Preparation of Nanocomposites for Antibacterial Orthodontic Invisible Appliance Based on Piezoelectric Catalysis
Abstract
1. Introduction
2. Materials and Methods
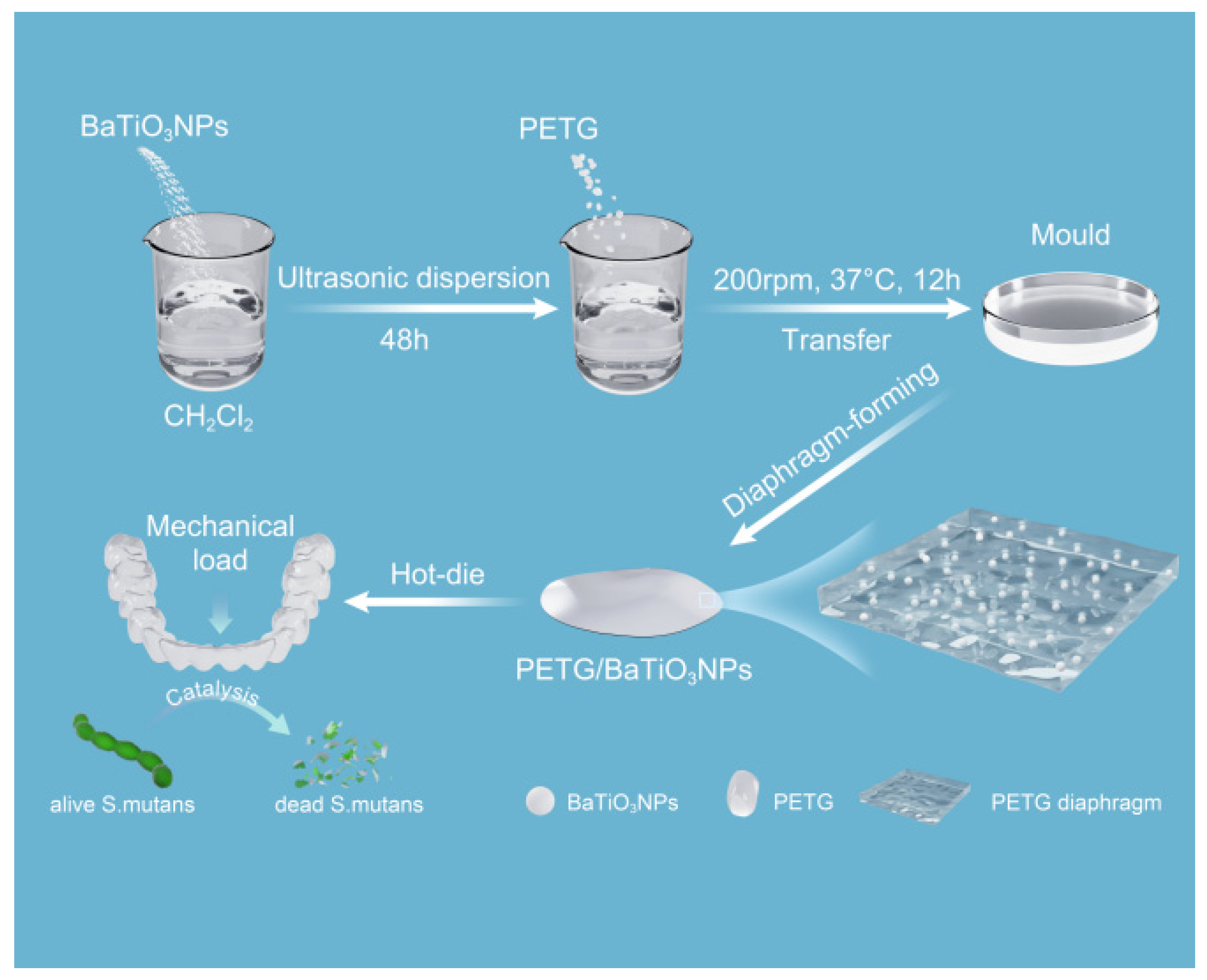
2.1. Fabrication of Nanocomposites Diaphragm
2.2. Characterization
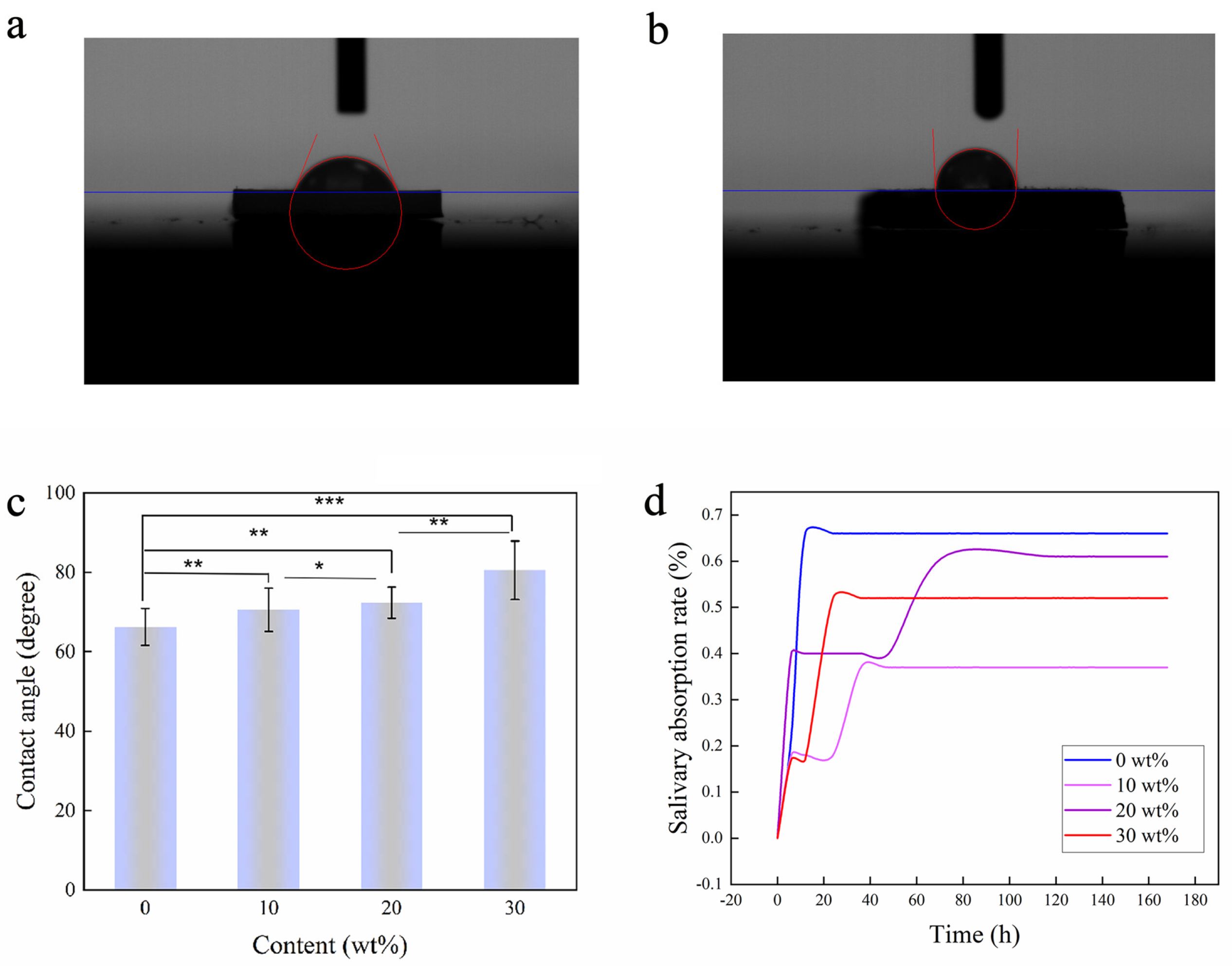
2.3. Contact Angle and Salivary Absorption
2.4. Mechanical Properties
2.5. In Vitro Antibacterial Test
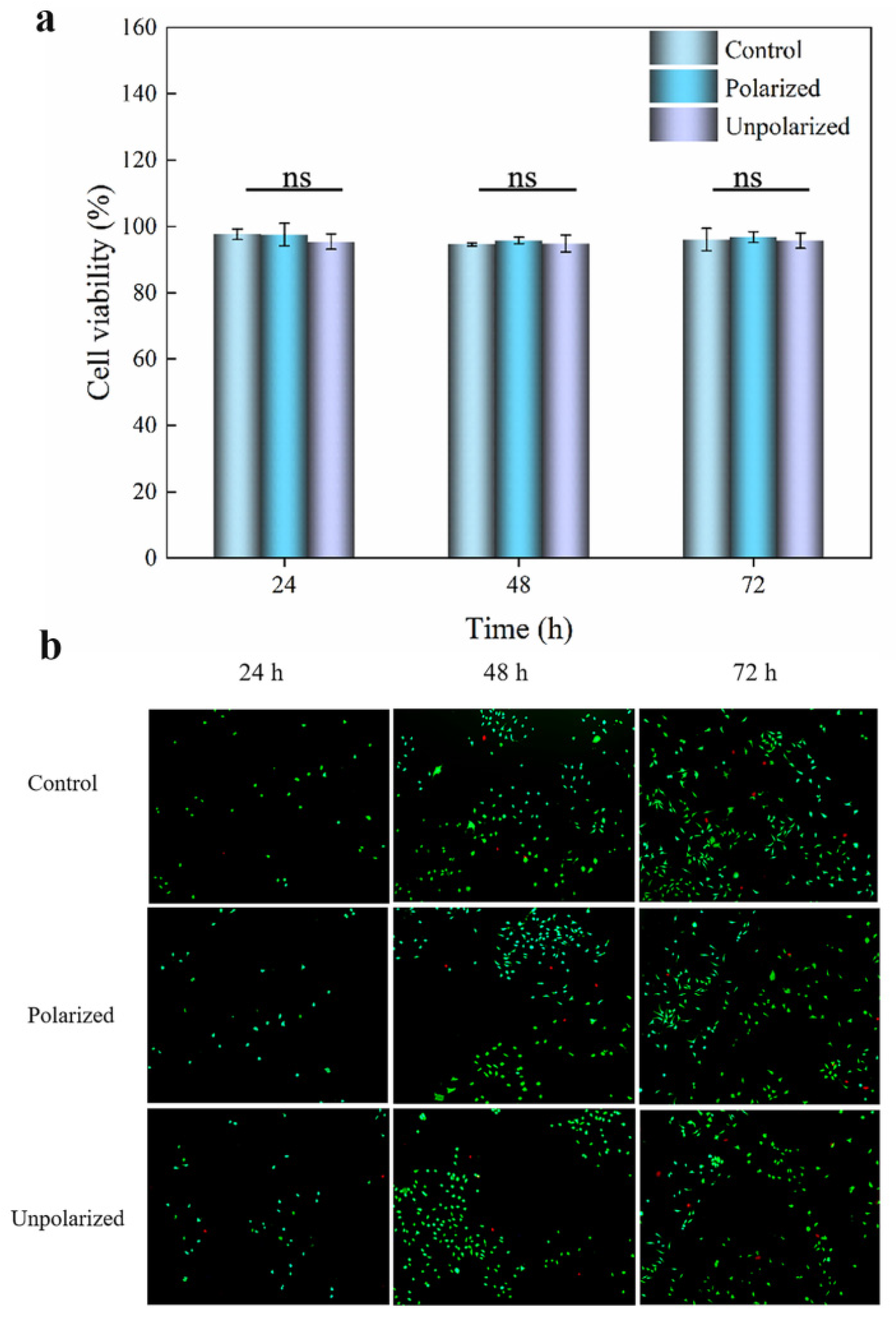
2.6. In Vitro Cytotoxicity
3. Results and Discussion
3.1. Characterization
3.2. Contact Angle and Salivary Absorption
3.3. Mechanical Properties
3.4. In Vitro Antibacterial Test
3.5. Cytotoxicity toward L-929
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, M.; Xie, C.; Yang, H.; Wu, C.; Ren, A. Prevalence of malocclusion in Chinese schoolchildren from 1991 to 2018: A systematic review and meta-analysis. Int. J. Paediatr. Dent. 2020, 30, 144–155. [Google Scholar] [CrossRef]
- Zou, J.; Meng, M.; Law, C.S.; Rao, Y.; Zhou, X. Common dental diseases in children and malocclusion. Int. J. Oral Sci. 2018, 10, 7. [Google Scholar] [CrossRef]
- Akbari, M.; Lankarani, K.B.; Honarvar, B.; Tabrizi, R.; Mirhadi, H.; Moosazadeh, M. Prevalence of malocclusion among Iranian children: A systematic review and meta-analysis. Dent. Res. J. 2016, 3, 387–395. [Google Scholar]
- Alvarado, K.; López, L.; Hanke, R.; Picón, F.; Rivas-Tumanyan, S. Prevalence of Malocclusion and Distribution of Occlusal Characteristics in 13- to 18-year-old Adolescents Attending Selected High Schools in the Municipality of San Juan, PR (2012–2013). Puerto Rico Health Sci. J. 2017, 36, 61–66. [Google Scholar]
- Mtaya, M.; Brudvik, P.; Astrøm, A.N. Prevalence of malocclusion and its relationship with socio-demographic factors, dental caries, and oral hygiene in 12 to 14-year-old Tanzanian schoolchildren. Eur. J. Orthod. 2009, 31, 467–476. [Google Scholar] [CrossRef]
- Zhao, R.; Huang, R.; Long, H.; Li, Y.; Gao, M.; Lai, W. The dynamics of the oral microbiome and oral health among patients receiving clear aligner orthodontic treatment. Oral Dis. 2020, 26, 473–483. [Google Scholar] [CrossRef]
- Heymann, G.C.; Grauer, D. A contemporary review of white spot lesions in orthodontics. J. Esthet. Restor. Dent. 2013, 25, 85–95. [Google Scholar] [CrossRef]
- Chang, H.S.; Walsh, L.J.; Freer, T.J. Enamel demineralization during orthodontic treatment. Aetiology and prevention. Aust. Dent. J. 1997, 42, 322–327. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Yang, S.B.; Moon, J.-H.; Ahn, H.-W.; Hong, J. A Polysaccharide-based antibacterial coating with improved durability for clear overlay Appliances. ACS Appl. Mater. Interfaces 2018, 10, 17714–17721. [Google Scholar] [CrossRef]
- Raja, T.A.; Littlewood, S.J.; Munyombwe, T.; Bubb, N.L. Wear resistance of four types of vacuum-formed retainer materials: A laboratory study. Angle Orthod. 2013, 84, 656–664. [Google Scholar] [CrossRef]
- Ahn, H.W.; Ha, H.R.; Lim, H.N.; Choi, S. Effects of aging procedures on the molecular, biochemical, morphological, and mechanical properties of vacuum-formed retainers. J. Mech. Behav. Biomed. Mater. 2015, 51, 356–366. [Google Scholar] [CrossRef]
- Tektas, S.; Thurnheer, T.; Eliades, T.; Attin, T.; Karygianni, L. Initial bacterial adhesion and biofilm formation on aligner materials. Antibiotics 2020, 9, 908. [Google Scholar] [CrossRef]
- Levrini, L.; Mangano, A.; Margherini, S.; Tenconi, C.; Vigetti, D.; Muollo, R.; Abbate, G.M. ATP bioluminometers analysis on the surfaces of removable orthodontic aligners after the use of different cleaning methods. Int. J. Dent. 2016, 2016, 5926941. [Google Scholar] [CrossRef]
- Albhaisi, Z.; Al-Khateeb, S.N.; Abu Alhaija, E.S. Enamel demineralization during clear aligner orthodontic treatment compared with fixed appliance therapy, evaluated with quantitative light-induced fluorescence: A randomized clinical trial. Am. J. Orthod. Dentofac. 2020, 157, 594–601. [Google Scholar] [CrossRef]
- Levrini, L.; Novara, F.; Margherini, S.; Tenconi, C.; Raspanti, M. Scanning electron microscopy analysis of the growth of dental plaque on the surfaces of removable orthodontic aligners after the use of different cleaning methods. Clin. Cosmet. Investig. Dent. 2015, 7, 125–131. [Google Scholar]
- Wu, M.; Zhang, Z.; Liu, Z.; Zhang, J.; Zhang, Y.; Ding, Y.; Huang, T.; Xiang, D.; Wang, Z.; Dai, Y.; et al. Piezoelectric nanocomposites for sonodynamic bacterial elimination and wound healing. Nano Today 2021, 37, 101104. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Jin, Y.M.; Ouyang, H.; Zou, Y.; Wang, X.X.; Xie, L.X.; Li, Z. Flexible piezoelectric nanogenerator in wearable self-powered active sensor for respiration and healthcare monitoring. Semicond. Sci. Technol. 2017, 32, 064004. [Google Scholar] [CrossRef]
- Jiang, D.; Shi, B.; Ouyang, H.; Fan, Y.; Wang, Z.L.; Chen, Z.-M.; Li, Z. A 25-year bibliometric study of implantable energy harvesters and self-powered implantable medical electronics researches. Mater Today Chem. 2020, 16, 100386. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Geng, W.; Su, H.; Long, M. Efficient bifunctional piezocatalysis of Au/BiVO4 for simultaneous removal of 4-chlorophenol and Cr(VI) in water. Appl. Catal. B Environ. 2019, 259, 118084. [Google Scholar] [CrossRef]
- Jiang, Y.; Parsonnet, E.; Qualls, A.; Zhao, W.; Susarla, S.; Pesquera, D.; Dasgupta, A.; Acharya, M.; Zhang, H.; Gosavi, T.; et al. Enabling ultra-low-voltage switching in BaTiO3. Nat. Mater. 2022, 21, 779–785. [Google Scholar] [CrossRef]
- Yan, X.; Lam, K.H.; Li, X.; Chen, R.; Ren, W.; Ren, X.; Zhou, Q.; Shung, K.K. Correspondence: Lead-free intravascular ultrasound transducer using BZT-50BCT ceramics. IEEE T Ultrason Ferr. 2013, 60, 1272–1276. [Google Scholar] [CrossRef]
- Yuan, M.; Cheng, L.; Xu, Q.; Wu, W.; Bai, S.; Gu, L.; Wang, Z.; Lu, J.; Li, H.; Qin, Y.; et al. Biocompatible nanogenerators through high piezoelectric coefficient 0.5Ba(Zr0.2Ti0.8)O3-0.5(Ba0.7Ca0.3)TiO3 nanowires for in-vivo applications. Adv. Mater. 2022, 26, 7432–7437. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, C.; Wu, Z.; Hu, L.; Zhang, W.; Zhao, K. Fabrication and in vitro biological properties of piezoelectric bioceramics for bone regeneration. Sci Rep. 2017, 7, 43360. [Google Scholar] [CrossRef]
- Busuioc, C.; Voicu, G.; Jinga, S.I.; Mitran, V.; Cimpean, A. The influence of barium titanate on the biological properties of collagen-hydroxiapatite composite scaffolds. Mater. Lett. 2019, 253, 317–322. [Google Scholar] [CrossRef]
- Fakhar-e-Alam, M.; Saddique, S.; Hossain, N.; Shahzad, A.; Ullah, I.; Sohail, A.; Khan, M.J.I.; Saadullah, M. Synthesis, characterization, and application of BaTiO3 nanoparticles for anti-cancer activity. J. Clust. Sci. 2022. [Google Scholar] [CrossRef]
- Shah, A.A.; Khan, A.; Dwivedi, S.; Musarrat, J.; Azam, A. Antibacterial and antibiofilm activity of barium titanate nanoparticles. Mater. Lett. 2018, 229, 130–133. [Google Scholar] [CrossRef]
- Dhall, A.; Islam, S.; Park, M.; Zhang, Y.; Kim, A.; Hwang, G. Bimodal Nanocomposite platform with antibiofilm and self-powering functionalities for biomedical applications. ACS Appl. Mater. Interfaces 2021, 13, 40379–40391. [Google Scholar] [CrossRef]
- Feng, Y.; Li, H.; Ling, L.; Yan, S.; Pan, D.; Ge, H.; Li, H.; Bian, Z. Enhanced photocatalytic degradation performance by fluid-Induced piezoelectric field. Environ. Sci. Technol. 2018, 52, 7842–7848. [Google Scholar] [CrossRef]
- Yao, G.; Kang, L.; Li, J.; Long, Y.; Wei, H.; Ferreira, C.A.; Jeffery, J.J.; Lin, Y.; Cai, W.; Wang, X. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat. Commun. 2018, 9, 5349. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Shi, Y.; Joe, P.; Ghaffari, R.; Balooch, G.; Usgaonkar, K.; Gur, O.; Tran, P.L.; Crosby, J.R.; Meyer, M.; et al. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat. Mater. 2015, 14, 728–736. [Google Scholar] [CrossRef]
- Montoya, C.; Jain, A.; Londoño, J.J.; Correa, S.; Lelkes, P.I.; Melo, M.A.; Orrego, S. Multifunctional dental composite with piezoelectric nanofillers for combined antibacterial and mineralization effects. ACS Appl. Mater. Interfaces 2021, 13, 43868–43879. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, X.; Jia, Y.; Huang, M.; Wang, F.; Zhang, X.; Bai, Y.; Yuan, G.; Wang, Y. Piezo-catalysis for nondestructive tooth whitening. Nat. Commun. 2020, 11, 1328. [Google Scholar] [CrossRef]
- Xu, J.; Ji, M.; Li, L.; Wu, Y.; Yu, Q.; Chen, M. Improving wettability, antibacterial and tribological behaviors of zirconia ceramics through surface texturing. Ceram. Int. 2022, 48, 3702–3710. [Google Scholar] [CrossRef]
- Wassmann, T.; Kreis, S.; Behr, M.; Buergers, R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int. J. Implant Dent. 2017, 3, 32. [Google Scholar] [CrossRef]
- Prabhu Suraj Nanduru, V.S.; Ramakrishna, N.S.; Babu, R.S.; Babu, P.D.; Marimuthu, P.; Miryala, S.; Srinandan, C. Laser surface texturing inhibits Biofilm formation. Mater. Chem. Phys. 2021, 271, 124909. [Google Scholar] [CrossRef]
- Ge, G.; Mandal, K.; Haghniaz, R.; Li, M.; Xiao, X.; Carlson, L.; Jucaud, V.; Dokmeci, M.R.; Ho, G.W.; Khademhosseini, A. Deep eutectic solvents-based ionogels with ultrafast gelation and high adhesion in harsh environments. Adv. Funct. Mater. 2023, 33, 2207388. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhang, N.; Liu, J.; Wang, J.; Shen, S.; Zhang, J.; An, X.; Si, Q. Preparation of Nanocomposites for Antibacterial Orthodontic Invisible Appliance Based on Piezoelectric Catalysis. Sensors 2023, 23, 5336. https://doi.org/10.3390/s23115336
Shi Y, Zhang N, Liu J, Wang J, Shen S, Zhang J, An X, Si Q. Preparation of Nanocomposites for Antibacterial Orthodontic Invisible Appliance Based on Piezoelectric Catalysis. Sensors. 2023; 23(11):5336. https://doi.org/10.3390/s23115336
Chicago/Turabian StyleShi, Yingying, Ningning Zhang, Jiajie Liu, Junbin Wang, Shuhui Shen, Jingxiang Zhang, Xiaoli An, and Qingzong Si. 2023. "Preparation of Nanocomposites for Antibacterial Orthodontic Invisible Appliance Based on Piezoelectric Catalysis" Sensors 23, no. 11: 5336. https://doi.org/10.3390/s23115336
APA StyleShi, Y., Zhang, N., Liu, J., Wang, J., Shen, S., Zhang, J., An, X., & Si, Q. (2023). Preparation of Nanocomposites for Antibacterial Orthodontic Invisible Appliance Based on Piezoelectric Catalysis. Sensors, 23(11), 5336. https://doi.org/10.3390/s23115336





