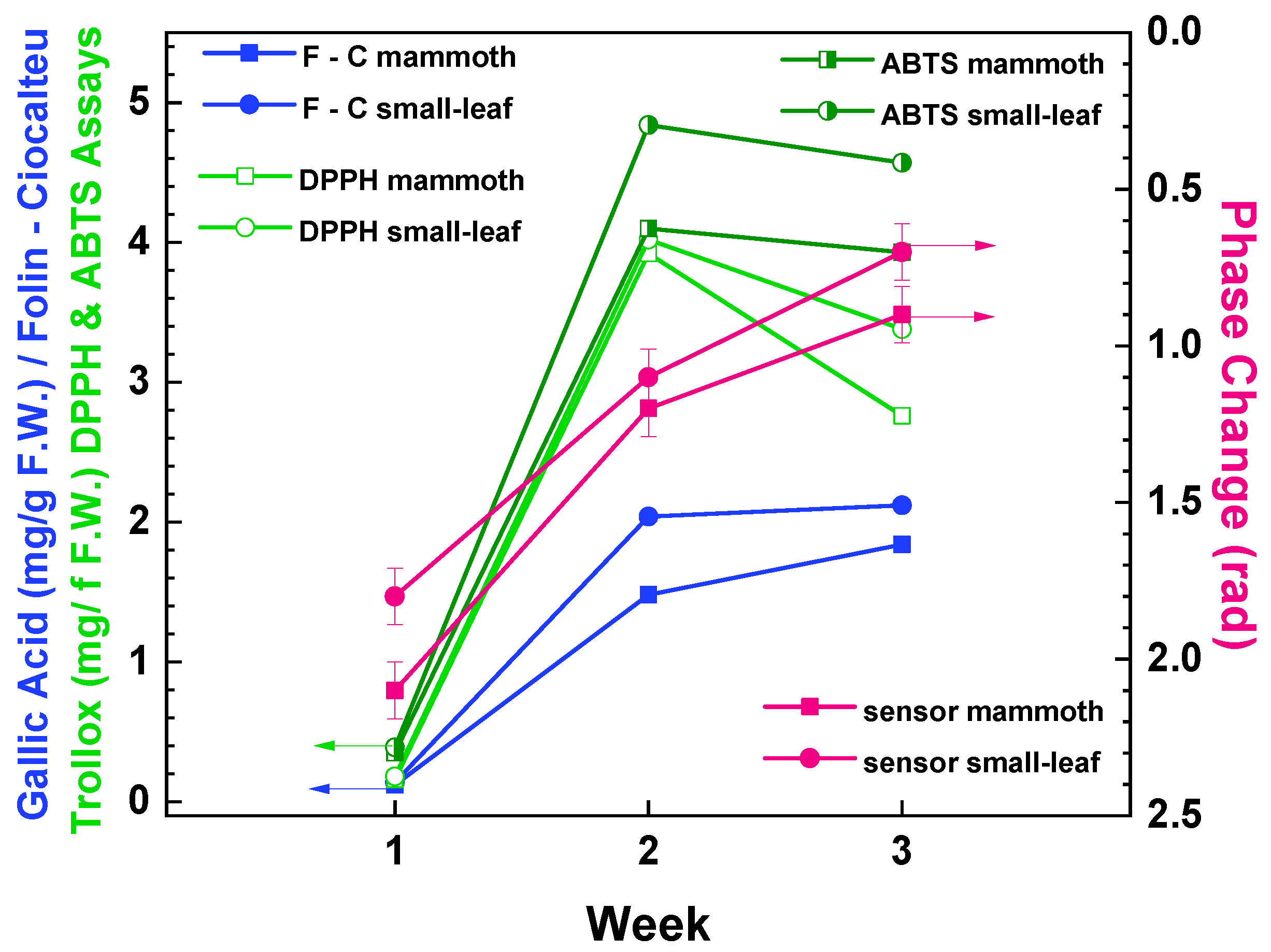
| Basil Type | Week | Folin–Ciocalteu Assay (mg Gallic Acid/g F.W.) | DPPH Assay (mg Trolox/g F.W.) | ABTS Assay (mg Trolox/g F.W.) | Immersible Sensors Phase Change (Rad) |
|---|---|---|---|---|---|
| mammoth | 1 | 0.12 | 0.16 | 0.35 | 2.1 |
| 2 | 1.48 | 3.92 | 4.1 | 1 | |
| 3 | 1.84 | 2.76 | 3.93 | 0.9 | |
| small-leaf | 1 | 0.14 | 0.18 | 0.39 | 1.8 |
| 2 | 2.04 | 4.02 | 4.84 | 1.3 | |
| 3 | 2.12 | 3.38 | 4.57 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christofi, A.; Margariti, G.; Salapatas, A.; Papageorgiou, G.; Zervas, P.; Karampiperis, P.; Koukourikos, A.; Tarantilis, P.A.; Kaparakou, E.H.; Misiakos, K.; et al. Determining the Nutrient Content of Hydroponically-Cultivated Microgreens with Immersible Silicon Photonic Sensors: A Preliminary Feasibility Study. Sensors 2023, 23, 5937. https://doi.org/10.3390/s23135937
Christofi A, Margariti G, Salapatas A, Papageorgiou G, Zervas P, Karampiperis P, Koukourikos A, Tarantilis PA, Kaparakou EH, Misiakos K, et al. Determining the Nutrient Content of Hydroponically-Cultivated Microgreens with Immersible Silicon Photonic Sensors: A Preliminary Feasibility Study. Sensors. 2023; 23(13):5937. https://doi.org/10.3390/s23135937
Chicago/Turabian StyleChristofi, Aristi, Georgia Margariti, Alexandros Salapatas, George Papageorgiou, Panagiotis Zervas, Pythagoras Karampiperis, Antonis Koukourikos, Petros A. Tarantilis, Eleftheria H. Kaparakou, Konstantinos Misiakos, and et al. 2023. "Determining the Nutrient Content of Hydroponically-Cultivated Microgreens with Immersible Silicon Photonic Sensors: A Preliminary Feasibility Study" Sensors 23, no. 13: 5937. https://doi.org/10.3390/s23135937
APA StyleChristofi, A., Margariti, G., Salapatas, A., Papageorgiou, G., Zervas, P., Karampiperis, P., Koukourikos, A., Tarantilis, P. A., Kaparakou, E. H., Misiakos, K., & Makarona, E. (2023). Determining the Nutrient Content of Hydroponically-Cultivated Microgreens with Immersible Silicon Photonic Sensors: A Preliminary Feasibility Study. Sensors, 23(13), 5937. https://doi.org/10.3390/s23135937









