A New Small-Size Camera with Built-In Specific-Wavelength LED Lighting for Evaluating Chlorophyll Status of Fruit Trees
Abstract
1. Introduction
2. Materials and Methods
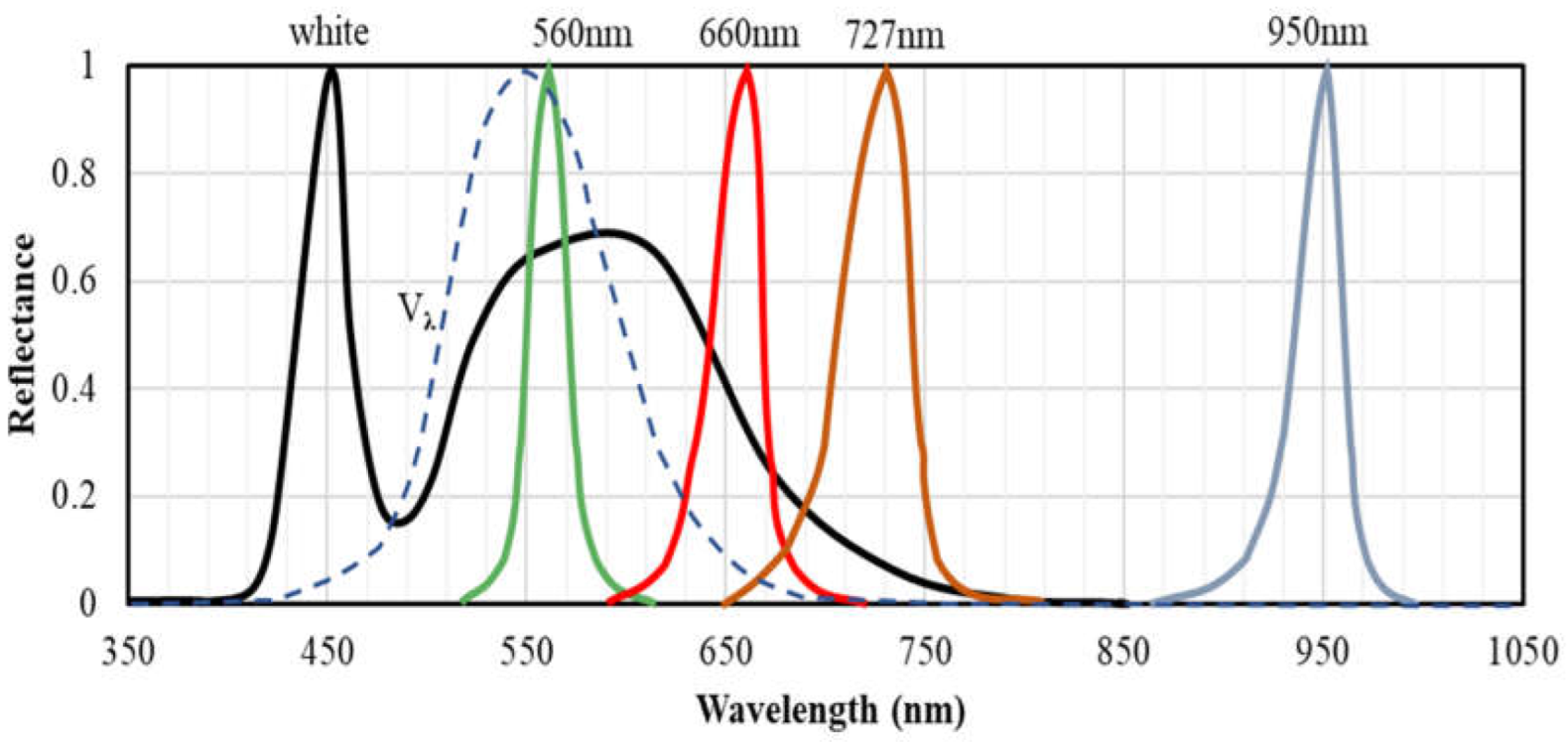
2.1. Selection of Key Wavelengths for Nutrient Status Evaluation
2.1.1. Experiment
2.1.2. Data Analysis
2.2. Design and Development of Camera Prototype and Software
2.3. Testing and Evaluation of Camera Prototype
3. Results and Discussion
3.1. Characteristics of Reflectance Spectra
3.2. Correlation between Spectral Reflectance and Chlorophyll Content
3.3. Chlorophyll Content Prediction Model
3.4. Design and Development of Camera Prototype
3.5. Design and Development of Software for Camera Prototype
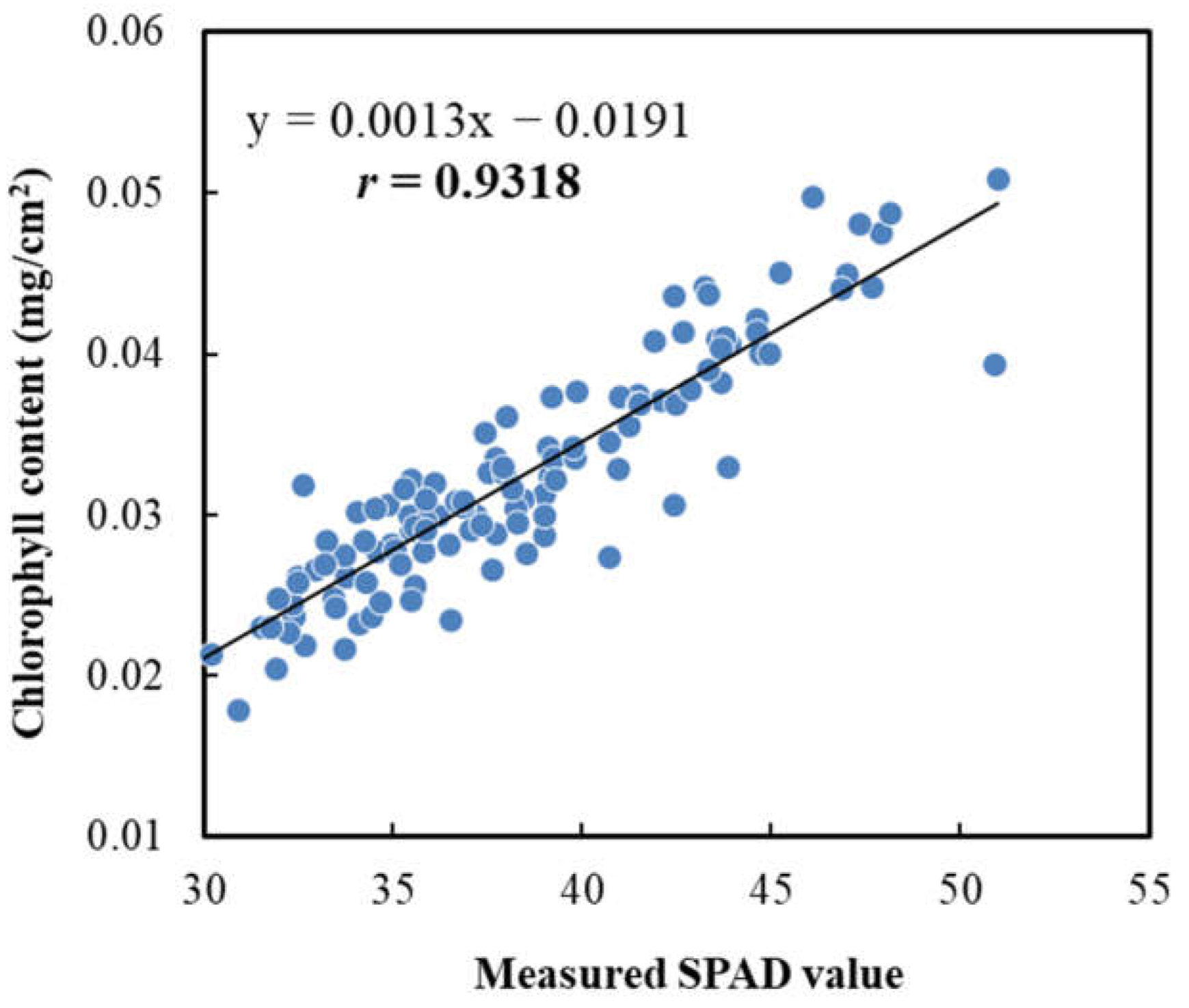
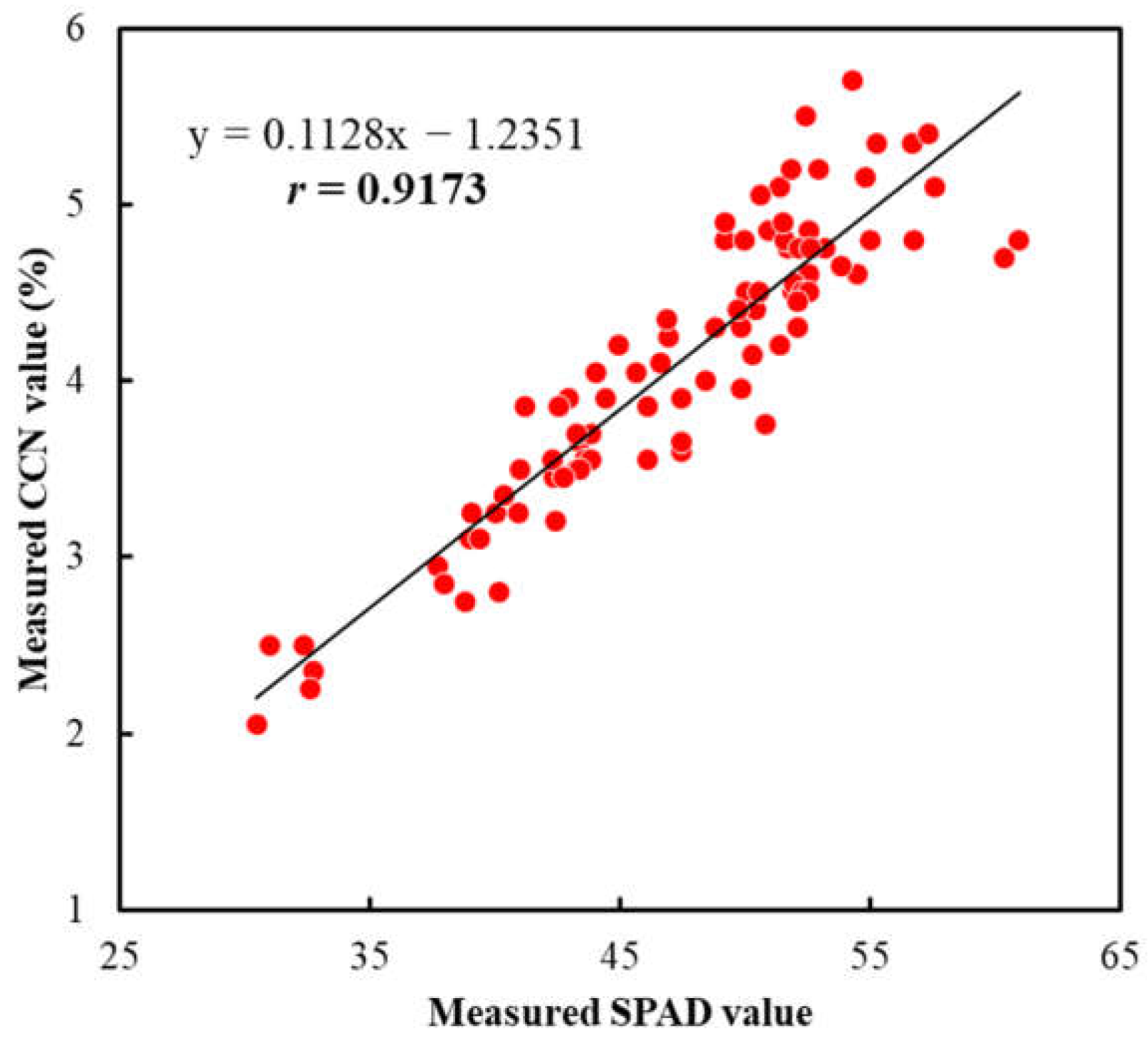
3.6. Relationships between SPAD, CCN Value and Chlorophyll Content
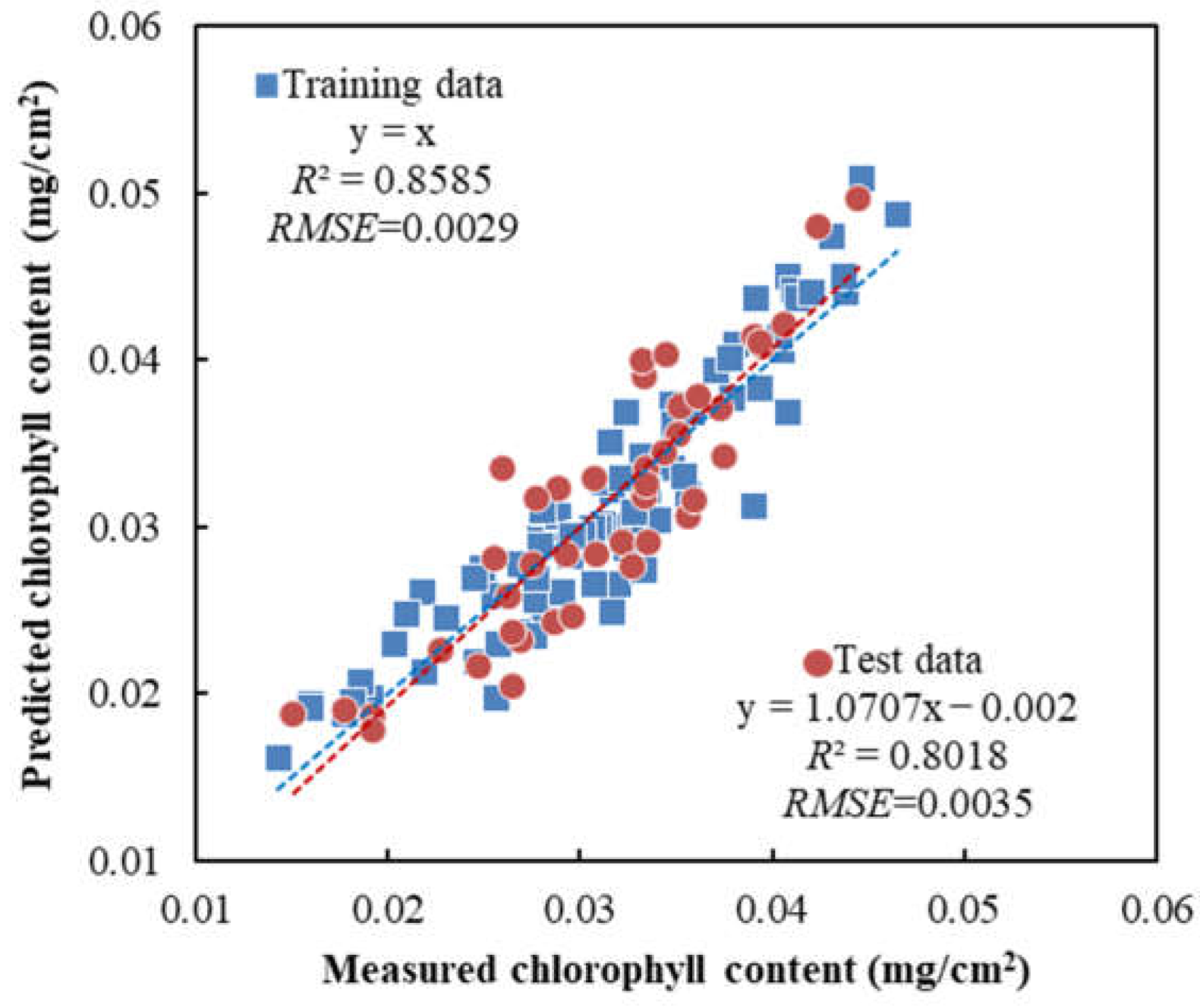
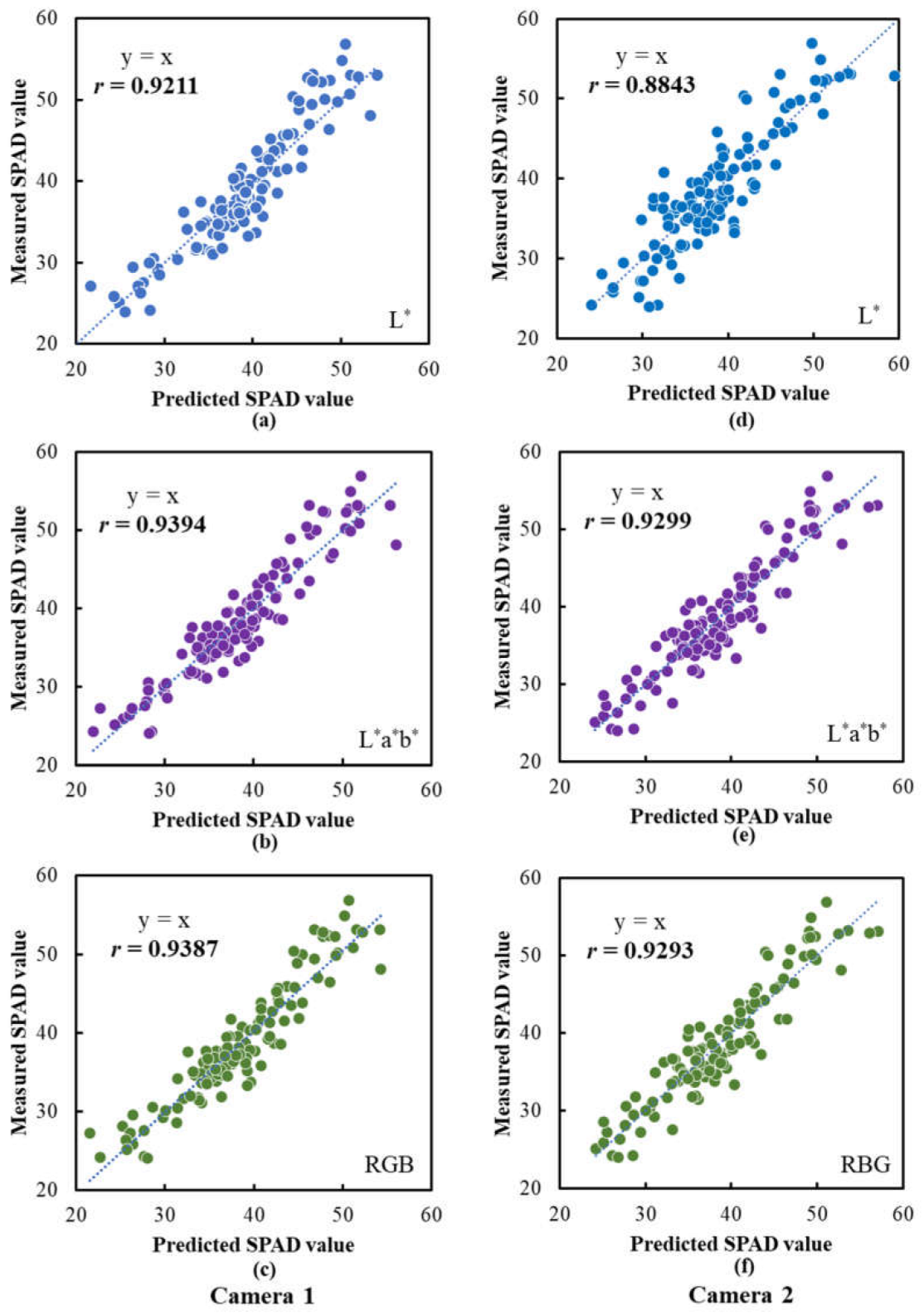
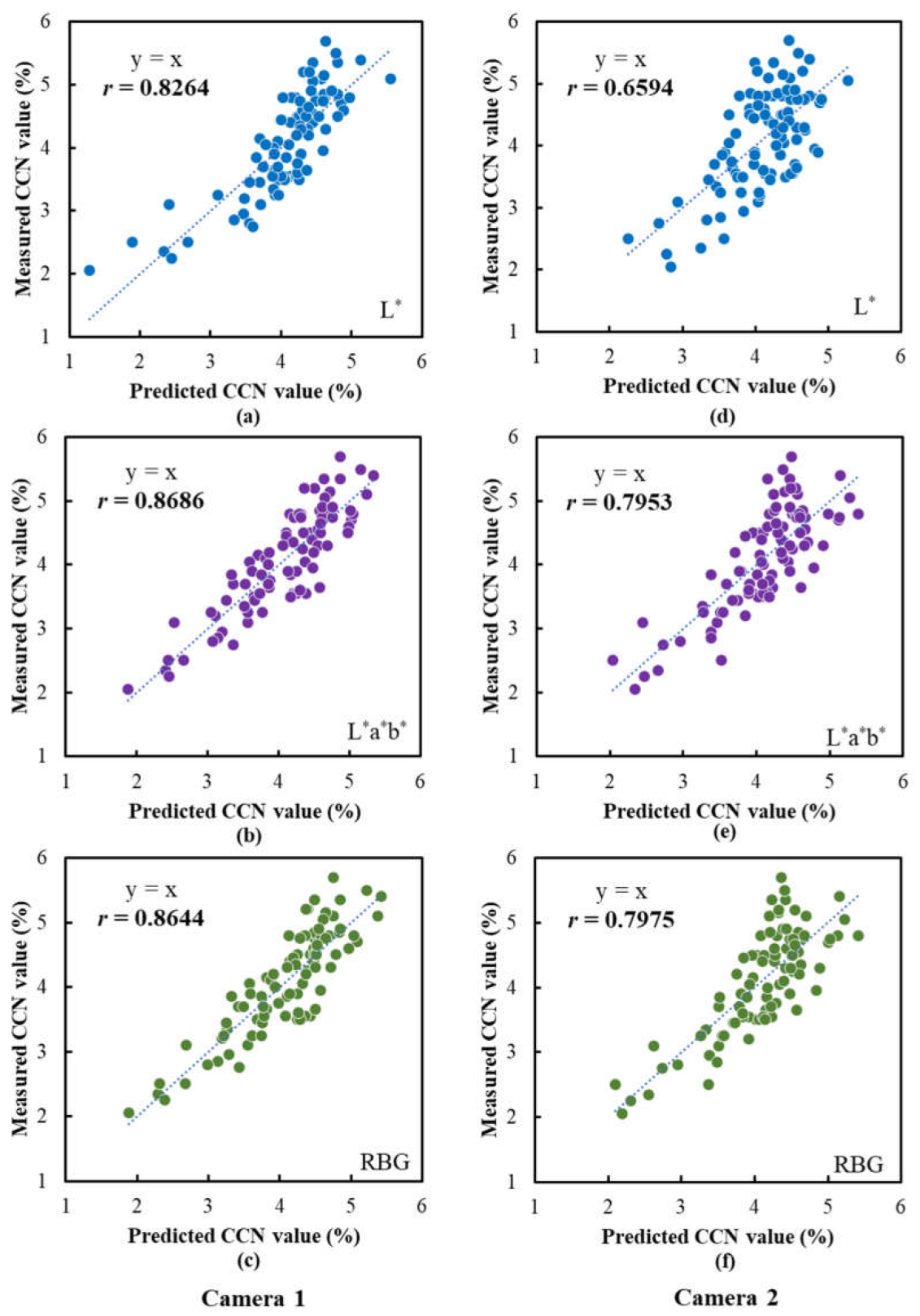
3.7. Performance of SPAD and CCN Prediction Models Based on Images Captured Using Camera Prototype
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aomori Prefecture Apple Countermeasures Council. One Year of Apple Cultivation. Available online: https://www.aomori-ringo.or.jp/kids/cultivation/ (accessed on 12 March 2023).
- Fukuda, H.; Masuda, T. Saving of labor inputs to grow apple fruit. Bull. Natl. Inst. Fruit Tree Sci. 2006, 5, 1–13. [Google Scholar]
- Zaman, Q.U.; Schumann, A.W. Nutrient management zones for citrus based on variation in soil properties and tree performance. Precis. Agric. 2006, 7, 45–63. [Google Scholar] [CrossRef]
- Aggelopoulou, K.; Pateras, D.; Fountas, S.; Gemtos, T.A.; Nanos, G.D. Soil spatial variability and site-specific fertilization maps in an apple orchard. Precis. Agric. 2011, 12, 118–129. [Google Scholar] [CrossRef]
- Aggelopooulou, K.; Castrignanò, A.; Gemtos, T.; Benedetto, D.D. Delineation of management zones in an apple orchard in Greece using a multivariate approach. Comput. Electron. Agric. 2013, 90, 119–130. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, P.; Sharma, M.; Shukla, A.K.; Butail, N.P. Spatial variability of soil nutrients in apple orchards and agricultural areas in Kinnaur region of cold desert, Trans-Himalaya, India. Environ. Monit. Assess. 2022, 194, 290. [Google Scholar] [CrossRef] [PubMed]
- Schumann, A.W. Precise placement and variable rate fertilizer application technologies for horticultural crop. HorTechnolgy 2010, 20, 30–40. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D.; Forge, T.; Hannam, K. Advances in soil and nutrient management in apple cultivation. In Achieving Sustainable Cultivation of Apples; Evans, K., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017. [Google Scholar]
- Takebe, M. Recent development in the plant nutritional diagnosis by nondestructive methods. Jpn. Soc. Soil Sci. Plant Nutr. 2009, 80, 63–65. [Google Scholar]
- Ye, X.; Abe, S.; Zhang, S. Estimation and mapping of nitrogen content in apple trees at leaf and canopy levels using hyperspectral imaging. Precis. Agric. 2020, 21, 198–225. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll content in slash pine. Tree Physiol. 1990, 7, 33–48. [Google Scholar] [CrossRef]
- Filella, I.; Serrano, I.; Serra, J.; Peñuelas, J. Evaluating wheat nitrogen status with canopy reflectance indices and discriminant analysis. Crop Sci. 1995, 35, 1400–1405. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 80, 97–198. [Google Scholar]
- Erel, R.; Dag, A.; Ben-Gal, A.; Schwartz, A.; Yermiyahu, U. Flowering and fruit set of olive trees in response to nitrogen, phosphorus, and potassium. J. Am. Soc. Hort. Sci. 2008, 133, 639–647. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Leonel, S.; Lima, G.P.P.; Leonel, M.; Minatel, I.O.; Souza, J.M.A.; Monteiro, G.C.; Silva, M.S. Contents of nitrogen compounds during bud break and peach tree performance in response to budburst-inducing products. Sci. Hortic. 2022, 305, 111388. [Google Scholar] [CrossRef]
- Xia, G.; Cheng, L.; Lakso, A.; Goffinet, M. Effects of nitrogen supply on source-sink balance and fruit size of ‘Gala’ apple trees. J. Am. Soc. Hort. Sci. 2009, 134, 126–133. [Google Scholar] [CrossRef]
- Albornoz, F. Crop responses to nitrogen overfertilization: A review. Sci. Hortic. 2016, 205, 79–83. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Shea, F.; Watts, C.E. Dumas method for organic nitrogen. Ind. Eng. Chem. Anal. Ed. 1939, 11, 333–334. [Google Scholar] [CrossRef]
- Abulaiti, Y.; Sawut, M.; Maimaitiaili, B.; Ma, C. A possible fractional order derivative and optimized spectral indices for assessing total nitrogen content in cotton. Comput. Electron. Agric. 2020, 171, 105275. [Google Scholar] [CrossRef]
- Kaniszewski, S.; Kowalski, A.; Dysko, J.; Agati, G. Application of a combined transmittance/fluorescence leaf clip sensor for the nondestructive determination of nitrogen status in white cabbage plants. Sensors 2021, 21, 482. [Google Scholar] [CrossRef]
- Bajwa, S.G.; Mishra, A.R.; Norman, R.J. Canopy reflectance response to plant nitrogen accumulation in rice. Precis. Agric. 2010, 11, 488–506. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, X.; Li, J.; Xiao, Y.; He, Y. Estimation and visualization of nitrogen content in citrus canopy based on two band vegetation index (TBVI). Spectrosc. Spectr. Anal. 2015, 35, 715–718. [Google Scholar]
- Li, J.; Ye, X.; Wang, Q.; Zhang, C.; Yong, H. Development of prediction models for determining N content in citrus leaves based on hyperspectral imaging technology. Spectrosc. Spectr. Anal. 2014, 34, 212–216. [Google Scholar]
- Padilla, F.M.; Gallardo, M.; Peña-Fleitas, M.T.; De Souza, R.; Thompson, R.B. Proximal optical sensors for nitrogen management of vegetable crops: A review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.M.; Davenport, J.R. Spectral and spatial differences in response of vegetation indices to nitrogen treatments on apple. Comput. Electron. Agric. 2007, 59, 56–65. [Google Scholar] [CrossRef]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahan, J.; Rundquist, D. Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 47–54. [Google Scholar] [CrossRef]
- Thorp, K.R.; Wang, G.; Bronson, K.F.; Badaruddin, M.; Mon, J. Hyperspectral data mining to identify relevant canopy spectral features for estimating durum wheat growth, nitrogen status, and grain yield. Comput. Electron. Agric. 2017, 136, 1–12. [Google Scholar] [CrossRef]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An investigation into robust spectral indices for leaf chlorophyll estimation. ISPRS J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index—The canopy chlorophyll content index (CCCI). Field Crop. Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Pérez-Patricio, M.; Camas-Anzueto, J.L.; Sanchez-Alegría, A.; Aguilar-González, A.; Gutiérrez-Miceli, F.; Escobar-Gómez, E.; Voisin, Y.; Rios-Rojas, C.; Grajales-Coutiño, R. Optical method for estimating the chlorophyll contents in plant leaves. Sensors 2018, 18, 650. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Quantitative estimation of chlorophyll a using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Yoder, B.J.; Pettigrew-Crosby, R.E. Predicting nitrogen and chlorophyll concentrations from reflectance spectra (400–2500 nm) at leaf and canopy scales. Remote Sens. Environ. 1995, 53, 199–211. [Google Scholar] [CrossRef]
- Buschmann, C.; Nagel, E. In vivo spectroscopy and internal optics of leaves as basis for remote sensing of vegetation. Int. J. Remote Sens. 1993, 14, 711–722. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and non-destructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- van den Berg, A.K.; Perkins, T.D. Evaluation of a portable chlorophyll meter to estimate chlorophyll and nitrogen contents in sugar maple (Acer saccharum Marsh.) leaves. For. Ecol. Manag. 2004, 200, 113–117. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crop. Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Wood, C.W.; Tracy, P.W.; Reeves, D.W.; Edmisten, K.L. Determination of cotton nitrogen status with a hand-held chlorophyll meter. J. Plant Nutr. 1992, 15, 1435–1448. [Google Scholar] [CrossRef]
- Simorte, V.; Bertoni, G.; Dupraz, C.; Masson, P. Assessment of nitrogen nutrition of walnut trees using foliar analysis and chlorophyll measurements. J. Plant Nutr. 2001, 24, 1645–1660. [Google Scholar] [CrossRef]
- Bijay-Singh; Ali, A.M. Using hand-held chlorophyll meters and canopy reflectance sensors for fertilizer nitrogen management in cereals in small farms in developing countries. Sensors 2020, 20, 1127. [Google Scholar] [CrossRef]
- de Souza, R.; Peña-Fleitas, M.T.; Thompson, R.B.; Gallardo, M.; Grasso, R.; Padilla, F.M. The use of chlorophyll meters to assess crop N status and derivation of sufficiency values for sweet pepper. Sensors 2019, 19, 2949. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.; Chandrasekaran, B.; Balasubramanian, T.N.; Bangarusamy, U.; Sivasamy, R.; Sankaran, N. Chlorophyll dynamics in rice (Oryza sativa) before and after flowering based on SPAD (chlorophyll) meter monitoring and its relation with grain yield. J. Agron. Crop Sci. 2002, 188, 102–105. [Google Scholar] [CrossRef]
- Shibayama, M.; Akiyama, T.A. A spectroradiometer for field use VII radiometric estimation of nitrogen levels in field rice canopies. Jpn. J. Crop Sci. 1986, 55, 439–445. [Google Scholar] [CrossRef]
- Ali, A.M.; Thind, H.S.; Sharma, S.; Varinderpal-Singh. Prediction of dry direct seeded rice yields using chlorophyll meter, leaf color chart and GreenSeeker optical sensor in northwestern India. Field Crop. Res. 2014, 161, 11–15. [Google Scholar] [CrossRef]
- Ye, X.; Abe, S.; Zhang, S.; Yoshimura, H. Rapid and non-destructive assessment of nutritional status in apple trees using a new smartphone-based wireless crop scanner system. Comput. Electron. Agric. 2020, 173, 105417. [Google Scholar] [CrossRef]
- Varinderpal-Singh; Bijay-Singh; Yadvinder-Singh; Thind, H.S.; Gobinder-Singh; Satwinderjit-Kaur; Kumar, A.; Vashistha, M. Establishment of threshold leaf colour greenness for need-based fertilizer nitrogen management in irrigated wheat (Triticum aestivum L.) using leaf colour chart. Field Crop. Res. 2012, 130, 109–119. [Google Scholar] [CrossRef]
- Bijay-Singh; Sharma, R.K.; Jaspreet-Kaur; Jat, M.L.; Martin, K.L.; Varinderpal-Singh; Yadvinder-Singh; Chandna, P.; Choudhary, O.P.; Gupta, R.K.; et al. Assessment of the nitrogen management strategy using an optical sensor for irrigated wheat. Agron. Sust. Dev. 2011, 31, 589–603. [Google Scholar] [CrossRef]
- Neilsen, D.; Hogue, E.J.; Neilsen, G.H.; Parchomchuk, P. Using SPAD-502 values to assess the nitrogen status of apple trees. HortScience 1995, 30, 508–512. [Google Scholar] [CrossRef]
- Chapman, S.C.; Barreto, H.J. Using a chlorophyll meter to estimate specific leaf nitrogen of tropical maize during vegetative growth. Agron. J. 1997, 89, 557–562. [Google Scholar] [CrossRef]
- Chang, S.X.; Robison, D.S. Nondestructive and rapid estimation of hardwood foliar nitrogen status using the SPAD-502 chlorophyll meter. For. Ecol. Manag. 2003, 181, 331–338. [Google Scholar] [CrossRef]
- Mackenney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Pearson, K. Notes on regression and inheritance in the case of two parents. Proc. R. Soc. Lond. 1895, 58, 240–242. [Google Scholar]
- Kotera, H. Development of color space. J. Imaging Soc. Jpn. 2004, 43, 73–81. [Google Scholar]
- Song, D.; Qiao, L.; Gao, D.; Li, S.; Li, M.; Sun, H.; Ma, J. Development of crop chlorophyll detector based on a type of interference filter optical sensor. Comput. Electron. Agric. 2021, 187, 106260. [Google Scholar] [CrossRef]
- Brown, L.A.; Williams, O.; Dash, J. Calibration and characterisation of four chlorophyll meters and transmittance spectroscopy for non-destructive estimation of forest leaf chlorophyll concentration. Agric. For. Meteorol. 2022, 323, 109059. [Google Scholar] [CrossRef]
- Sawada, T.; Tanaka, M.; Yoshikawa, T. Relationship between values of leaf nitrogen content analyser and values of a chlorophyll meter. Bull. Hyogo Pre. Agri. Inst. 2001, 49, 14–16. [Google Scholar]
- Ida, R. Measurement of nitrogen content in rice fresh leaf by the near infrared transmission method. Jpn. Soc. Soil Sci. Plant Nutr. 2001, 72, 676–678. [Google Scholar]
- Ida, R. Relationship between chemical analyzed nitrogen content, CCN value and SPAD value in flag leaves of cv. Koshihikari for the ripening period. Jpn. J. Crop Sci. 2006, 75, 550–553. [Google Scholar] [CrossRef]


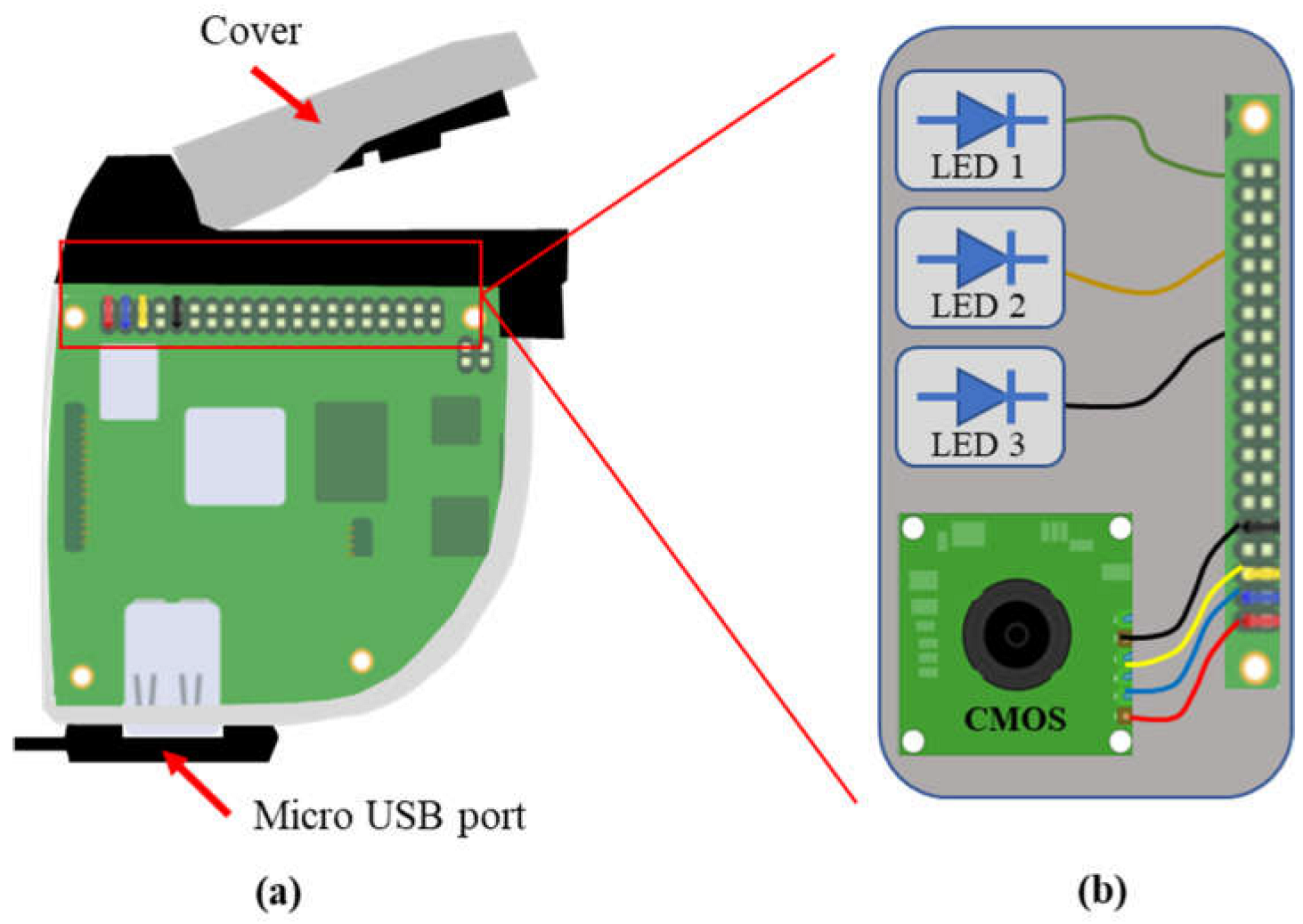
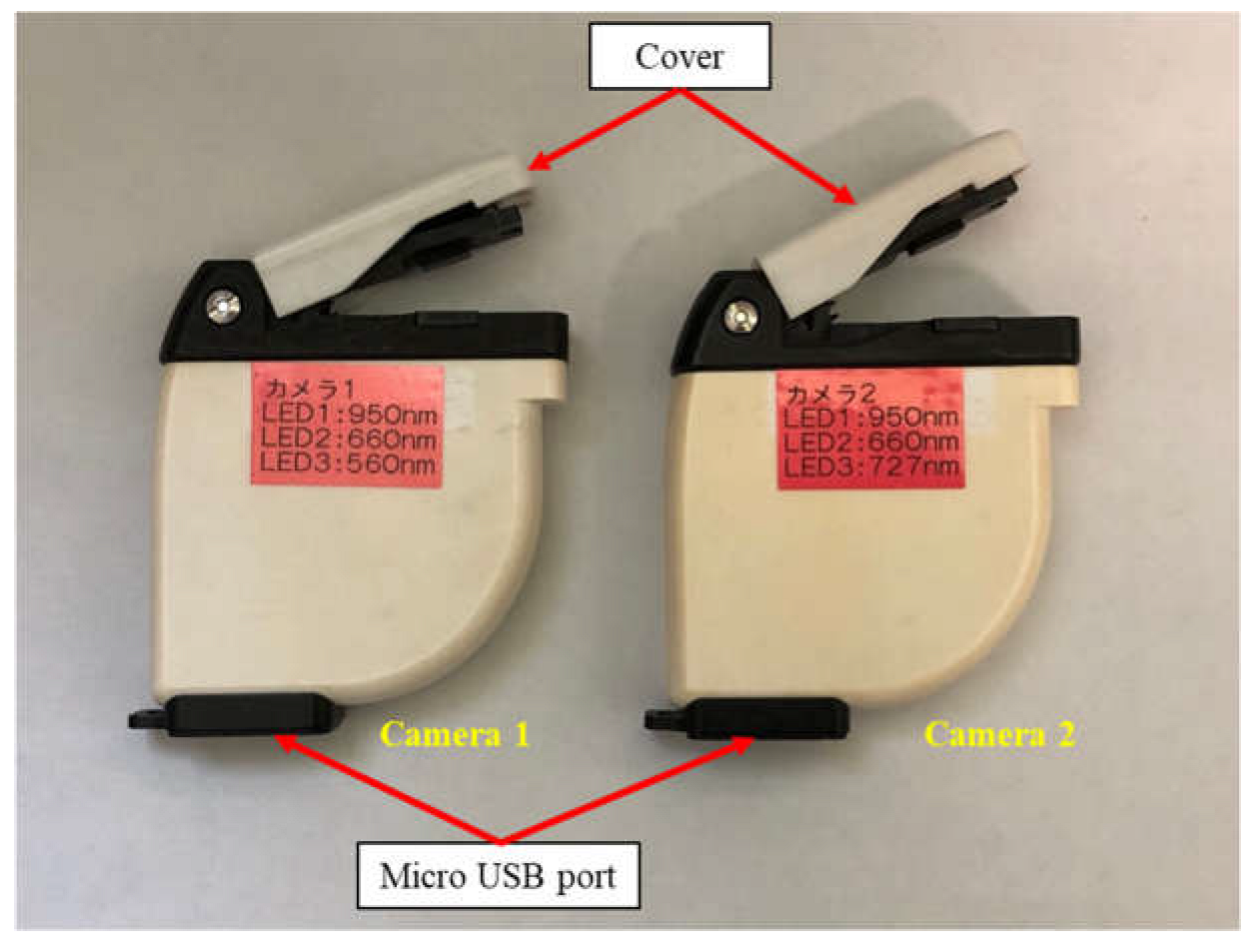
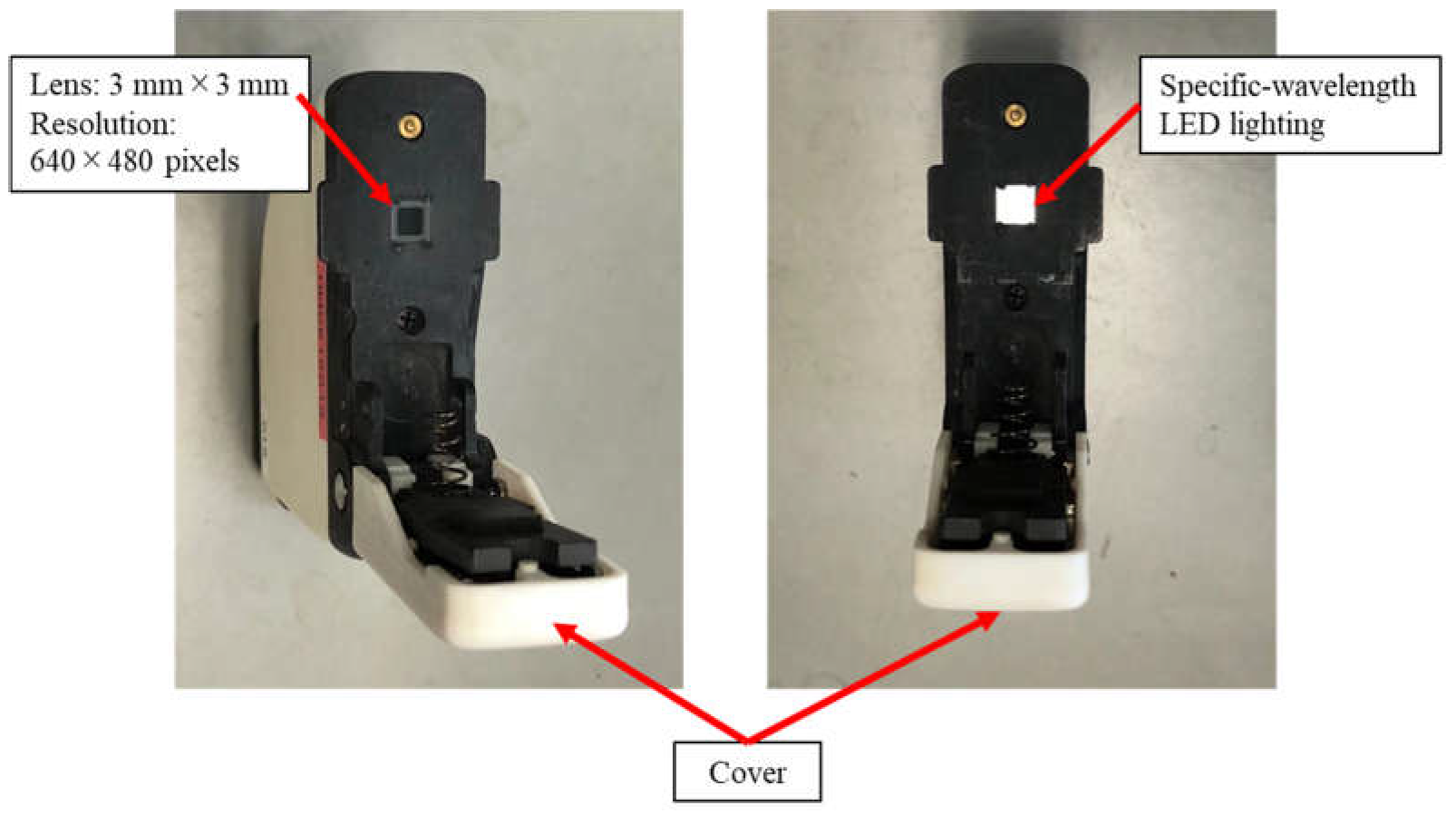
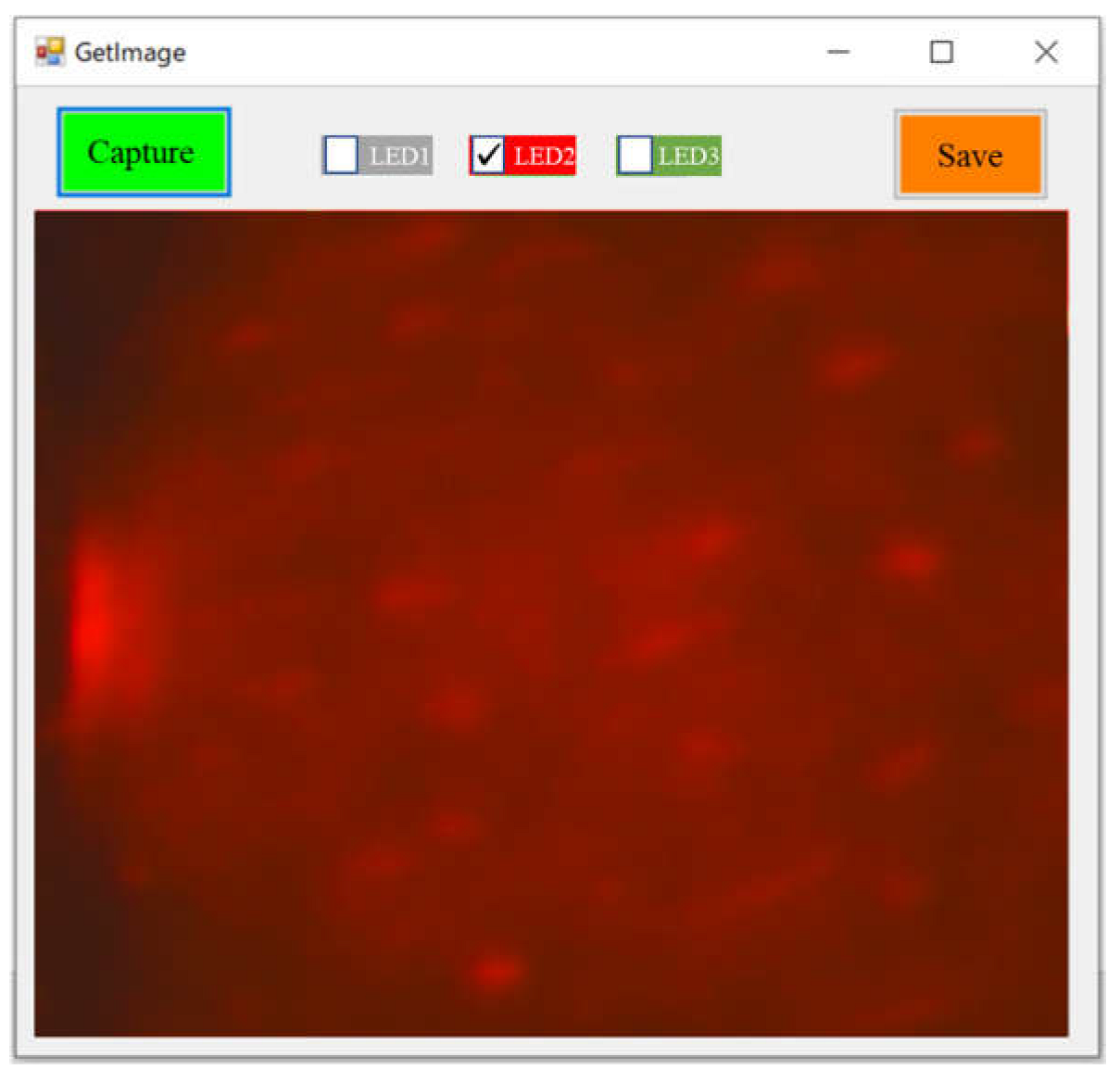

| LED No. | Camera Prototype 1 | Camera Prototype 2 | ||
|---|---|---|---|---|
| λ (nm) | Product No. | λ (nm) | Product No. | |
| LED 1 | 950 | SFH4646 | 950 | SFH4646 |
| LED 2 | 660 | GH DASPA2.24 | 660 | GH DASPA2.24 |
| LED 3 | 560 | LP M675 | 727 | GF DASPA2.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Kitaya, M.; Abe, S.; Sheng, F.; Zhang, S. A New Small-Size Camera with Built-In Specific-Wavelength LED Lighting for Evaluating Chlorophyll Status of Fruit Trees. Sensors 2023, 23, 4636. https://doi.org/10.3390/s23104636
Ye X, Kitaya M, Abe S, Sheng F, Zhang S. A New Small-Size Camera with Built-In Specific-Wavelength LED Lighting for Evaluating Chlorophyll Status of Fruit Trees. Sensors. 2023; 23(10):4636. https://doi.org/10.3390/s23104636
Chicago/Turabian StyleYe, Xujun, Marin Kitaya, Shiori Abe, Fanxing Sheng, and Shuhuai Zhang. 2023. "A New Small-Size Camera with Built-In Specific-Wavelength LED Lighting for Evaluating Chlorophyll Status of Fruit Trees" Sensors 23, no. 10: 4636. https://doi.org/10.3390/s23104636
APA StyleYe, X., Kitaya, M., Abe, S., Sheng, F., & Zhang, S. (2023). A New Small-Size Camera with Built-In Specific-Wavelength LED Lighting for Evaluating Chlorophyll Status of Fruit Trees. Sensors, 23(10), 4636. https://doi.org/10.3390/s23104636






