NanoFN10: A High-Contrast Turn-On Fluorescence Nanoprobe for Multiphoton Singlet Oxygen Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. General Materials
2.2. General Synthetic Remarks
2.3. Synthesis of FN10 [(E)-(5-(2-(Naphtho[1,2-d]oxazol-2-yl)vinyl)furan-2-yl)methanol]
2.4. Isolation and Identification of the FN10 Oxidation Product (FN10-ox)
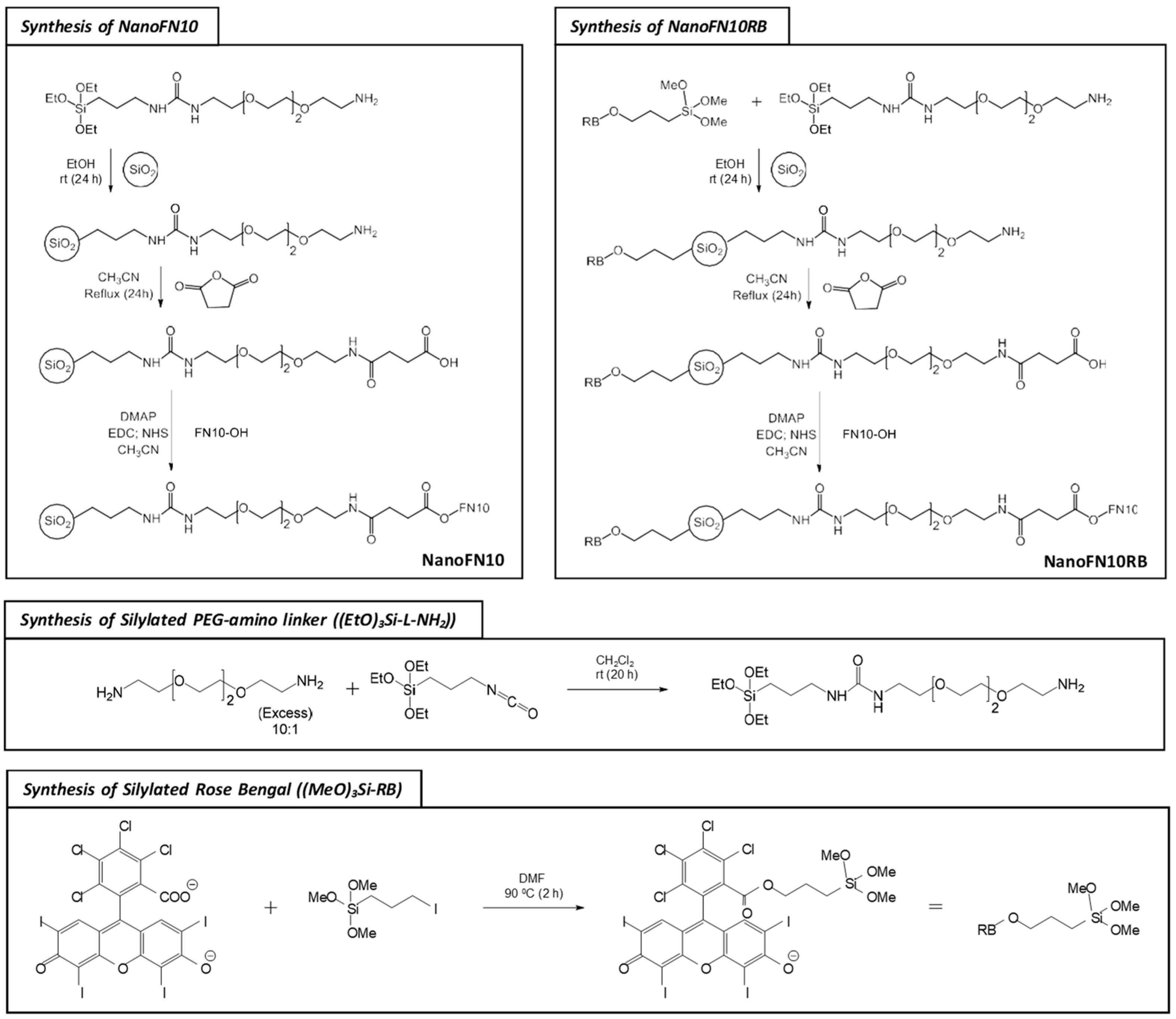
2.5. Synthesis of the Silylated PEG-Amino Linker [(EtO)3Si-L-NH2)]
2.6. Synthesis of Silylated Rose Bengal [(MeO)3Si-RB: 2,4,5,7-Tetraiodo-3-oxo-9-(2,3,4,5-tetrachloro-6-((3-(trimethoxysilyl)-propoxy)-carbonyl)-phenyl)-3H-xanthen-6-olate]
2.7. Synthesis of Mesoporous Silica Nanoparticles (MSNP)
2.8. Grafting of Rose Bengal onto Mesoporous Silica Nanoparticles (MSNP-RB)
2.9. Grafting of the Sylilated PEG-Amino Linker onto Mesoporous Silica Nanoparticles
2.10. Grafting of Carboxylic-Acid Groups on the MSNP-L-NH-CO-CH2-CH2-COOH and RB-MSNP-L-NH-CO-CH2-CH2-COOH Nanoparticles
2.11. Synthesis of NanoFN10 [MSNP-L-NH-CO-CH2-CH2-CO-O-FN10] and NanoFN10RB [MSNP-L-NH-CO-CH2-CH2-CO-O-FN10]
2.12. Determination of the Hydrodynamic Size, ζ-Potential, and Organic Elemental Analysis of the Synthesized Nanoparticles
2.13. One-Photon Spectroscopy
2.14. Two-Photon Spectroscopy
2.15. Cell Culture
2.16. Microscopy Analysis
3. Results and Discussion
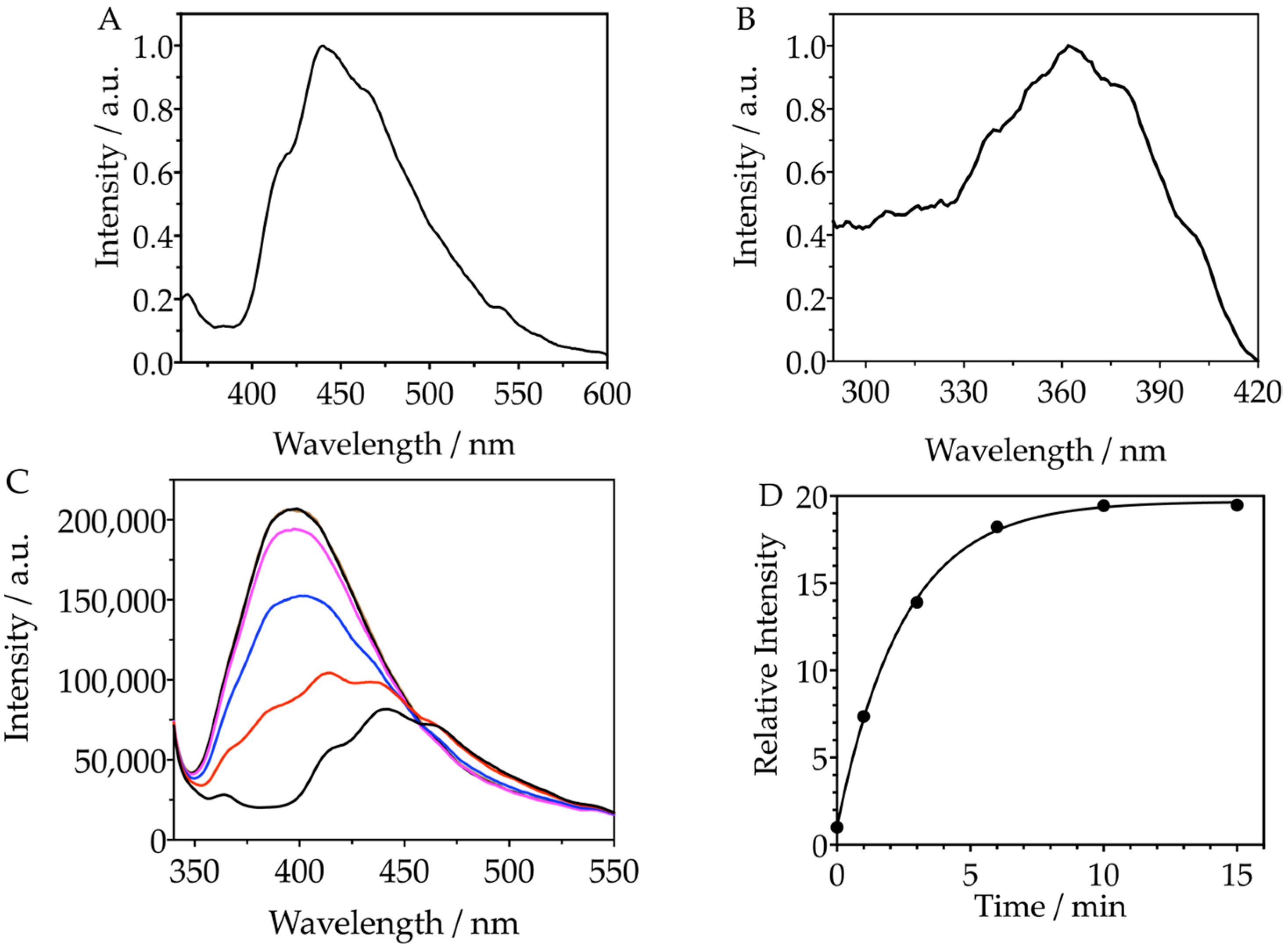
3.1. Synthesis and Characterization of the Molecular Probe FN10
3.2. Reactivity of FN10 with Singlet Oxygen
3.3. Synthesis and Characterization of the Nanoprobe NanoFN10
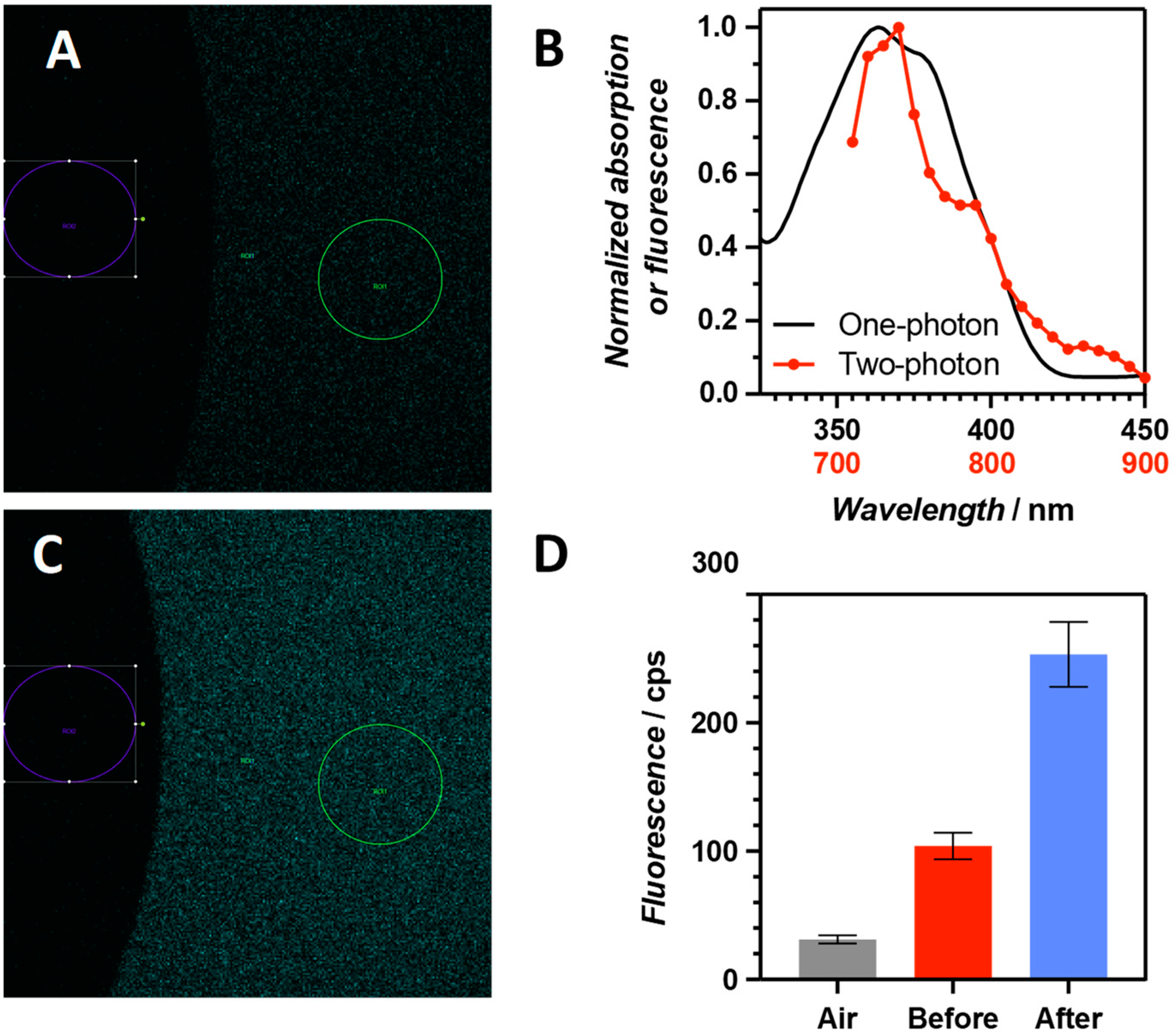
3.4. Imaging of Singlet Oxygen in Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nonell, S.; Flors, C. Singlet Oxygen: Applications in Biosciences and Nanosciences; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Ogilby, P.R. Singlet oxygen: There is indeed something new under the sun. Chem. Soc. Rev. 2010, 39, 3181–3209. [Google Scholar] [CrossRef]
- Davies, M.J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Kopetzki, D.; Lévesque, F.; Seeberger, P.H. A continuous-flow process for the synthesis of artemisinin. Chem. Eur. J. 2013, 19, 5450–5456. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. J. Am. Cancer Soc. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Kim, C.; Meskauskiene, R.; Apel, K.; Laloi, C. No single way to understand singlet oxygen signalling in plants. EMBO Rep. 2008, 9, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Flors, C.; Nonell, S. Light and singlet oxygen in plant defense against pathogens: Phototoxic phenalenone phytoalexins. Acc. Chem. Res. 2006, 39, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Salokhiddinov, K.I.; Byteva, I.M.; Dzhagarov, B.M. Duration of luminescence of singlet oxygen in solutions with laser excitation. Opt. Spektrosk. 1979, 47, 881–887. [Google Scholar]
- Jiménez-Banzo, A.; Ragàs, X.; Kapusta, P.; Nonell, S. Time-resolved methods in biophysics. 7. Photon counting vs. analog time-resolved singlet oxygen phosphorescence detection. Photochem. Photobiol. Sci. 2008, 7, 1003–1010. [Google Scholar] [CrossRef]
- Nonell, S.; Braslavsky, S.E. Chapter 4: Time-resolved singlet oxygen detection. In Methods in Enzymology 310; Packer, L., Sies, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 37–49. [Google Scholar]
- Rabello, B.R.; Gerola, A.P.; Pellosi, D.S.; Tessaro, A.L.; Aparício, J.L.; Caetano, W.; Hioka, N. Singlet oxygen dosimetry using uric acid as a chemical probe: Systematic evaluation. J. Photochem. Photobiol. A Chem. 2012, 238, 53–62. [Google Scholar] [CrossRef]
- Umezawa, N.; Tanaka, K.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Nagano, T. Novel Fluorescent Probes for Singlet Oxygen. Angew. Chem. Int. Ed. 1999, 38, 2899–2901. [Google Scholar] [CrossRef]
- Kálai, T.; Hideg, E.; Vass, I.; Hideg, K. Double (fluorescent and spin) sensors for detection of reactive oxygen species in the thylakoid membrane. Free Radic. Biol. Med. 1998, 24, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Flors, C.; Fryer, M.J.; Waring, J.; Reeder, B.; Bechtold, U.; Mullineaux, P.M.; Nonell, S.; Wilson, M.T.; Baker, N.R. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green. J. Exp. Bot. 2006, 57, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Miura, T.; Umezawa, N.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Nagano, T. Rational design of fluorescein-based fluorescence probes. Mechanism-based design of a maximum fluorescence probe for singlet oxygen. J. Am. Chem. Soc. 2001, 123, 2530–2536. [Google Scholar] [CrossRef]
- Price, M.; Reiners, J.J.; Santiago, A.M.; Kessel, D. Monitoring singlet oxygen and hydroxyl radical formation with fluorescent probes during photodynamic therapy. Photochem. Photobiol. 2009, 85, 1177–1181. [Google Scholar] [CrossRef]
- Kim, S.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Far-red fluorescence probe for monitoring singlet oxygen during photodynamic therapy. J. Am. Chem. Soc. 2014, 136, 11707–11715. [Google Scholar] [CrossRef]
- Kim, S.; Fujitsuka, M.; Majima, T. Photochemistry of singlet oxygen sensor green. J. Phys. Chem. B 2013, 117, 13985–13992. [Google Scholar] [CrossRef]
- Lindig, B.A.; Rodgers, M.A.J.; Schaap, A.P. Determination of the lifetime of singlet oxygen in water-d2 using 9,10-anthracenedipropionic acid, a water-soluble probe. J. Am. Chem. Soc. 1980, 102, 5590–5593. [Google Scholar] [CrossRef]
- Ragàs, X.; Jiménez-Banzo, A.; Sánchez-García, D.; Batllori, X.; Nonell, S. Singlet oxygen photosensitisation by the fluorescent probe Singlet Oxygen Sensor Green®. Chem. Commun. 2009, 20, 2920–2922. [Google Scholar] [CrossRef]
- Gollmer, A.; Arnbjerg, J.; Blaikie, F.H.; Pedersen, B.W.; Breitenbach, T.; Daasbjerg, K.; Glasius, M.; Ogilby, P.R. Singlet Oxygen Sensor Green®: Photochemical behavior in solution and in a mammalian cell. Photochem. Photobiol. 2011, 87, 671–679. [Google Scholar] [CrossRef]
- Ruiz-González, R.; Cortajarena, A.L.; Mejias, S.H.; Agut, M.; Nonell, S.; Flors, C. Singlet Oxygen Generation by the Genetically Encoded Tag miniSOG. J. Am. Chem. Soc. 2013, 135, 9564–9567. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-González, R.; Bresolí-Obach, R.; Gulías, O.; Agut, M.; Savoie, H.; Boyle, R.W.; Nonell, S.; Giuntini, F. NanoSOSG: A nanostructured fluorescent probe for the detection of intracellular singlet oxygen. Angew. Chem. Int. Ed. 2017, 56, 2885–2888. [Google Scholar] [CrossRef]
- Bresolí-Obach, R.; Nos, J.; Mora, M.; Sagristà, M.L.; Ruiz-González, R.; Nonell, S. Anthracene-based fluorescent nanoprobes for singlet oxygen detection in biological media. Methods 2016, 109, 64–72. [Google Scholar] [CrossRef]
- Ruiz-González, R.; Zanocco, R.; Gidi, Y.; Zanocco, A.L.; Nonell, S.; Lemp, E. Naphthoxazole-Based Singlet Oxygen Fluorescent Probes. Photochem. Photobiol. 2013, 89, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-González, R.; Agut, M.; Nonell, S. Synthesis, Photophysical Characterization, and Photoinduced Antibacterial Activity of Methylene Blue-loaded Amino- and Mannose-Targeted Mesoporous Silica Nanoparticles. Molecules 2015, 20, 6284–6298. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to imageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saafeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Neises, B.; Steglich, W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chem. Int. Ed. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Zanocco, R.P.; Bresoli-Obach, R.; Nonell, S.; Lemp, E.; Zanocco, A.L. Structure-activity study of furyl aryloxazole fluorescent probes for the detection of singlet oxygen. PLoS ONE 2018, 13, e0200006. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J. Phys. Chem. Ref. Data 1995, 24, 663–1021. [Google Scholar] [CrossRef]
- Clennan, E.L.; Mehrsheikh-Mohammadi, M.E. Mechanism of endoperoxide formation. 3. Utilization of the Young and Carlsson kinetic techniques. J. Am. Chem. Soc. 1984, 106, 7112–7118. [Google Scholar] [CrossRef]
- Denk, W.; Strickler, J.; Webb, W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Hoover, E.E.; Squier, J.A. Advances in multiphoton microscopy technology. Nat. Photonics 2013, 7, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Xu, S.; Wang, P.; Hu, X.X.; Zhang, J.; Yuan, L.; Zhang, X.B.; Tan, W. An efficient two-photon fluorescent probe for monitoring mitochondrial singlet oxygen in tissues during photodynamic therapy. Chem. Commun. 2016, 52, 12330–12333. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.A. Quinine sulfate as a fluorescence quantum yield standard. Photochem. Photobiol. 1969, 9, 439–444. [Google Scholar] [CrossRef]
- NIST Standard Reference Database 35. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C116165&Mask=80#IR-Spec (accessed on 30 January 2018).
- Günther, G.S.; Lemp, E.; Zanocco, A.L. On the use of 9,10-dimethylanthracene as chemical rate constant actinometer in singlet molecular oxygen reactions. Bol. Soc. Chil. Quím. 2000, 45, 637–644. [Google Scholar] [CrossRef]
- Schmidt, R.; Tanielian, C.; Dunsbach, R.; Wolff, C.; Womb, C.J. Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen O2(1Δg) sensitization. Photochem. Photobiol. A Chem. 1994, 79, 11–17. [Google Scholar] [CrossRef]
- Marti, C.; Jürgens, O.; Cuenca, O.; Casals, M.; Nonell, S. Aromatic ketones as standards for singlet molecular oxygen photosensitization. Time-resolved photoacoustic and near-IR emission studies. J. Photochem. Photobiol. A Chem. 1996, 97, 11–18. [Google Scholar] [CrossRef]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skid, W.M. UFF, a Full periodic table force field for molecular mechanics and dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]

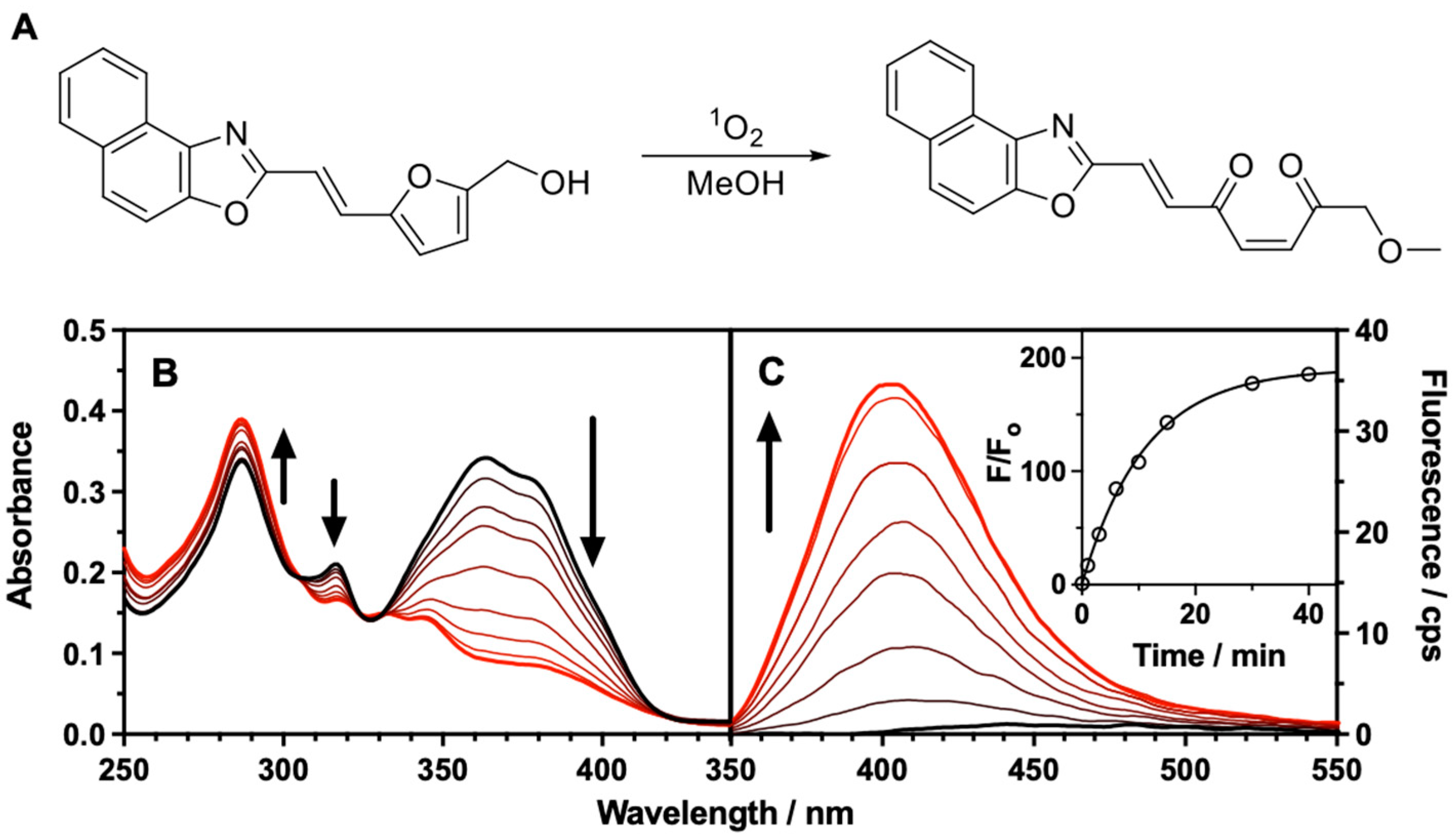


| FN10 | |
| λabs/nm | 363 |
| ε/cm−1M−1 | 4.3 × 104 |
| λflu/nm | 439 |
| ΦF | 0.005 |
| ΦΔ | 0.003 |
| kq/M−1s−1 | 3.0 × 107 |
| kr/M−1s−1 | 1.7 × 107 |
| FN10-ox | |
| λabs/nm | 335 |
| λflu/nm | 405 |
| Fluorescence enhancement | 180 (at 400 nm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanocco, R.P.; Bresolí-Obach, R.; Nájera, F.; Pérez-Inestrosa, E.; Zanocco, A.L.; Lemp, E.; Nonell, S. NanoFN10: A High-Contrast Turn-On Fluorescence Nanoprobe for Multiphoton Singlet Oxygen Imaging. Sensors 2023, 23, 4603. https://doi.org/10.3390/s23104603
Zanocco RP, Bresolí-Obach R, Nájera F, Pérez-Inestrosa E, Zanocco AL, Lemp E, Nonell S. NanoFN10: A High-Contrast Turn-On Fluorescence Nanoprobe for Multiphoton Singlet Oxygen Imaging. Sensors. 2023; 23(10):4603. https://doi.org/10.3390/s23104603
Chicago/Turabian StyleZanocco, Renzo P., Roger Bresolí-Obach, Francisco Nájera, Ezequiel Pérez-Inestrosa, Antonio L. Zanocco, Else Lemp, and Santi Nonell. 2023. "NanoFN10: A High-Contrast Turn-On Fluorescence Nanoprobe for Multiphoton Singlet Oxygen Imaging" Sensors 23, no. 10: 4603. https://doi.org/10.3390/s23104603
APA StyleZanocco, R. P., Bresolí-Obach, R., Nájera, F., Pérez-Inestrosa, E., Zanocco, A. L., Lemp, E., & Nonell, S. (2023). NanoFN10: A High-Contrast Turn-On Fluorescence Nanoprobe for Multiphoton Singlet Oxygen Imaging. Sensors, 23(10), 4603. https://doi.org/10.3390/s23104603






