Langmuir–Blodgett Films with Immobilized Glucose Oxidase Enzyme Molecules for Acoustic Glucose Sensor Application
Abstract
1. Introduction
2. Materials and Methods
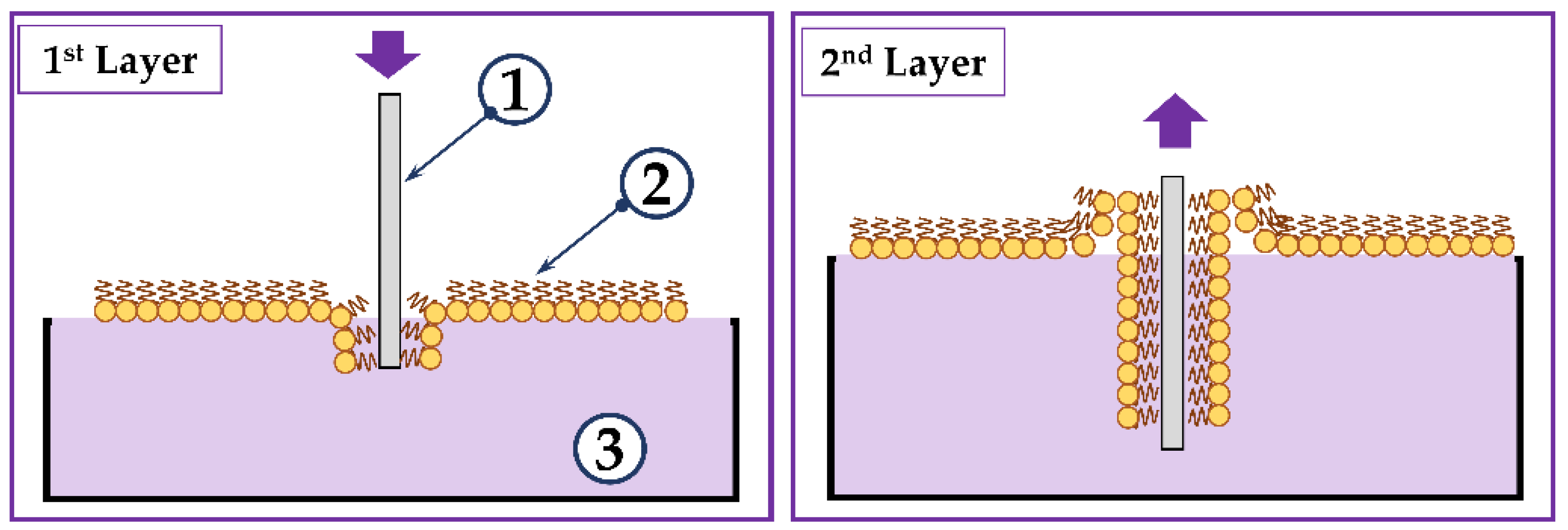
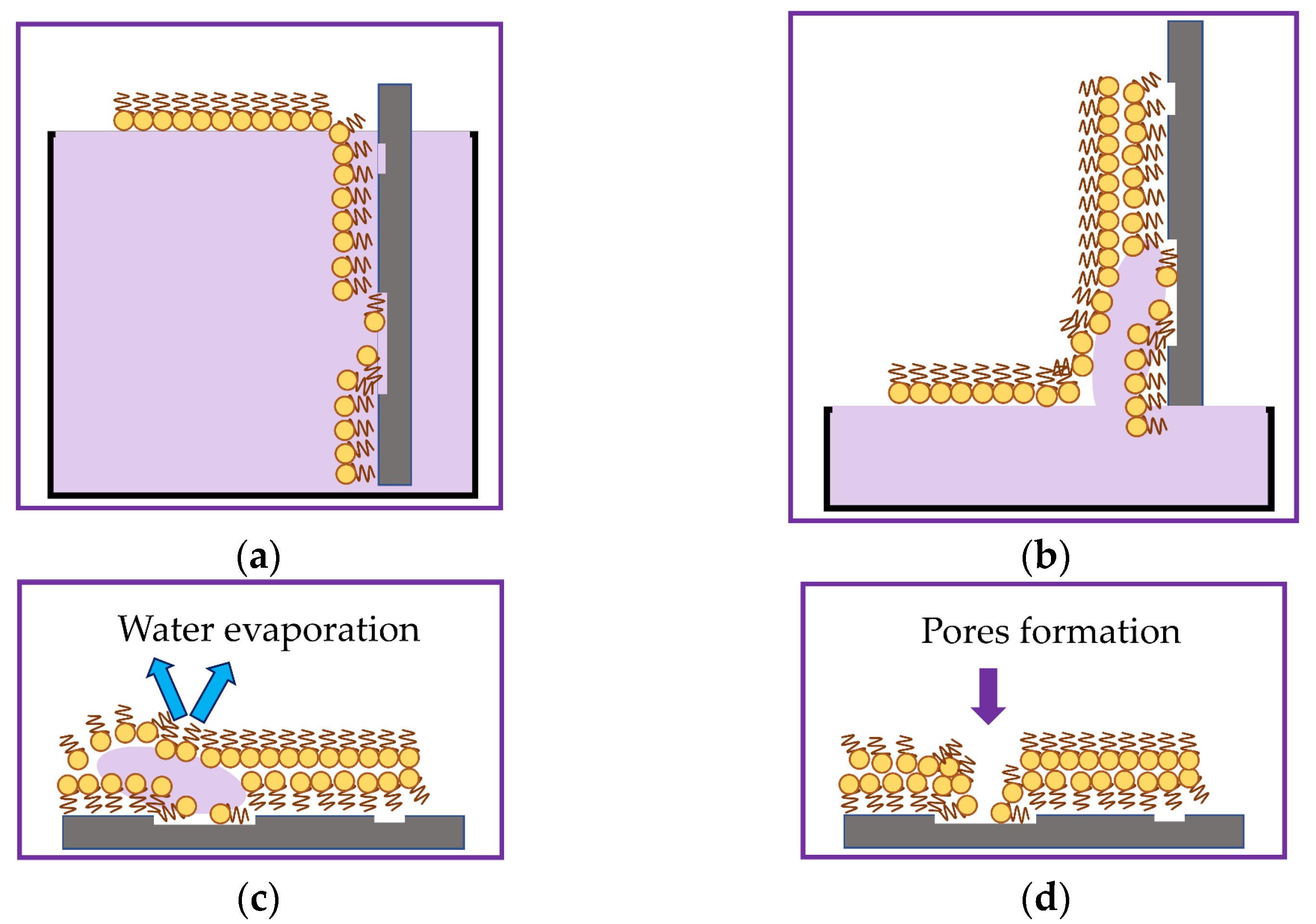
2.1. Formation of a Sensitive Coating Based on the LB Film of Phospholipid Molecules with Immobilized Molecules of the GOx Enzyme
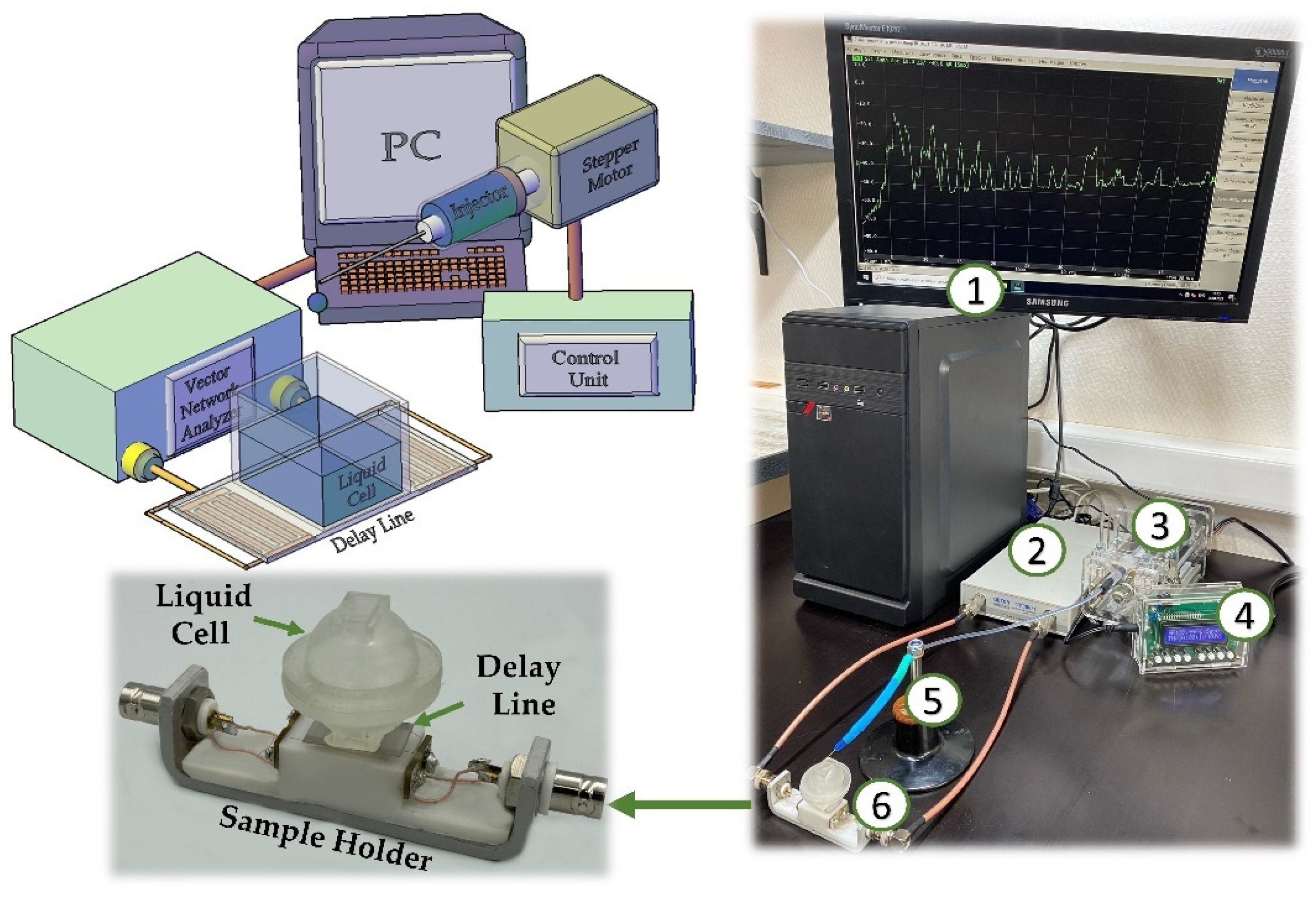
2.2. Production of an Acoustic Delay Line
2.3. Setup and Methods for the Study of Glucose-Sensitive Properties of the LB Film
3. Results and Discussion
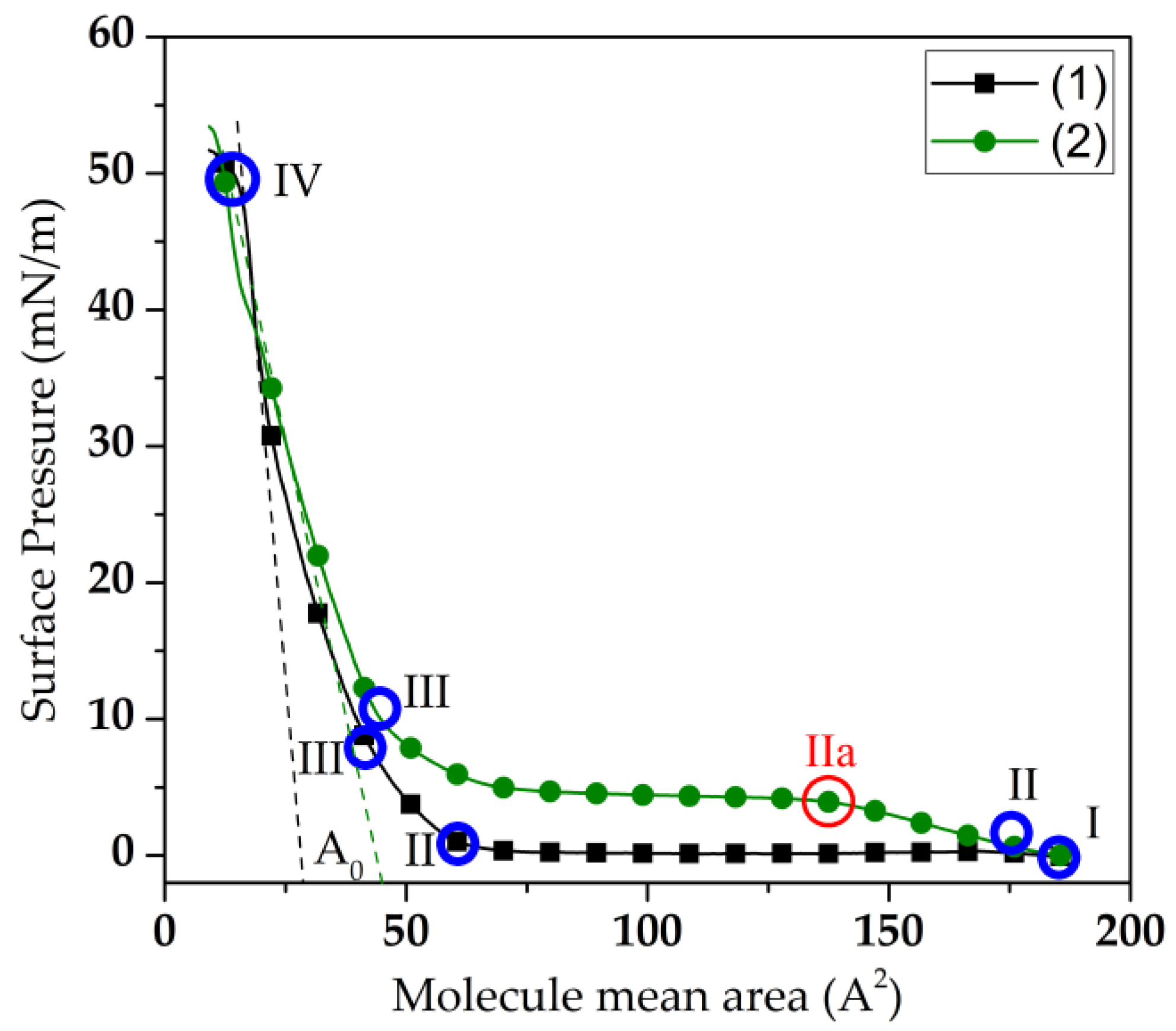
3.1. Influence of Adsorption of GOx Enzyme Molecules on the Surface Properties of a Langmuir DPPE Monolayer
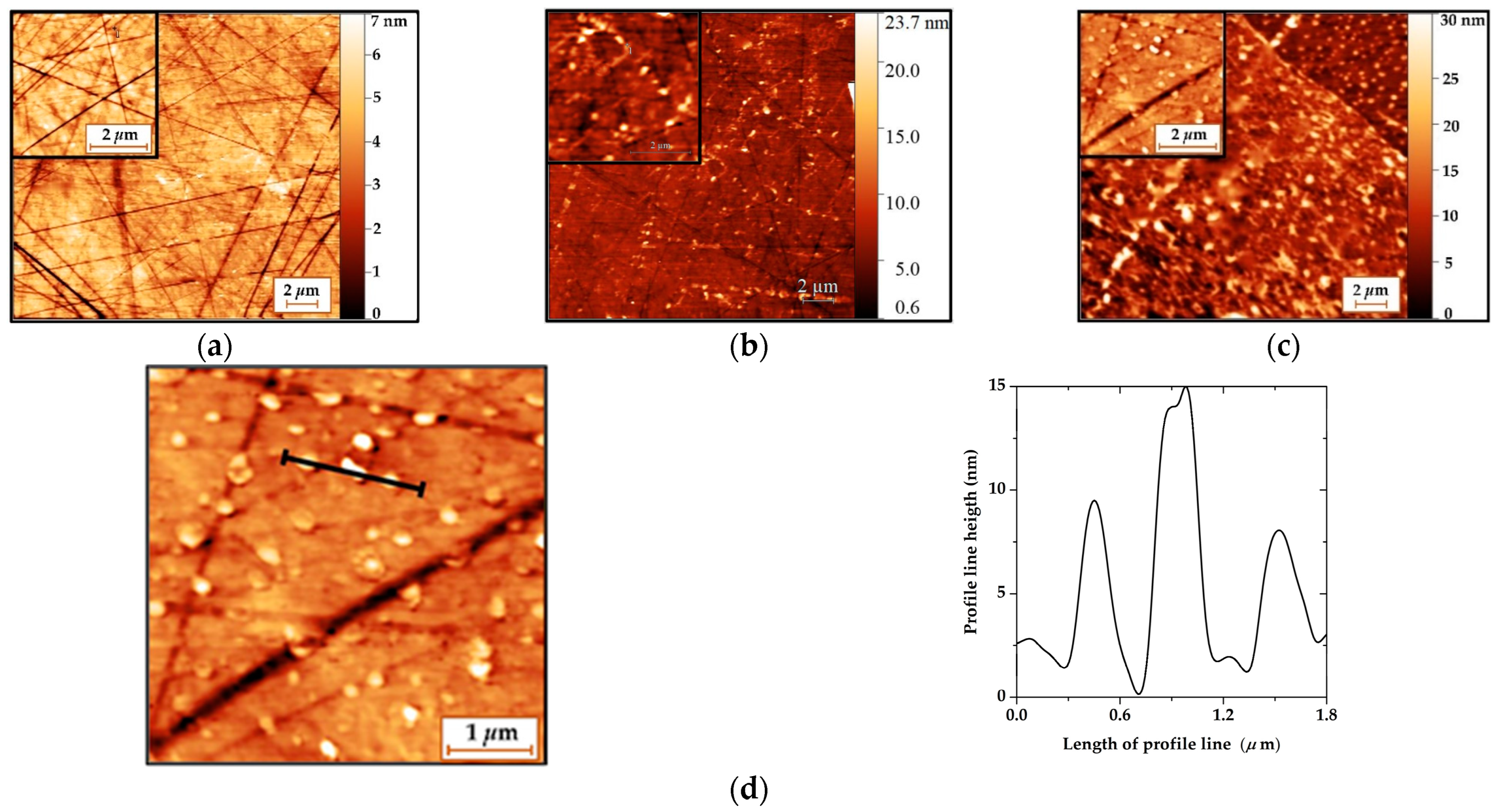
3.2. Study of the Morphology of a Sensitive Coating Based on an LB DPPE Film with Immobilized GOx Enzyme Molecules
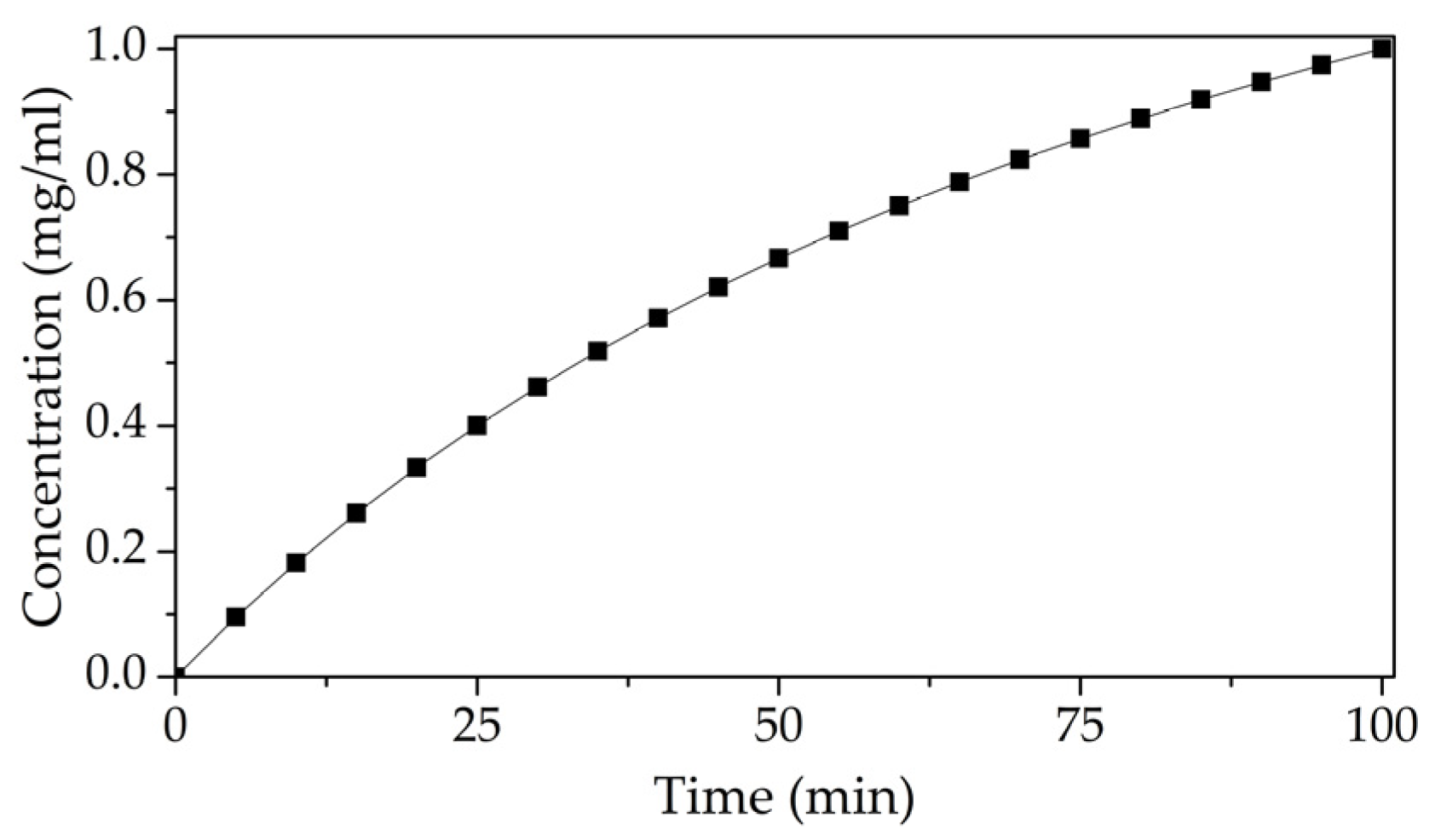
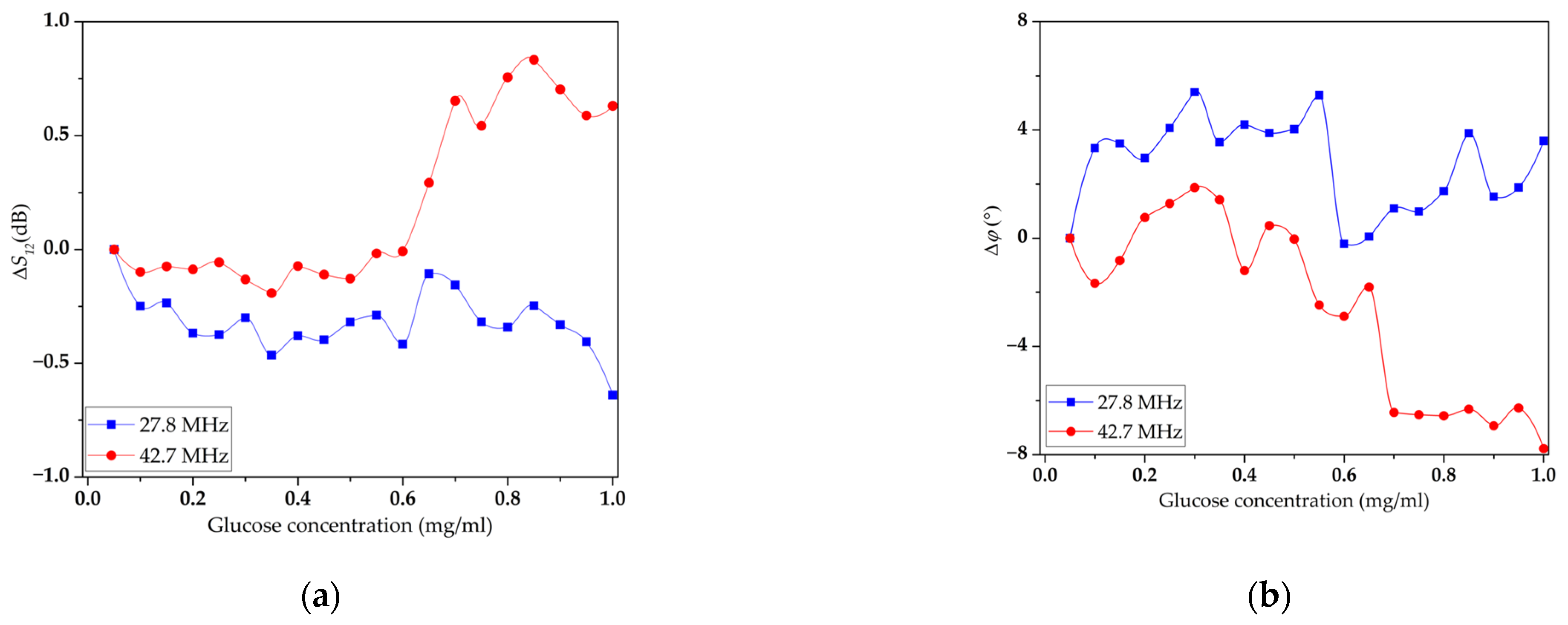
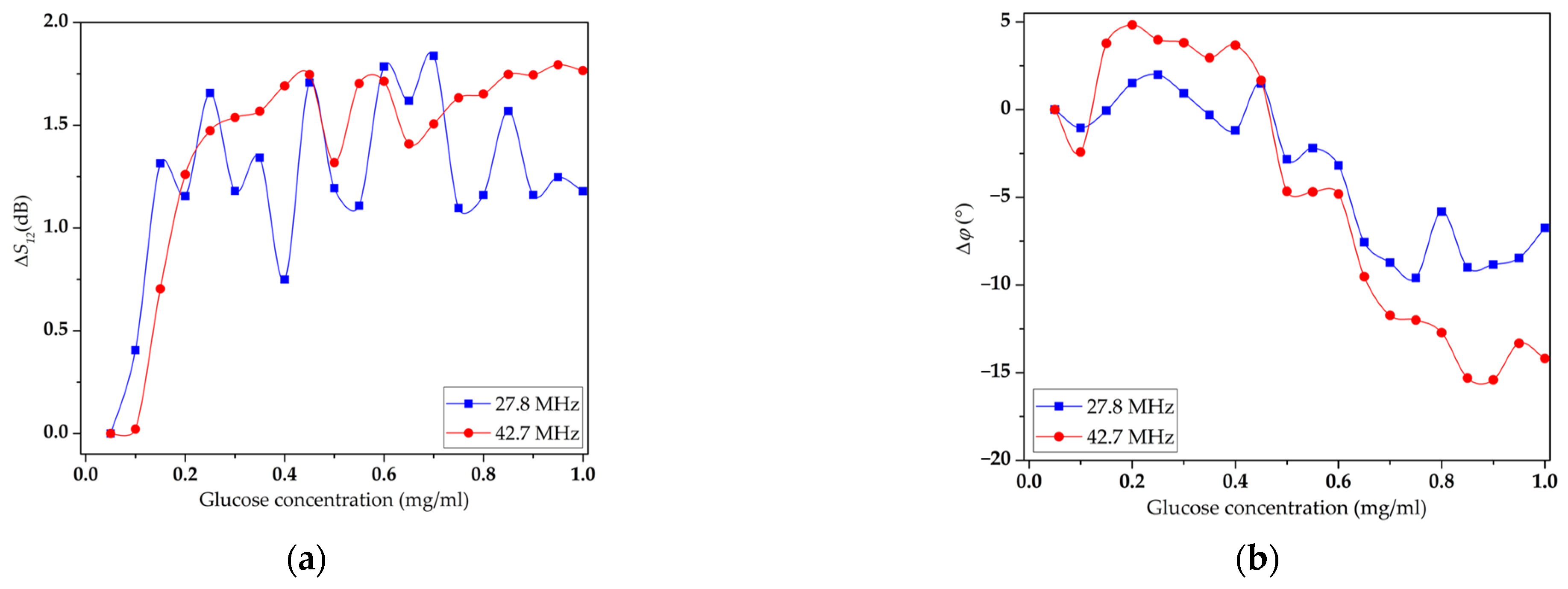
3.3. Study of the Sensitivity of LB Film with DPPE and Immobilized GOx Enzyme to Glucose Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parker, K.; Salas, M.; Nwosu, V.C. High fructose corn syrup: Production, uses and public health concerns. Biotechnol. Mol. Biol. Rev. 2010, 5, 71–78. [Google Scholar]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J. 2014, 14, 203–207. [Google Scholar] [PubMed]
- Chaiwut, P.; Jirarat, A.; Tiensri, N.; Sangthong, S.; Pintathong, P. Green Synthesis Optimization of Glucose Palm Oleate and Its Potential Use as Natural Surfactant in Cosmetic Emulsion. Cosmetics 2022, 9, 76. [Google Scholar] [CrossRef]
- Megias-Sayago, C.; Navarro-Jaen, S.; Drault, F.; Ivanova, S. Recent advances in the brønsted/lewis acid catalyzed Conversion of glucose to HMF and lactic acid: Pathways toward bio-based plastics. Catalysts 2021, 11, 1395. [Google Scholar] [CrossRef]
- Fagan, P.; Pokhrel, P.; Herzog, T.A.; Moolchan, E.T.; Cassel, K.D.; Franke, A.A.; Li, X.; Pagano, I.; Trinidad, D.R.; Sakuma, K.K.; et al. Sugar and aldehyde content in flavored electronic cigarette liquids. Nicotine Tob. Res. 2018, 20, 985–992. [Google Scholar] [CrossRef]
- Chadha, U.; Bhardwaj, P.; Agarwal, R.; Rawat, P.; Agarwal, R.; Gupta, I.; Panjwani, M.; Singh, S.; Ahuja, C.; Selvaraj, S.K.; et al. Recent progress and growth in biosensors technology: A critical review. J. Ind. Eng. Chem. 2022, 109, 21–51. [Google Scholar] [CrossRef]
- Escalona-Villalpando, A.R.; Sandoval-Garcia, A.; Espinosa, L.J.R.; Miranda-Silva, M.G.; Arriaga, L.G.; Minteer, S.D.; Ledesma-Garcia, J. A self-powered glucose biosensor device based on microfluidics using human blood. J. Power Source 2021, 515, 230631. [Google Scholar] [CrossRef]
- White, J.R., Jr.; Campbell, R.K.; Freerksen, A.; Gould, B. A comprehensive evaluation of a blood glucose self-monitoring system for diabetes care. Curr. Ther. Res. 1994, 55, 1127–1135. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Dai, J.; Zhang, W.; Jiang, Y.; Zhou, A. A low-cost mobile platform for whole blood glucose monitoring using colorimetric method. Microchem. J. 2021, 162, 105814. [Google Scholar] [CrossRef]
- Peng, Z.; Xie, X.; Tan, Q.; Kang, H.; Cui, J.; Zhang, X.; Li, W.; Feng, G. Blood glucose sensors and recent advances: A review. J. Innov. Opt. Health Sci. 2022, 15, 2230003. [Google Scholar] [CrossRef]
- Hammour, G.; Mandic, D.P. An In-Ear PPG-Based Blood Glucose Monitor: A Proof-of-Concept Study. Sensors 2023, 23, 3319. [Google Scholar] [CrossRef] [PubMed]
- Buehler, L.A.; Balasubramanian, V.; Baskerville, S.; Baylei, R.; McCarthy, K.; Rippen, M.; Bena, J.F.; Lansang, M.C. Noninvasive Glucose Monitor Using Dielectric Spectroscopy. Endocr. Pract. 2022, 28, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.; Chorage, S. Investigational Outcomes of Normal and Diabetic Human Volunteers using Microwave based Non-invasive Blood Glucometer. Int. J. Emerg. Technol. Adv. Eng. 2022, 12, 177–184. [Google Scholar] [CrossRef]
- Moon, J.-M.; Del Cano, R.; Moonla, C.; Sakdaphetsiri, K.; Saha, T.; Francine Mendes, L.; Yin, L.; Chang, A.-Y.; Seker, S.; Wang, J. Self-Testing of Ketone Bodies, along with Glucose, Using Touch-Based Sweat Analysis. ACS Sens. 2022, 7, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Lin, X.; Feng, J.; Min, X.; Ni, Y. NiNP/Cu-MOF-C/GCE for the the noninvasive detection of glucose in natural saliva samples. Microchem. J. 2023, 190, 108657. [Google Scholar] [CrossRef]
- Franco, F.F.; Hogg, R.A.; Manjakkal, L. Cu2O-Based Electrochemical Biosensor for Non-Invasive and Portable Glucose Detection. Biosensors 2022, 12, 174. [Google Scholar] [CrossRef]
- Lin, C.W.; Tsai, Y.H.; Lu, Y.P.; Yang, J.T.; Chen, M.Y.; Huang, T.J.; Weng, R.C.; Tung, C.W. Application of a Novel Biosensor for Salivary Conductivity in Detecting Chronic Kidney Disease. Biosensors 2022, 12, 178. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, I.; Campo-Valera, M.; Rodríguez, J.V.; Frisa-Rubio, A. Constrained IoT-Based Machine Learning for Accurate Glycemia Forecasting in Type 1 Diabetes Patients. Sensors 2023, 23, 3665. [Google Scholar] [CrossRef]
- Nor, M.N.; Ridhuan, N.S.; Razak, A.K. Progress of Enzymatic and Non-Enzymatic Electrochemical Glucose Biosensor Based on Nanomaterial-Modified Electrode. Biosensors 2022, 12, 1136. [Google Scholar]
- Reshetilov, A.N. Models of Biosensors Based on Principles of Potentiometric and Amperometric Transducers: Use in Medicine, Biotechnology, and Environmental Monitoring (Review). Appl. Biochem. Microbiol. 1996, 32, 72–85. [Google Scholar]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, H.; Istif, E.; Abbasiasl, T.; Mirlou, F.; Özkahraman, E.E.; Hasanreisoglu, M.; Beker, L. Femtosecond Laser Ablation Assisted NFC Antenna Fabrication for Smart Contact Lenses. Adv. Mater. Technol. 2022, 7, 2101629. [Google Scholar] [CrossRef]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanovic, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef] [PubMed]
- Thust, M.; Schöning, M.J.; Vetter, J.; Kordos, P.; Lüth, H. A long-term stable penicillin-sensitive potentiometric biosensor with enzyme immobilized by heterobifunctional cross-linking. Anal. Chim. Acta 1996, 323, 115–121. [Google Scholar] [CrossRef]
- Welden, M.; Poghossian, A.; Vahidpour, F.; Wendlandt, T.; Keusgen, M.; Wege, C.; Schöning, M.J. Capacitive field-effect biosensor modified with a stacked bilayer of weak polyelectrolyte and plant virus particles as enzyme nanocarriers. Bioelectrochemistry 2023, 151, 108397. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Development and Practical Application of Glucose Biosensor Based on Dendritic Gold Nanostructures Modified by Conducting Polymers. Biosensors 2022, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Hidouri, S.; Errachid, A.H.; Baussels, J.; Korpan, Y.I.; Ruiz-Sanchez, O.; Baccar, Z.M. Potentiometric sensing of histamine using immobilized enzymes on layered double hydroxides. J. Food Sci. Technol. 2021, 58, 2936–2942. [Google Scholar] [CrossRef] [PubMed]
- Osuna, V.; Vega-Rios, A.; Zaragoza-Contreras, E.A.; Estrada-Moreno, I.A.; Dominguez, R.B. Progress of Polyaniline Glucose Sensors for Diabetes Mellitus Management Utilizing Enzymatic and Non-Enzymatic Detection. Biosensors 2022, 12, 137. [Google Scholar] [CrossRef]
- Li, G.; Xu, Q.; Singh, R.; Zhang, W.; Marques, C.; Xie, Y.; Zhang, B.; Kumar, S. Graphene Oxide/Multiwalled Carbon Nanotubes Assisted Serial Quadruple Tapered Structure-Based LSPR Sensor for Glucose Detection. IEEE Sens. J. 2022, 22, 16904–16911. [Google Scholar] [CrossRef]
- Yu, Y.; Pan, M.; Peng, J.; Hu, D.; Hao, Y.; Qian, Z. A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection. Chin. Chem. Lett. 2022, 33, 4133–4145. [Google Scholar] [CrossRef]
- Yao, B.; Yao, J.; Fan, Z.; Zhao, D.J.; Zhang, D.K.; Huang, D.W. Rapid Advances of Versatile MXenes for Electrochemical Enzyme-Based Biosensors Immunosensors, and Nucleic Acid-Based Biosensors. ChemElectroChem 2022, 9, e202200103. [Google Scholar] [CrossRef]
- Manwar, R.; Saint-Martin, L.; Avanaki, K. Couplants in Acoustic Biosensing Systems. Chemosensors 2022, 10, 181. [Google Scholar] [CrossRef]
- Ariga, K.; Nakanishi, T.; Michinobu, T. Immobilization of Biomaterials to Nano-Assembled Films (Self-Assembled Monolayers, Langmuir-Blodgett Films, and Layer-by-Layer Assemblies) and Their Related Functions. J. Nanosci. Nanotechnol. 2006, 6, 2278–2301. [Google Scholar] [CrossRef] [PubMed]
- Caseli, L. Enzymes immobilized in Langmuir-Blodgett films: Why determining the surface properties in Langmuir monolayer is important? An. Acad. Bras. Cienc. 2018, 90, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Torres Rodrigues, R.; Nordi, C.F.S.; Siqueira Junior, J.R.; Caseli, L. Effect of interfering agents for urease immobilized in Langmuir-Blodgett films of controlled molecular architecture. Thin Solid Films 2020, 704, 138043. [Google Scholar] [CrossRef]
- Zaitsev, S.Y. Functional Langmuir films with glucose oxidase. Colloids Surf. A Physicochem. Eng. Asp. 1993, 75, 211–216. [Google Scholar] [CrossRef]
- Li, J.; Rosilio, V.; Boissonnade, M.-M.; Baszkin, A. Adsorption of glucose oxidase into lipid monolayers: Effect of a lipid headgroup charge. Colloids Surf. B Biointerfaces 2003, 29, 13–20. [Google Scholar] [CrossRef]
- Javadi, A.; Dowlati, S.; Miller, R.; Schneck, E.; Eckert, K.; Kraume, M. Dynamics of Competitive Adsorption of Lipase and Ionic Surfactants at the Water-Air Interface. Langmuir 2020, 36, 12010–12022. [Google Scholar] [CrossRef]
- Zhang, J.; Rosilio, V.; Goldmann, M.; Boissonnade, M.-M.; Baszkin, A. Adsorption of Glucose Oxidase into Lipid Monolayers. Effect of Lipid Chain Lengths on the Stability and Structure of Mixed Enzyme/Phospholipid Films. Langmuir 2000, 16, 1226–1232. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y. Affinity Biosensors Based on Preconcentration/Voltammetric Analysis. Detection of Phenothiazine Drugs at Langmuir-Blodgett Films of Tyrosine Hydroxylase. Anal. Chem. 1993, 65, 513–518. [Google Scholar] [CrossRef]
- Ohnuki, H.; Saiki, T.; Kusakari, A.; Endo, H.; Ichihara, M.; Izumi, M. Incorporation of Glucose Oxidase into Langmuir-Blodgett Films Based on Prussian Blue Applied to Amperometric Glucose Biosensor. Langmuir 2007, 23, 4675–4681. [Google Scholar] [CrossRef] [PubMed]
- Matharu, Z.; Sumana, G.; Sunil, K.; Arya, S.; Singh, P.; Gupta, V.; Malhotra, B.D. Polyaniline Langmuir-Blodgett Film Based Cholesterol Biosensor. Langmuir 2007, 23, 13188–13192. [Google Scholar] [CrossRef] [PubMed]
- Scholl, F.; Morais, P.; Gabriel, R.; Schöning, M.J.; Siqueira, J.R., Jr.; Caseli, L. Carbon nanotubes arranged as smart interfaces in lipid Langmuir-Blodgett films enhancing the enzymatic properties of penicillinase for biosensing applications. ACS Appl. Mater. Interfaces 2017, 9, 31054–31066. [Google Scholar] [CrossRef]
- Gur, F.; Kaya, E.D.; Gur, B.; Türkhan, A.; Onganer, Y. Preparation of bio-electrodes via Langmuir-Blodgett technique for pharmaceutical and waste industries and their biosensor application. Colloids Surf. A 2019, 583, 124005. [Google Scholar] [CrossRef]
- Ballantine, D.S.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zeller, E.T.; Frye, G.C.; Wohltjen, H. Acoustic Wave Sensors and Responses. In Acoustic Wave Sensors; Academic Press: San Diego, CA, USA, 1997; Chapter 3; pp. 36–149. [Google Scholar]
- Mandal, D.; Banerjee, S. Surface Acoustic Wave (SAW) Sensors: Physics, Materials, and Applications. Sensors 2022, 22, 820. [Google Scholar] [CrossRef]
- Yuan, Q.; Mirzajani, H.; Evans, B.; Greenbaum, E.; Wu, J. A Disposable Bulk-Acoustic-Wave Microalga Trapping Device for Real-time Water Monitoring. Sens. Actuators B Chem. 2020, 304, 127388. [Google Scholar] [CrossRef]
- Länge, K. Bulk and Surface Acoustic Wave Biosensors for Milk Analysis. Biosensors 2022, 12, 602. [Google Scholar] [CrossRef]
- Spagnolo, S.; De La Franier, B.; Davoudian, K.; Hianik, T.; Thompson, M. Detection of E. coli Bacteria in Milk by an Acoustic Wave Aptasensor with an Anti-Fouling Coating. Sensors 2022, 22, 1853. [Google Scholar] [CrossRef]
- Luo, T.; Liu, W.; Wen, Z.; Xie, Y.; Tong, X.; Cai, Y.; Liu, Y.; Sun, C. A High-Sensitivity Gravimetric Biosensor Based on S1 Mode Lamb Wave Resonator. Sensors 2022, 22, 5912. [Google Scholar] [CrossRef]
- Richardson, M.; Das, P.K.; Morrill, S.; Suthar, K.J.; Sankaranarayanan, S.K.R.S.; Bhethanabotla, V.R. Removal of Non-Specifically Bound Proteins Using Rayleigh Waves Generated on ST-Quartz Substrates. Sensors 2022, 22, 4096. [Google Scholar] [CrossRef]
- Holcroft, B.; Roberts, G.G.; Barraud, A.; Richard, J. Surface-acoustic-wave device incorporating conducting Langmuir-Blodgett-films. Electron. Lett. 1987, 23, 336–447. [Google Scholar] [CrossRef]
- Ivanov, G.R.; Avramov, I. Langmuir-Blodgett Films from Fluorescently Labelled Phospholipids Deposited on Surface Acoustic Wave Devices. J. Phys. Conf. Ser. 2019, 1186, 012007. [Google Scholar] [CrossRef]
- Acikbas, Y.; Tetik, G.D.; Ozkaya, C.; Bozkurt, S.; Capan, R.; Erdogan, M. Developing of N-(4-methylpyrimidine-2-yl)methacrylamide Langmuir–Blodgett thin film chemical sensor via quartz crystal microbalance technique. Microsc. Res. Tech. 2020, 83, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Gizeli, E.; Goddard, N.J.; Lowe, C.R.; Stevenson, A.C. A Love plate biosensor utilising a polymer layer. Sens. Actuators B Chem. 1992, 6, 131–137. [Google Scholar] [CrossRef]
- Lu, Y.; Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Pang, W.; Zhang, D.; Duan, X. Detection of Volatile Organic Compounds Using Microfabricated Resonator Array Functionalized with Supramolecular Monolayers. ACS Appl. Mater. Interfaces 2015, 7, 17893–17903. [Google Scholar] [CrossRef]
- Gorbachev, I.; Smirnov, A.; Ivanov, G.; Avramov, I.; Datsuk, E.; Venelinov, T.; Bogdanova, E.; Anisimkin, V.; Kolesov, V.; Kuznetsova, I. Langmuir-Blodgett Films of Arachidic and Stearic Acids as Sensitive Coatings for Chloroform HF SAW Sensors. Sensors 2023, 23, 100. [Google Scholar] [CrossRef]
- Penza, M.; Tagliente, M.A.; Aversa, P.; Re, M.; Cassano, G. The effect of purification of single-walled carbon nanotube bundles on the alcohol sensitivity of nanocomposite Langmuir-Blodgett films for SAW sensing applications. Nanotechnology 2007, 18, 185502. [Google Scholar] [CrossRef]
- Balcerzak, A.; Aleksiejuk, M.; Zhavnerko, G.; Agabekov, V. Sensing properties of two-component Langmuir-Blodgett layer and its porous derivative in SAW sensor for vapors of methanol and ethanol. Thin Solid Films 2010, 518, 3402–3406. [Google Scholar] [CrossRef]
- Maji, S.; Asrey, R.; Kumar, S.; Saxena, C.; Kumar, N.; Vyas, K.D.; Banerjee, S. Polymer-Coated Piezoelectric Quartz Crystal Sensor for Sensing the Nerve Agent Simulant Dimethyl Methylphosphonate Vapor. J. Appl. Polym. Sci. 2010, 116, 3708–3717. [Google Scholar] [CrossRef]
- Nanduri, V.; Sorokulova, I.B.; Samoylov, A.M.; Simonian, A.L.; Petrenko, V.A.; Vodyanoy, V. Phage as a molecular recognition element in biosensors immobilized by physical adsorption. Biosens. Bioelectron. 2007, 22, 986–992. [Google Scholar] [CrossRef]
- Ivanov, G.R. Investigation of Langmuir-Blodgett films from fluorescently labelled phospholipids with fluorescence lifetime spectroscopy and atomic force microscopy. AIP Conf. Proc. 2019, 2075, 020027. [Google Scholar]
- Raba, J.; Mottola, H.A. Glucose Oxidase as an Analytical Reagent. Crit. Rev. Anal. Chem. 1995, 25, 1–42. [Google Scholar] [CrossRef]
- Kolašinac, R.; Kleusch, C.; Braun, T.; Merkel, R.; Csiszár, A. Deciphering the Functional Composition of Fusogenic Liposomes. Int. J. Mol. Sci. 2018, 19, 346. [Google Scholar] [CrossRef] [PubMed]
- Benavidez, T.E.; Torrente, D.; Marucho, M.; Garcia, C.D. Adsorption and Catalytic Activity of Glucose Oxidase Accumulated on OTCE upon the Application of External Potential. J. Colloid Interface Sci. 2014, 435, 164–170. [Google Scholar] [CrossRef]
- Vu, T.T.; Kharitonova, N.V.; Maiorova, L.A.; Gromova, O.A.; Torshin, I.Y.; Koifman, O.I. Compression Speed as a Parameter Changing the Dimensionality of CorroleNanostructures in Layers at the Air-Water Interface. Macroheterocycles 2018, 11, 286–292. [Google Scholar] [CrossRef]
- Gaines, G.L. A thorough and comprehensive review of the literature on floating monolayers with a short section on LB films. In Insoluble Monolayers at Liquid-Gas Interface; Interscience Publishers: New York, NY, USA, 1966; p. 386. [Google Scholar]
- Barmentlo, M. Reorientation of liquid-crystal (LC) molecules in mixed surfactant/LC Langmuir films. Chem. Phys. Lett. 1993, 209, 347–351. [Google Scholar] [CrossRef]
- Sthoer, A.; Tyrode, E. Interactions of Na+ Cations with a Highly Charged Fatty AcidLangmuir Monolayer: Molecular Description of the Phase Transition. J. Phys. Chem. C 2019, 123, 23037–23048. [Google Scholar] [CrossRef]
- Necas, D.; Klapetek, P.; Valtr, M. Estimation of roughness measurement bias originating from background subtraction. Meas. Sci. Technol. 2020, 31, 094010. [Google Scholar] [CrossRef]
- Necas, D.; Valtr, M.M.; Klapetek, P. How levelling and scan line corrections ruin roughness measurement and how to prevent it. Sci. Rep. 2020, 10, 15294. [Google Scholar] [CrossRef]
- Hecht, H.J.; Kalisz, H.M.; Hendle, J.; Schmid, R.D.; Schomburg, D. Crystal Structure of Glucose Oxidase from Aspergillus niger Refined at 2·3 Å Resolution. J. Mol. Biol. 1993, 229, 153–172. [Google Scholar] [CrossRef]
- Anisimkin, V.; Shamsutdinova, E.; Li, P.; Wang, B.; Zhu, B.; Qian, Z.; Kuznetsova, I. Selective Detection of Liquid Viscosity Using Acoustic Plate Waves with In-Plane Polarization. Sensors 2022, 22, 2727. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, B.D.; Kuznetsova, I.E.; Joshi, S.G.; Borodina, I.A. Shear-horizontal acoustic waves in piezoelectric plates bordered with conductive liquid. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.A.; Zámocká, M.; Majtán, J.; Bauerová-Hlinková, V. Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbachev, I.; Smirnov, A.; Ivanov, G.R.; Venelinov, T.; Amova, A.; Datsuk, E.; Anisimkin, V.; Kuznetsova, I.; Kolesov, V. Langmuir–Blodgett Films with Immobilized Glucose Oxidase Enzyme Molecules for Acoustic Glucose Sensor Application. Sensors 2023, 23, 5290. https://doi.org/10.3390/s23115290
Gorbachev I, Smirnov A, Ivanov GR, Venelinov T, Amova A, Datsuk E, Anisimkin V, Kuznetsova I, Kolesov V. Langmuir–Blodgett Films with Immobilized Glucose Oxidase Enzyme Molecules for Acoustic Glucose Sensor Application. Sensors. 2023; 23(11):5290. https://doi.org/10.3390/s23115290
Chicago/Turabian StyleGorbachev, Ilya, Andrey Smirnov, George R. Ivanov, Tony Venelinov, Anna Amova, Elizaveta Datsuk, Vladimir Anisimkin, Iren Kuznetsova, and Vladimir Kolesov. 2023. "Langmuir–Blodgett Films with Immobilized Glucose Oxidase Enzyme Molecules for Acoustic Glucose Sensor Application" Sensors 23, no. 11: 5290. https://doi.org/10.3390/s23115290
APA StyleGorbachev, I., Smirnov, A., Ivanov, G. R., Venelinov, T., Amova, A., Datsuk, E., Anisimkin, V., Kuznetsova, I., & Kolesov, V. (2023). Langmuir–Blodgett Films with Immobilized Glucose Oxidase Enzyme Molecules for Acoustic Glucose Sensor Application. Sensors, 23(11), 5290. https://doi.org/10.3390/s23115290















