Experimental Analysis of Tear Fluid and Its Processing for the Diagnosis of Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Biological Material
2.2. Infrared Spectroscopy
2.3. Drop-Coating Deposition Raman Spectroscopy
2.4. Atomic-Force Microscopy
2.5. HPLC Mass Spectrometry
3. Results
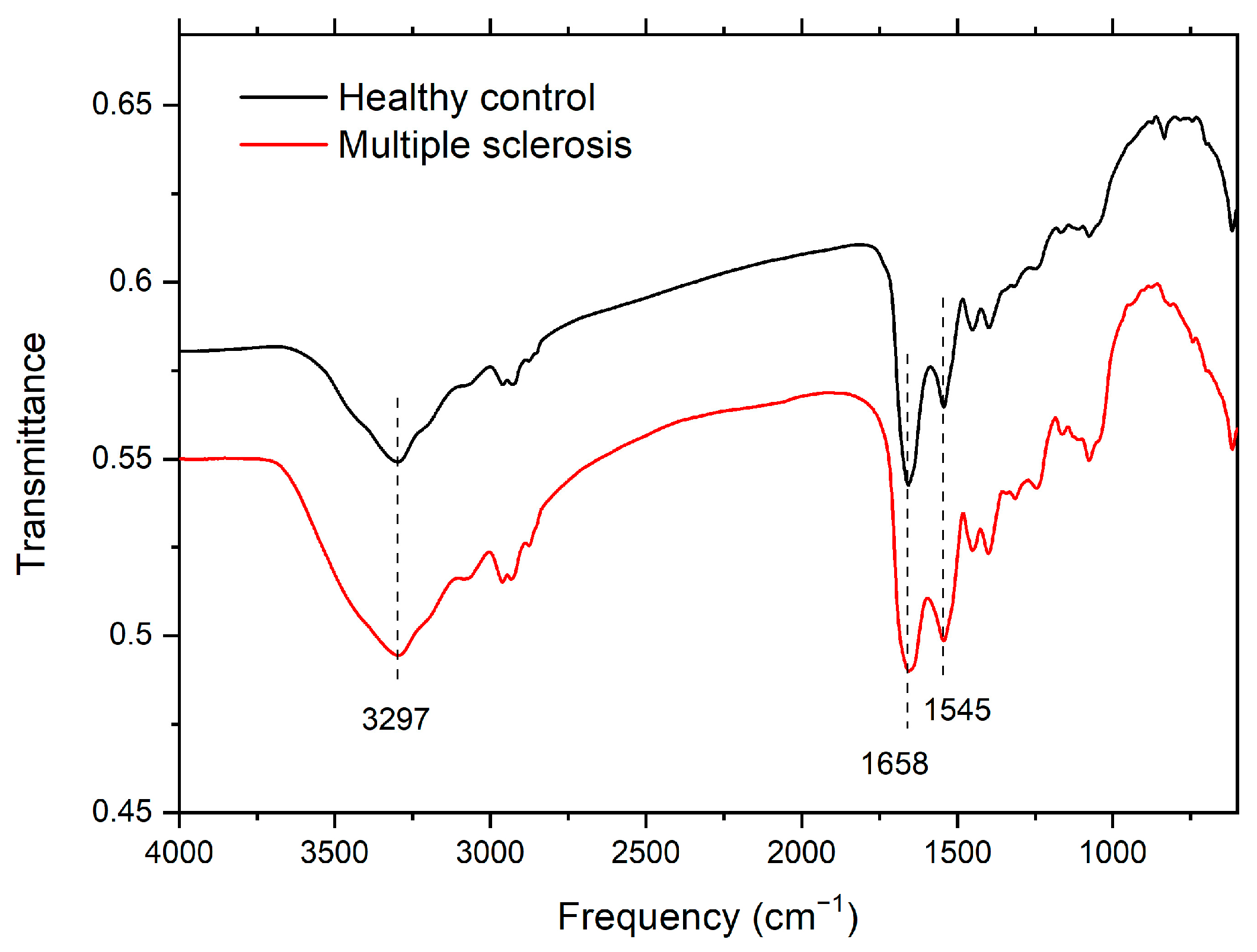
3.1. Infrared Spectroscopy
3.2. Drop-Coating Deposition Raman (DCDR) Spectroscopy
3.3. Atomic-Force Microscopy
3.4. HPLC Mass Spectrometry—Bottom Up Proteomic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J.; MSCOI Study Group; European Multiple Sclerosis Platform. New insights into the burden and costs of multiple sclerosis in Europe. Mult. Scler. J. 2017, 23, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Farias, A.S.; Santos, L.M. How can proteomics elucidate the complexity of multiple sclerosis? Proteom. Clin. Appl. 2015, 9, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Salvisberg, C.; Tajouri, N.; Hainard, A.; Burkhard, P.R.; Lalive, P.H.; Turck, N. Exploring the human tear fluid: Discovery of new biomarkers in multiple sclerosis. Proteom. Clin. Appl. 2014, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Babajani, A.; Rezaei-Tavirani, M. Multiple Sclerosis Biomarker Discoveries by Proteomics and Metabolomics Approaches. Biomark. Insights 2021, 16, 11772719211013352. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Tseng, M.Y.; Ro, L.S.; Lyu, R.K.; Tai, Y.H.; Chang, H.S.; Wu, Y.R.; Huang, C.C.; Hsu, W.C.; Kuo, H.C.; et al. Analyses of haptoglobin level in the cerebrospinal fluid and serum of patients with neuromyelitis optica and multiple sclerosis. Clin. Chim. Acta 2013, 417, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Zhang, G.X.; Zhang, W.T.; Yu, W.J.; Du, L.; Toledo, M.C.; Olivera Leal, I.R.; O’Farrill, Z.L.; Izquierdo, G. Ocular manifestations of multiple sclerosis in patients from three countries: A Web-based survey. Eur. J. Ophthal. 2022, 32, 2975–2981. [Google Scholar] [CrossRef]
- Muñoz-Ortiz, J.; Reyes-Guanes, J.; Zapata-Bravo, E.; Mora-Muñoz, L.; Reyes-Hurtado, J.A.; Tierradentro-García, L.O.; Rojas-Carabali, W.; Gómez-Suarez, M.; de-la-Torre, A. Ocular adverse events from pharmacological treatment in patients with multiple sclerosis—A systematic review of the literature. Syst. Rev. 2021, 10, 280. [Google Scholar] [CrossRef]
- Szucs, G.; Szekanecz, Z.; Aszalos, Z.; Gesztelyi, R.; Zsuga, J.; Szodoray, P.; Kemeny-Beke, A. A Wide Spectrum of Ocular Manifestations Signify Patients with Systemic Sclerosis. Ocul. Immunol. Inflamm. 2021, 29, 81–89. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Chou, Y.; Ma, J.; Zhong, Y. Significant retinal microvascular impairments in multiple sclerosis assessed through optical coherence tomography angiography. Mult. Scler. Relat. Disord. 2023, 70, 104505. [Google Scholar] [CrossRef]
- Segars, K.L.; Azzari, N.A.; Gomez, S.; Machen, C.; Rich, C.B.; Trinkaus-Randall, V. Age Dependent Changes in Corneal Epithelial Cell Signaling. Front. Cell Dev. Biol. 2022, 10, 886721. [Google Scholar] [CrossRef]
- Tursic, A.; Vaessen, M.; Zhan, M.; Vingerhoets, A.J.J.M.; de Gelder, B. The power of tears: Observers’ brain responses show that tears provide unambiguous signals independent of scene context. Neuroimage Rep. 2022, 2, 100105. [Google Scholar] [CrossRef]
- Jesuthasan, A.; Ali, A.; Lee, J.K.W.; Rutherfurd-Markwick, K. Assessment of Changes in Physiological Markers in Different Body Fluids at Rest and after Exercise. Nutrients 2022, 14, 4685. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Stern, M.E. Biological functions of tear film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef]
- Singh, S.; Srivastav, S.; Mohamed, A.; Basu, S. Non-invasive Tear Film Assessment in Normal Population: Effect of Age, Sex, and Interparametric Relationship. Front. Med. 2022, 9, 894184. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W. The power of tears: How tear proteomics research could revolutionize the clinic. Expert Rev. Proteom. 2017, 14, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Masoud, S. Biochemistry of human tear film: A review. Exp. Eye Res. 2022, 220, 109101. [Google Scholar] [CrossRef] [PubMed]
- Farias, A.S.; Pradella, F.; Schmitt, A.; Santos, L.M.; Martins-de-Souza, D. Ten years of proteomics in multiple sclerosis. Proteomics 2014, 14, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Almuslehi, M.S.M.; Shortland, P.J.; Mahns, D.A.; Coorssen, J.R. Proteomics of Multiple Sclerosis: Inherent Issues in Defining the Pathoetiology and Identifying (Early) Biomarkers. Int. J. Mol. Sci. 2021, 22, 7377. [Google Scholar] [CrossRef] [PubMed]
- Król-Grzymała, A.; Sienkiewicz-Szłapka, E.; Fiedorowicz, E.; Rozmus, D.; Cieślińska, A.; Grzybowski, A. Tear Biomarkers in Alzheimer’s and Parkinson’s Diseases, and Multiple Sclerosis: Implications for Diagnosis (Systematic Review). Int. J. Mol. Sci. 2022, 23, 10123. [Google Scholar] [CrossRef]
- Liland, K.H.; Almøy, T.; Mevik, B.H. Optimal choice of baseline correction for multivariate calibration of spectra. Appl. Spectrosc. 2010, 64, 1007–1016. [Google Scholar] [CrossRef]
- Sang, S.; Zhao, Y.; Zhang, W.; Li, P.; Hu, J.; Li, G. Surface stress-based biosensors. Biosens. Bioelectron. 2014, 51, 124–135. [Google Scholar] [CrossRef]
- Gadegaard, N. Atomic force microscopy in biology: Technology and techniques. Biotech. Histochem. 2006, 81, 87–97. [Google Scholar] [CrossRef]
- Glinská, G.; Krajčíková, K.; Zakuťanská, K.; Shylenko, O.; Kondrakhova, D.M.; Tomašovičová, N.; Komanický, V.; Mašlanková, J.; Tomečková, V. Noninvasive diagnostic methods for diabetes mellitus from tear fluid. RSC Adv. 2019, 9, 18050–18059. [Google Scholar] [CrossRef] [PubMed]
- Fabian, H.; Mäntele, W. Infrared Spectroscopy of Proteins. In Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Wen, Z.Q. Raman spectroscopy of protein pharmaceuticals. J. Pharm. Sci. 2007, 96, 2861–2878. [Google Scholar] [CrossRef] [PubMed]
- Niaura, G. Raman Spectroscopy in Analysis of Biomolecules. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2008; pp. 1–34. [Google Scholar]
- Kocherbitov, V.; Latynis, J.; Misiunas, A.; Barauskas, J.; Niaura, G. Hydration of lysozyme studied by Raman spectroscopy. J. Phys. Chem. B 2013, 117, 4981–4992. [Google Scholar] [CrossRef] [PubMed]
- Frontzek, K.; Lutz, M.I.; Aguzzi, A.; Kovacs, G.G.; Budka, H. Amyloid-β pathology and cerebral amyloid angiopathy are frequent in iatrogenic Creutzfeldt-Jakob disease after dural grafting. Swiss Med. Wkly. 2016, 146, w14287. [Google Scholar] [CrossRef]
- Di Foggia, M.; Boga, C.; Micheletti, G.; Nocentini, B.; Taddei, P. Vibrational Raman and IR data on brown hair subjected to bleaching. Data Brief 2021, 38, 107439. [Google Scholar] [CrossRef]
- Kijlstra, A.; Kuizenga, A. Analysis and function of the human tear proteins. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes; Springer: Boston, MA, USA, 1994; pp. 299–308. [Google Scholar]
- O’Sullivan, N.L.; Montgomery, P.C. Ocular Mucosal Immunity. In Mucosal Immunology; Academic Press: Cambridge, MA, USA, 2015; pp. 1873–1897. [Google Scholar]
- Willcox, M.D.; Lan, J. Secretory immunoglobulin A in tears: Functions and changes during contact lens wear. Clin. Exp. Optom. 1999, 82, 1–3. [Google Scholar] [CrossRef]
- Hümmert, M.W.; Wurster, U.; Bönig, L.; Schwenkenbecher, P.; Sühs, K.W.; Alvermann, S.; Gingele, S.; Skripuletz, T.; Stangel, M. Investigation of Oligoclonal IgG Bands in Tear Fluid of Multiple Sclerosis Patients. Front. Immunol. 2019, 10, 1110. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef] [PubMed]
- McHeyzer-Williams, M.; Okitsu, S.; Wang, N.; McHeyzer-Williams, L. Molecular programming of B cell memory. Nat. Rev. Immunol. 2011, 12, 24–34. [Google Scholar] [CrossRef]
- Teng, G.; Papavasiliou, F.N. Immunoglobulin somatic hypermutation. Annu. Rev. Genet. 2007, 41, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Rüge, A.; Putscher, E.; Boxberger, N.; Rommer, P.S.; Fitzner, B.; Zettl, U.K. Aberrant expression of alternative splicing variants in multiple sclerosis—A systematic review. Autoimmun. Rev. 2019, 18, 721–732. [Google Scholar] [CrossRef]
- Putscher, E.; Hecker, M.; Fitzner, B.; Boxberger, N.; Schwartz, M.; Koczan, D.; Lorenz, P.; Zettl, U.K. Genetic risk variants for multiple sclerosis are linked to differences in alternative pre-mRNA splicing. Front. Immunol. 2022, 13, 931831. [Google Scholar] [CrossRef] [PubMed]
- Krajčíková, K.; Mašlanková, J.; Shylenko, O.; Zakuťanská, K.; Glinská, G.; Tomečková, V.; Komanický, V.; Tomašovičová, N. Novel strategies in diagnostics of eye diseases from tear fluid. Quaere 2018, 8, 577–586. [Google Scholar]
- Krajčíková, K.; Semančíková, E.; Zakuťanská, K.; Kondrakhova, D.; Mašlanková, J.; Stupák, M.; Talian, I.; Tomašovičová, N.; Kimáková, T.; Komanický, V.; et al. Tear fluid biomarkers in major depressive disorder: Potential of spectral methods in biomarker discovery. J. Psychiatr. Res. 2021, 138, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kori, M.; Aydin, B.; Unal, S.; Arga, K.Y.; Kazan, D. Metabolic biomarkers and neurodegeneration: A pathway enrichment analysis of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. OMICS J. Integr. Biol. 2016, 20, 645–661. [Google Scholar] [CrossRef]
- Cocco, E.; Murgia, F.; Lorefice, L.; Barberini, L.; Poddighe, S.; Frau, J.; Fenu, G.; Coghe, G.; Murru, M.R.; Murru, R.; et al. 1H-NMR analysis provides a metabolomic profile of patients with multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e185. [Google Scholar] [CrossRef]
- Aeinehband, S.; Brenner, P.; Ståhl, S.; Bhat, M.; Fidock, M.D.; Khademi, M.; Olsson, T.; Engberg, G.; Jokinen, J.; Erhardt, S.; et al. Cerebrospinal fluid kynurenines in multiple sclerosis; relation to disease course and neurocognitive symptoms. Brain Behav. Immun. 2016, 51, 47–55. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; Platero, J.L.; Benlloch, M.; Franco-Martinez, L.; Tvarijonaviciute, A.; Escribá-Alepuz, J.; Sancho-Castillo, S. Role of Haptoglobin as a Marker of Muscular Improvement in Patients with Multiple Sclerosis after Administration of Epigallocatechin Gallate and Increase of Beta-Hydroxybutyrate in the Blood: A Pilot Study. Biomolecules 2021, 11, 617. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Bao, T.; Jia, F.; Ou, R.; Wei, Q.; Chen, Y.; Liu, J.; Yang, J.; Shang, H. Changes in Serum Cystatin C Levels and the Associations with Cognitive Function in Alzheimer’s Disease Patients. Front. Aging Neurosci. 2022, 13, 790939. [Google Scholar] [CrossRef] [PubMed]
- De Bondt, M.; Hellings, N.; Opdenakker, G.; Struyf, S. Neutrophils: Underestimated Players in the Pathogenesis of Multiple Sclerosis (MS). Int. J. Mol. Sci. 2020, 21, 4558. [Google Scholar] [CrossRef] [PubMed]
- Filep János, G. Targeting Neutrophils for Promoting the Resolution of Inflammation. Front. Immunol. 2022, 13, 866747. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Arnhold, J. Heme Peroxidases at Unperturbed and Inflamed Mucous Surfaces. Antioxidants 2021, 10, 1805. [Google Scholar] [CrossRef]
- Yamakaze, J.; Lu, Z. Deletion of the lactoperoxidase gene causes multisystem inflammation and tumors in mice. Sci. Rep. 2021, 11, 12429. [Google Scholar] [CrossRef]
- Pegoretti, V.; Swanson, K.A.; Bethea, J.R.; Probert, L.; Eisel, U.L.M.; Fischer, R. Inflammation and Oxidative Stress in Multiple Sclerosis: Consequences for Therapy Development. Oxid. Med. Cell. Longev. 2020, 12, 7191080. [Google Scholar] [CrossRef]
- Michaličková, D.; Šíma, M.; Slanař, O. New insights in the mechanisms of impaired redox signaling and its interplay with inflammation and immunity in multiple sclerosis. Physiol. Res. 2020, 69, 1–19. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Du, C.; Sang, L.; Liu, L.; Li, Y.; Wang, F.; Fan, W.; Tang, P.; Zhang, S.; et al. Evidence of pyroptosis and ferroptosis extensively involved in autoimmune diseases at the single-cell transcriptome level. J. Transl. Med. 2022, 20, 363. [Google Scholar] [CrossRef]
- Verdelli, A.; Caproni, M.; Coi, A.; Corrà, A.; Degl’Innocenti, D.; Vasarri, M.; Quintarelli, L.; Volpi, V.; Cipollini, E.M.; Barletta, E. Neutrophil Gelatinase-Associated Lipocalin as Potential Predictive Biomarker of Melanoma and Non-Melanoma Skin Cancers in Psoriatic Patients: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 12291. [Google Scholar] [CrossRef]
- Schreiber, A.; Rousselle, A.; Klocke, J.; Bachmann, S.; Popovic, S.; Bontscho, J.; Schmidt-Ott, K.M.; Siffrin, V.; Jerke, U.; Ashraf, M.I.; et al. Neutrophil Gelatinase-Associated Lipocalin Protects from ANCA-Induced GN by Inhibiting TH17 Immunity. J. Am. Soc. Nephrol. 2020, 31, 1569–1584. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, Y.; Midorikawa, Y.; Furukawa, T.; Shimoyama, S.; Takekawa, D.; Kitayama, M.; Ueno, S.; Kushikata, T.; Hirota, K. The role of neutrophil gelatinase-associated lipocalin and iron homeostasis in object recognition impairment in aged sepsis-survivor rats. Sci. Rep. 2022, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Hobbs, R.P.; Coulombe, P.A. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell Biol. 2013, 25, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Trotter, A.; Anstadt, E.; Clark, R.B.; Nichols, F.; Dwivedi, A.; Aung, K.; Cervantes, J.L. The role of phospholipase A2 in Multiple Sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2019, 27, 206–213. [Google Scholar] [CrossRef]
- Momchilova, A.; Pankov, R.; Alexandrov, A.; Markovska, T.; Pankov, S.; Krastev, P.; Staneva, G.; Vassileva, E.; Krastev, N.; Pinkas, A. Sphingolipid Catabolism and Glycerophospholipid Levels Are Altered in Erythrocytes and Plasma from Multiple Sclerosis Patients. Int. J. Mol. Sci. 2022, 23, 7592. [Google Scholar] [CrossRef]
- Pineda-Torra, I.; Siddique, S.; Waddington, K.E.; Farrell, R.; Jury, E.C. Disrupted Lipid Metabolism in Multiple Sclerosis: A Role for Liver X Receptors? Front. Endocrinol. 2021, 12, 639757. [Google Scholar] [CrossRef]
- Garvin, R.A. Elevated Phospholipid Transfer Protein in Subjects with Multiple Sclerosis. J. Lipids 2015, 2015, 518654. [Google Scholar] [CrossRef]
- Ventura, R.; Martínez-Ruiz, I.; Hernández-Alvarez, M.I. Phospholipid Membrane Transport and Associated Diseases. Biomedicines 2022, 10, 1201. [Google Scholar] [CrossRef]
- Glasgow, B.J.; Abduragimov, A.R. Lipocalin-1 is the acceptor protein for phospholipid transfer protein in tears. Biochem. Biophys. Res. Commun. 2021, 548, 35–38. [Google Scholar] [CrossRef]
- van Leent, M.M.T.; Beldman, T.J.; Toner, Y.C.; Lameijer, M.A.; Rother, N.; Bekkering, S.; Teunissen, A.J.P.; Zhou, X.; van der Meel, R.; Malkus, J.; et al. Prosaposin mediates inflammation in atherosclerosis. Sci. Transl. Med. 2021, 13, eabe1433. [Google Scholar] [CrossRef]
- Sandin, S.I.; de Alba, E. Quantitative Studies on the Interaction between Saposin-like Proteins and Synthetic Lipid Membranes. Methods Protoc. 2022, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Witte, D.P.; Ran, H.; Zamzow, M.; Barnes, S.; Cheng, H.; Han, X.; Williams, M.T.; Skelton, M.R.; Vorhees, C.V.; et al. Neurological deficits and glycosphingolipid accumulation in saposin B deficient mice. Hum. Mol. Genet. 2008, 17, 2345–2356. [Google Scholar] [CrossRef]
- Kaiser, R.; Escaig, R.; Erber, J.; Nicolai, L. Neutrophil-Platelet Interactions as Novel Treatment Targets in Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 8, 824112. [Google Scholar] [CrossRef] [PubMed]
- Darmoise, A.; Maschmeyer, P.; Winau, F. The Immunological Functions of Saposins. Adv. Immunol. 2010, 105, 25–62. [Google Scholar] [PubMed]
- Nally, F.K.; De Santi, C.; McCoy, C.E. Nanomodulation of Macrophages in Multiple Sclerosis. Cells 2019, 8, 543. [Google Scholar] [CrossRef]
- Prineas, J.W.; Parratt, J.D.E. Multiple Sclerosis: Microglia, Monocytes, and Macrophage-Mediated Demyelination. J. Neuropathol. Exp. Neurol. 2021, 80, 975–996. [Google Scholar] [CrossRef]
- Perga, S.; Martire, S.; Montarolo, F.; Navone, N.D.; Calvo, A.; Fuda, G.; Marchet, A.; Leotta, D.; Chiò, A.; Bertolotto, A. A20 in Multiple Sclerosis and Parkinson’s Disease: Clue to a Common Dysregulation of Anti-Inflammatory Pathways? Neurotox. Res. 2017, 32, 1–7. [Google Scholar] [CrossRef]
- Pitchford, S.; Pan, D.; Welch, H.C. Platelets in neutrophil recruitment to sites of inflammation. Curr. Opin. Hematol. 2017, 24, 23–31. [Google Scholar] [CrossRef]
- Najafi, M.R.; Shaygannajad, V.; Mirpourian, M.; Gholamrezaei, A. Vitamin B12 deficiency and multiple sclerosis; Is there any association? Int. J. Prev. Med. 2012, 4, 286–289. [Google Scholar]
- Sandi, D.; Kokas, Z.; Biernacki, T.; Bencsik, K.; Klivényi, P.; Vécsei, L. Proteomics in Multiple Sclerosis: The Perspective of the Clinician. Int. J. Mol. Sci. 2022, 23, 5162. [Google Scholar] [CrossRef] [PubMed]



| Average | CTRL | MS |
|---|---|---|
| Patients | 10 | 20 |
| Females/Males | 10/0 | 18/2 |
| Age (years) | 26.8 ± 3.02 | 33.3 ± 5.3 |
| Retinal nerve fiber layer (μm) | - | 101.4 ± 15.8 |
| Ganglion cell complex (μm) | - | 90.5 ± 11.9 |
| Medication | 0 | 20 |
| No medication | 10 | - |
| REBIF (interferon β-1a) | - | 10 |
| AVONEX (interferon β-1a) | - | 7 |
| TECFIDERA (dimethyl fumarate) | - | 1 |
| OCREVUS (ocrelizumab) | - | 2 |
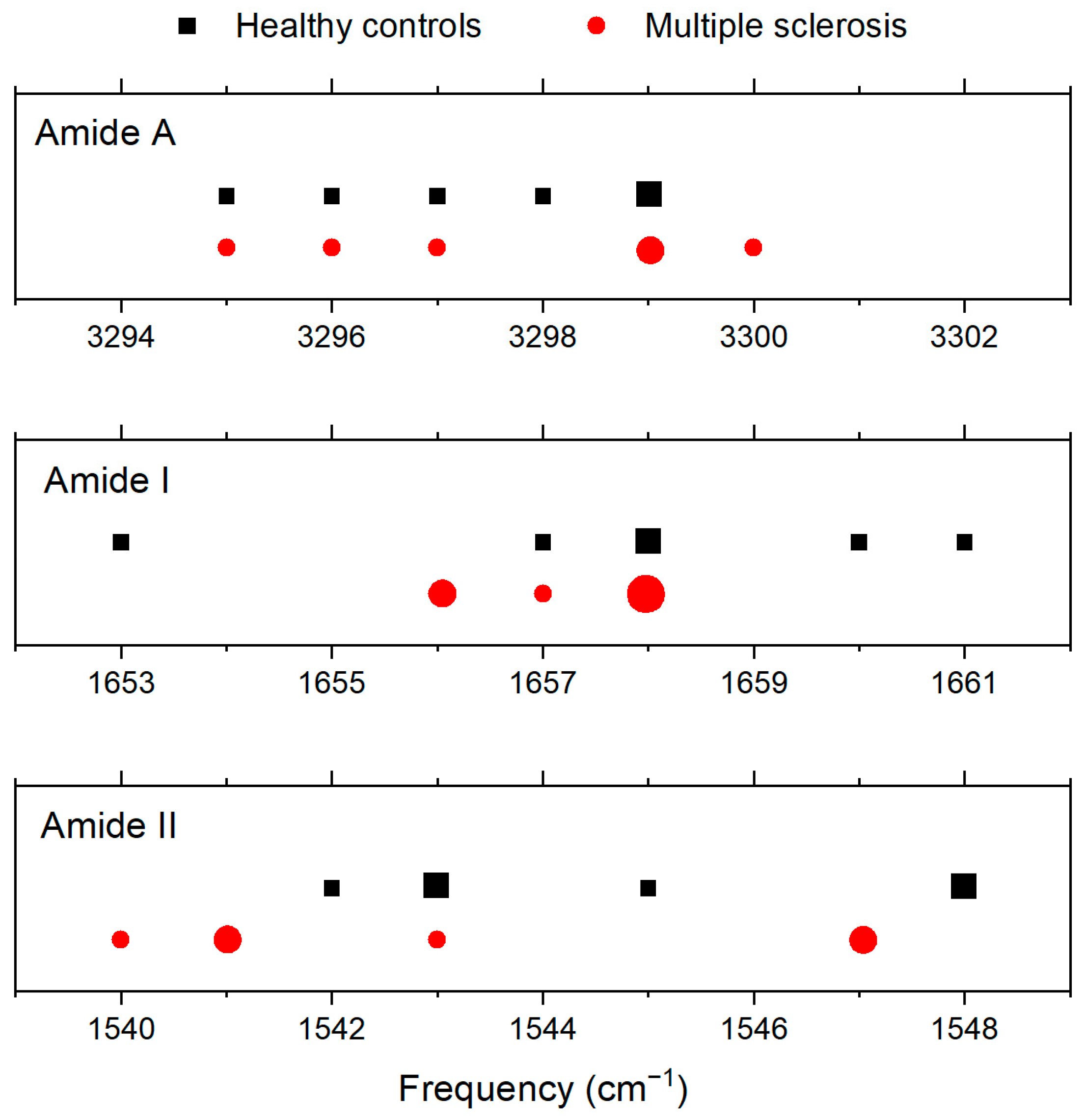
| Amide A | Amide I | Amide II | |
|---|---|---|---|
| Frequency (cm−1) | Frequency (cm−1) | Frequency (cm−1) | |
| CTRL | 3299 | 1658 | 1543 |
| 3295 | 1653 | 1543 | |
| 3298 | 1657 | 1542 | |
| 3297 | 1658 | 1545 | |
| 3296 | 1660 | 1548 | |
| 3295 | 1661 | 1548 | |
| Average | 3297 | 1658 | 1545 |
| MS | 3295 | 1658 | 1547 |
| 3295 | 1658 | 1547 | |
| 3300 | 1658 | 1541 | |
| 3297 | 1656 | 1543 | |
| 3296 | 1656 | 1541 | |
| 3299 | 1657 | 1540 | |
| Average | 3297 | 1657 | 1543 |
| Peak Position (cm−1) | Assignment | Ref. |
|---|---|---|
| 1666 | Amid I: stretching C=O | [25,28,29] |
| 1616 | Trp, Phe, Tyr: n8a, ring stretching | [25,29,30] |
| 1554 | Trp: stretching C2=C3, W3 mode | [25,29] |
| 1448 | bending CH2 | [25,28] |
| 1358 | Trp: Fermi resonance between in-plane N−C stretching and combination bands of ring out-of-plane deformations, W7 mode | [27,29] |
| 1336 | Trp, C−Cα−H bending, Cα−C stretching | [25,29] |
| 1317 sh | aliphatic sidechain stretching vibrations | [25] |
| 1263 sh | amid III (α) | [25,29] |
| 1242 | amid III (β) | [25,29] |
| 1206 | Tyr: n7a, C−CH2, Phe | [28,29] |
| 1172 | Tyr: n9a, CH in-plane bending | [29,30] |
| 1100/1125 | stretching C−N | [25] |
| 1030 | Phe | [25] |
| 1002 | Phe | [25] |
| 954 | Trp, Val | [25] |
| 935 | stretching N−Cα−C (α) | [25,28,29] |
| 877 | Trp: benzene ring breathing and deformation N−H, W17 mode | [28] |
| 853/829 | Tyr doublet: Fermi resonance between ring breathing mode and overtone of out-of-plane ring bending mode | [25,28,29] |
| 757 | Trp: W18 mode | [25,29] |
| 641 | Tyr: n6b, ring deformation | [25,30] |
| 620 | Phe: in-plane ring deformation | [28] |
| 539 | S-S stretching (trans-gauche-trans) | [25] |
| 520 | S-S stretching (gauche-gauche-trans) | [25,29] |
| 505 | S-S stretching (gauche-gauche-gauche) | [25,29] |
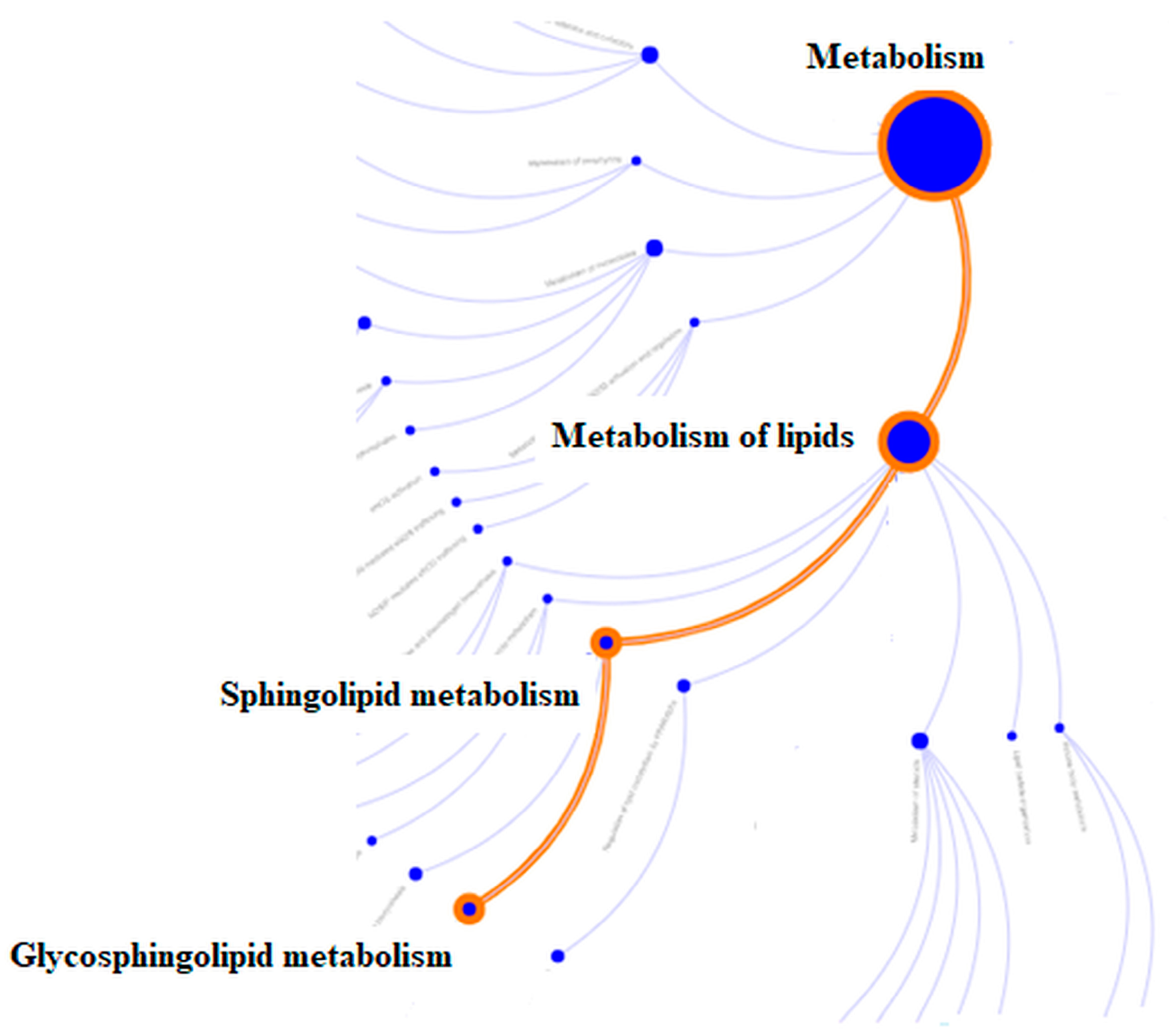
| Identifier | Regulation | FDR | Av.FC | N Genes |
|---|---|---|---|---|
| R-HSA-1660662 Glycosphingolipid metabolism | down | 0.033 | −1.662 | 1 |
| R-HSA-428157 Sphingolipid metabolism | down | 0.033 | −1.662 | 1 |
| R-HSA-556833 Lipid metabolism | down | 0.044 | −1.086 | 3 |
| Identifier | logFC | p.Value | adj.P.Val |
|---|---|---|---|
| PIP_ HUMAN | −0.5873613 | 0.0000270 | 0.0014060 |
| SAP_HUMAN | −1.6620236 | 0.0016957 | 0.0440891 |
| PIGR_HUMAN | −0.3813822 | 0.0045466 | 0.0788082 |
| HPT_HUMAN | −1.8960652 | 0.0067941 | 0.0883235 |
| PRP17_HUMAN | −1.0899125 | 0.0132627 | 0.1379316 |
| K2C1B_HUMAN | −0.6535084 | 0.0281095 | 0.1995068 |
| K1C13_HUMAN | −1.1428253 | 0.0276666 | 0.1995068 |
| NGAL_HUMAN | −0.9558929 | 0.0383667 | 0.1995068 |
| PA2GA_HUMAN | −0.8169733 | 0.0380019 | 0.1995068 |
| CLUS_HUMAN | −0.4132970 | 0.0503125 | 0.2378411 |
| Identifier | logFC | p.Value | adj.P.Val |
|---|---|---|---|
| TCO1_HUMAN | 0.6126178 | 0.0373045 | 0.1995068 |
| CYTC_HUMAN | 1.0627939 | 0.0658102 | 0.2444378 |
| PLTP_HUMAN | 0.7180146 | 0.0641231 | 0.2444378 |
| PERL_HUMAN | 0.5038272 | 0.0763513 | 0.2646845 |
| FSP1_HUMAN | 0.4001445 | 0.2080562 | 0.4626503 |
| LV147_HUMAN | 0.5034786 | 0.3074830 | 0.4996598 |
| ZA2G_HUMAN | 0.1295051 | 0.5520604 | 0.7176785 |
| VP35L_HUMAN | 0.2350438 | 0.6123376 | 0.7614320 |
| IGKC_HUMAN | 0.0340178 | 0.8323604 | 0.9017238 |
| B2MG_HUMAN | 0.0037347 | 0.9874597 | 0.9874597 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomečková, V.; Tkáčiková, S.; Talian, I.; Fabriciová, G.; Hovan, A.; Kondrakhova, D.; Zakutanská, K.; Skirková, M.; Komanický, V.; Tomašovičová, N. Experimental Analysis of Tear Fluid and Its Processing for the Diagnosis of Multiple Sclerosis. Sensors 2023, 23, 5251. https://doi.org/10.3390/s23115251
Tomečková V, Tkáčiková S, Talian I, Fabriciová G, Hovan A, Kondrakhova D, Zakutanská K, Skirková M, Komanický V, Tomašovičová N. Experimental Analysis of Tear Fluid and Its Processing for the Diagnosis of Multiple Sclerosis. Sensors. 2023; 23(11):5251. https://doi.org/10.3390/s23115251
Chicago/Turabian StyleTomečková, Vladimíra, Soňa Tkáčiková, Ivan Talian, Gabriela Fabriciová, Andrej Hovan, Daria Kondrakhova, Katarína Zakutanská, Miriama Skirková, Vladimír Komanický, and Natália Tomašovičová. 2023. "Experimental Analysis of Tear Fluid and Its Processing for the Diagnosis of Multiple Sclerosis" Sensors 23, no. 11: 5251. https://doi.org/10.3390/s23115251
APA StyleTomečková, V., Tkáčiková, S., Talian, I., Fabriciová, G., Hovan, A., Kondrakhova, D., Zakutanská, K., Skirková, M., Komanický, V., & Tomašovičová, N. (2023). Experimental Analysis of Tear Fluid and Its Processing for the Diagnosis of Multiple Sclerosis. Sensors, 23(11), 5251. https://doi.org/10.3390/s23115251






