Highly Sensitive Carbon Monoxide Sensor Element with Wide-Range Humidity Resistance by Loading Pd Nanoparticles on SnO2 Surface
Abstract
:1. Introduction
2. Materials and Methods
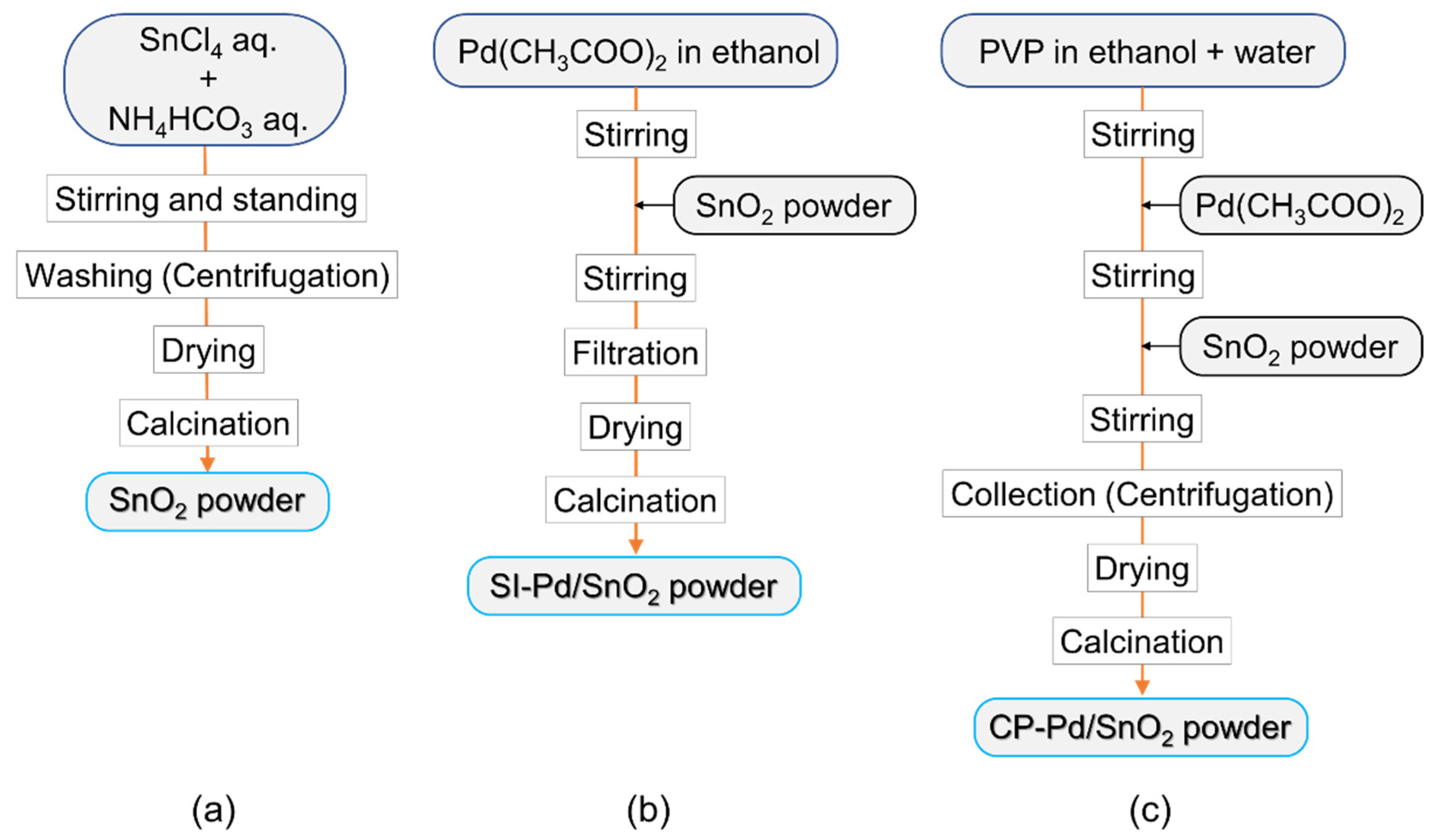
2.1. Synthesis of SnO2 and Pd/SnO2 Nanoparticles and Sensor Fabrication
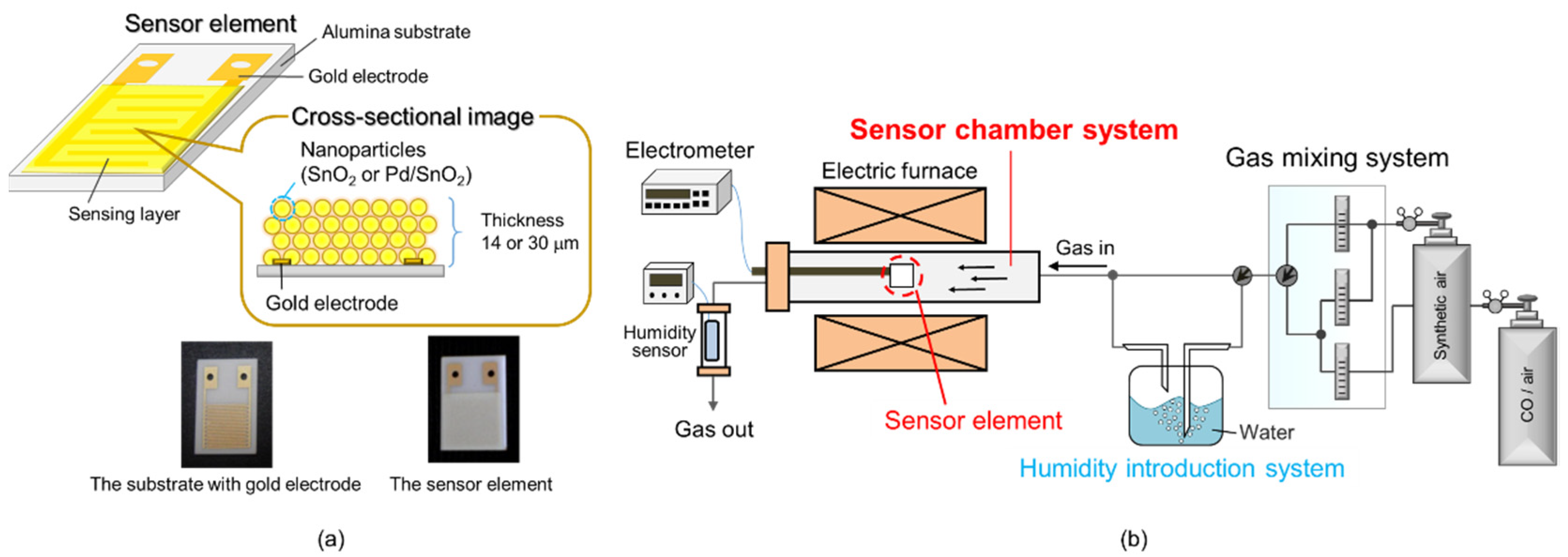
2.2. Materials and Sensor Evaluation
3. Results and Discussion
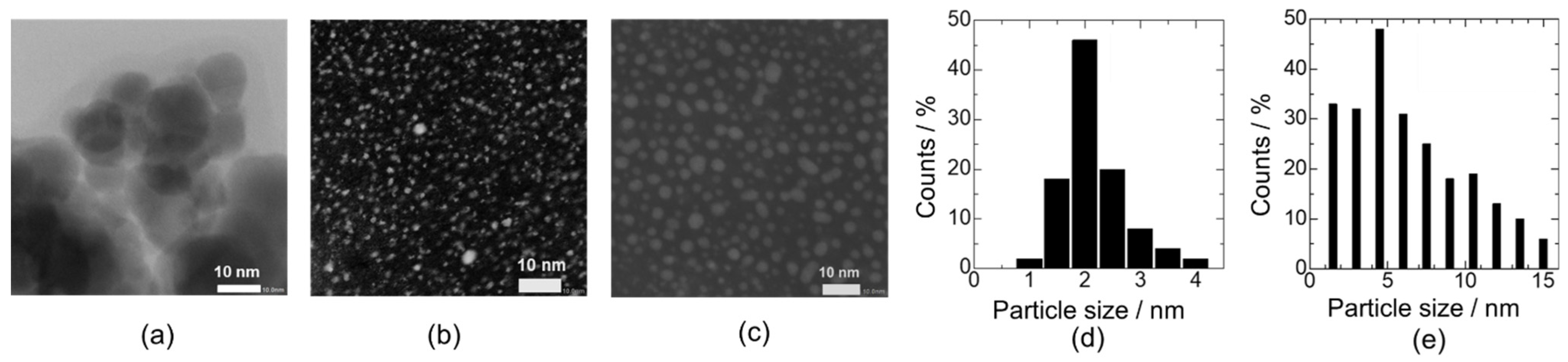
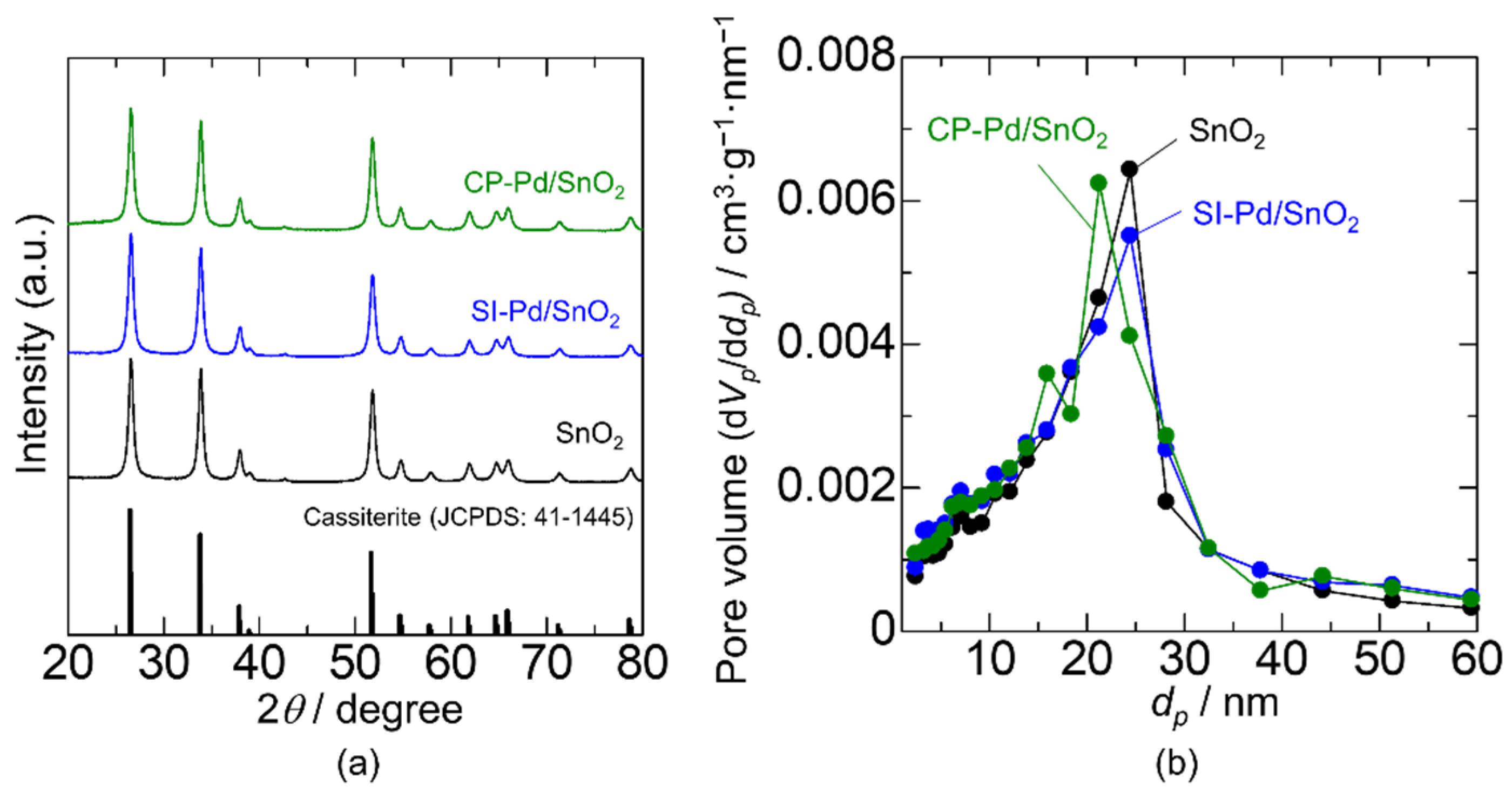
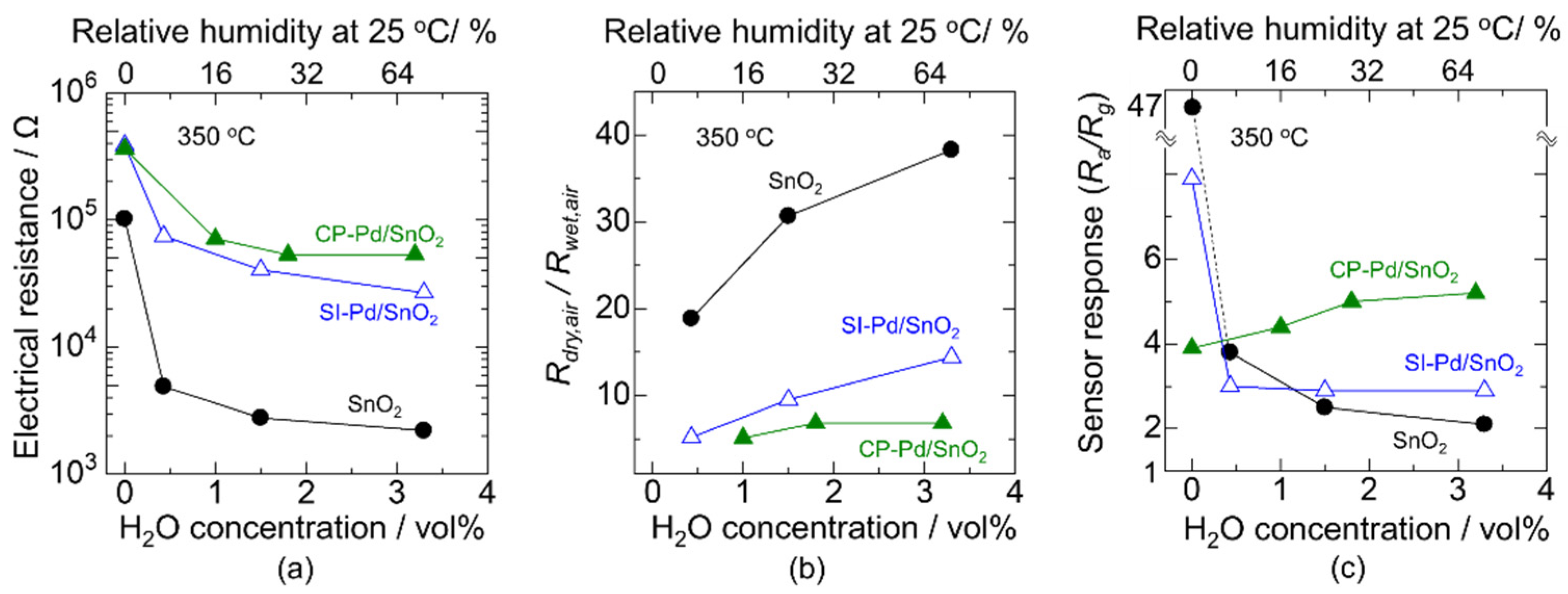
3.1. Effect of Pd Loading Method on Materials and Electrical Characteristics
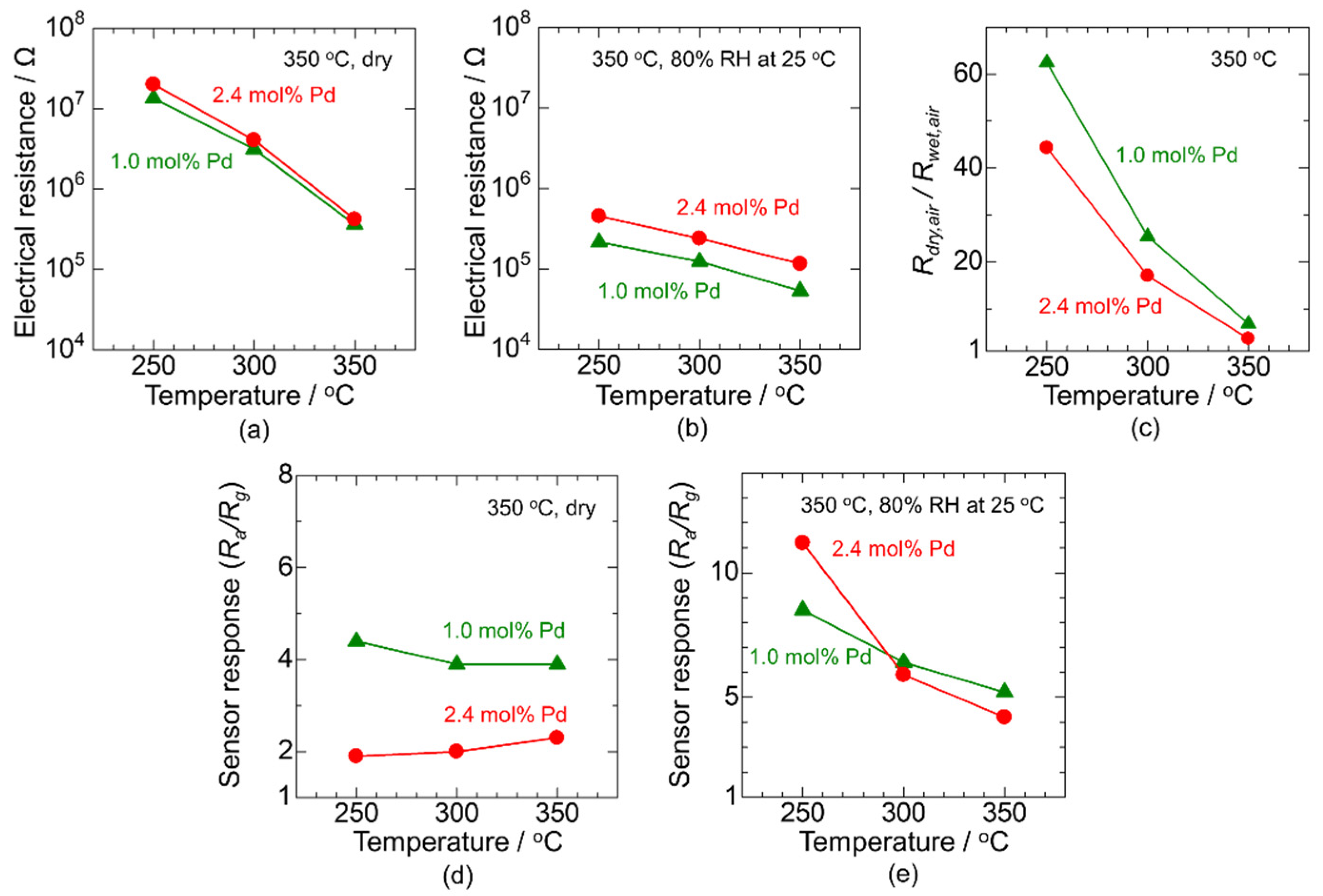
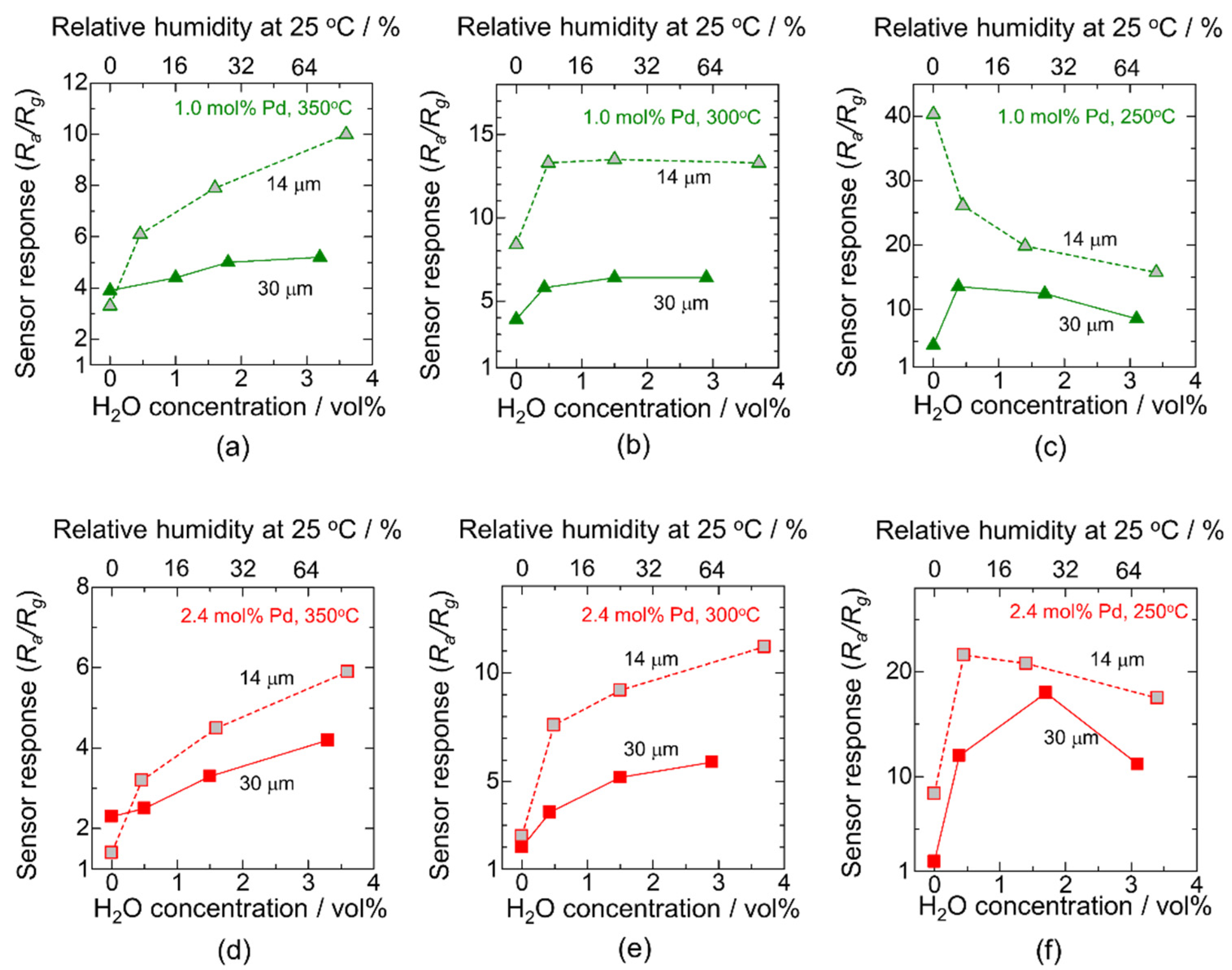
3.2. Improving the Sensor Response in a Humid Atmosphere
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Taguchi, N. Metal Oxide Gas Sensor. Japan Patent No. S45-38200, 1962. [Google Scholar]
- Tanaka, K.; Morimoto, S.; Sonoda, S.; Matsuura, S.; Moriya, K.; Egashira, M. Combustion monitoring sensor using tin dioxide semiconductor. Sens. Actuators B 1991, 3, 247–253. [Google Scholar] [CrossRef]
- Matsuura, Y.; Takahata, K. Stabilization of SnO2 sintered gas sensors. Sens. Actuators B 1991, 5, 205–209. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Yamazoe, N. Toward Innovations of Gas Sensors Technology. Sens. Actuators B 2005, 108, 2–14. [Google Scholar]
- Bârsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Kida, T.; Kuroiwa, T.; Yuasa, M.; Shimanoe, K.; Yamazoe, N. Study on the response and recovery properties of semiconductor gas sensors using a high-speed gas-switching system. Sens. Actuators B 2008, 134, 928–933. [Google Scholar] [CrossRef]
- Heiland, G. Homogeneous semiconducting gas sensors. Sens. Actuators 1981, 2, 343–361. [Google Scholar] [CrossRef]
- Bârsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter. 2003, 15, R813. [Google Scholar]
- Meixner, H.; Lampe, U. Metal oxide sensors. Sens. Actuators B 1996, 33, 198–202. [Google Scholar] [CrossRef]
- Santarossa, G.; Hahn, K.; Baiker, A. Free Energy and Electronic Properties of Water Adsorption on the SnO2 (110) Surface. Langmuir 2013, 29, 5487–5499. [Google Scholar] [CrossRef]
- Koziej, D.; Bârsan, N.; Weimar, U.; Szuber, J.; Shimanoe, K.; Yamazoe, N. Water-Oxygen Interplay on Tin Dioxide Surface: Implication on Gas Sensing. Chem. Phys. Lett. 2005, 410, 321–323. [Google Scholar] [CrossRef]
- Degler, D.; Junker, B.; Allmendinger, F.; Weimar, U.; Barsan, N. Investigations on the Temperature-Dependent Interaction of Water Vapor with Tin Dioxide and Its Implications on Gas Sensing. ACS Sens. 2020, 5, 3207–3216. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of tin oxide surface with O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen ionosorption and spectroscopic evidence for adsorbed oxygen. ChemPhysChem 2006, 7, 2041–2052. [Google Scholar] [CrossRef]
- Suematsu, K.; Yuasa, M.; Kida, T.; Yamazoe, N.; Shimanoe, K. Determination of Oxygen Adsorption on SnO2: Exact Analysis of Gas Sensing Properties Using a Sample Gas Pretreatment System. J. Electrochem. Soc. 2014, 161, B123–B128. [Google Scholar] [CrossRef]
- Mizokawa, Y.; Nakamura, S. ESR and Electric Conductance Studies of Fine-Powdered SnO2. Jpn. J. Appl. Phys. 1975, 14, 779–788. [Google Scholar] [CrossRef]
- Degker, D.; Wicker, S.; Weimar, U.; Barsan, N. Identifying the Active Oxigen Species in SnO2 Based Gas Sensing Materials: An Operando IR Spectrsocopy Study. J. Phys. Chem. C 2015, 119, 11792–11799. [Google Scholar]
- Suematsu, K.; Ma, N.; Watanabe, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of Humid Aging on the Oxygen Adsorption in SnO2 Gas Sensors. Sensors 2018, 18, 254. [Google Scholar] [CrossRef] [Green Version]
- Pavelko, R.G.; Daly, H.; Hardacre, C.; Vasiliev, A.A.; Llobet, E. Interaction of water, hydrogen and their mixtures with SnO2 based materials: The role of surface hydroxyl groups in detection mechanisms. Phys. Chem. Chem. Phys. 2010, 12, 2639–2647. [Google Scholar] [CrossRef]
- Kim, H.-R.; Haensch, A.; Kim, I.-D.; Barsan, N.; Weimar, U.; Lee, J.-H. The Role of NiO Doping in Reducing the Impact of Humidity on the Performance of SnO2-Based Gas Sensors: Synthesis Strategies, and Phenomenological and Spectroscopic Studies. Adv. Funct. Mater. 2011, 21, 4456–4463. [Google Scholar] [CrossRef]
- Choi, K.-I.; Kim, H.-J.; Lang, Y.-C.; Lee, J.-H. Ultraselective and ultrasensitive detection of H2S in highly humid atmosphere using CuO-loaded SnO2 hollow spheres for real-time diagnosis of halitosis. Sens. Actuator B 2014, 194, 371–376. [Google Scholar] [CrossRef]
- Suematsu, K.; Ma, N.; Yuasa, M.; Kida, T.; Shimanoe, K. Surface-modification of SnO2 nanoparticles by incorporation of Al for the detection of combustible gases in humid atmosphere. RSC Adv. 2015, 5, 86347–86354. [Google Scholar] [CrossRef]
- Suematsu, K.; Sasaki, M.; Ma, N.; Yuasa, M.; Shimanoe, K. Antimony-Doped Tin Dioxide Gas Sensors Exhibiting High Stability in the Sensitivity to Humidity Changes. ACS Sens. 2016, 1, 913–920. [Google Scholar] [CrossRef]
- Großmann, K.; Wicker, S.; Weimar, U.; Barsan, N. Impact of Pt additives on the surface reactions between SnO2, water vapour, CO and H2; An operand investigation. Phys. Chem. Chem. Phys. 2013, 15, 19151–19158. [Google Scholar] [CrossRef] [PubMed]
- Marikutsa, A.V.; Rumyantseva, M.N.; Yashina, L.V.; Gaskov, A.M. Role of surface hydroxyl groups in promoting room temperature CO sensing by Pd-modified nanocrystalline SnO2. J. Solid State Chem. 2010, 183, 2389–2399. [Google Scholar] [CrossRef]
- Ma, N.; Suematsu, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of Water Vapor on Pd-Loaded SnO2 Nanoparticles Gas Sensor. ACS Appl. Mater. Interfaces 2015, 7, 5863–5869. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N. Recent development of semiconductor gas sensor technology. Shokubai 1986, 28, 555–559. [Google Scholar]
- Matsushima, S.; Teraoka, Y.; Miura, N.; Yamazoe, N. Electronic interaction between metal additives and tin dioxide in tin dioxide-based gas sensors. Jpn. J. Appl. Phys. 1988, 27, 1798–1802. [Google Scholar] [CrossRef]
- Matsushima, S.; Maekawa, T.; Tamaki, J.; Miura, N.; Yamazoe, N. Dispersion and Electronic Interaction of Palladium Particles Supported on Tin Oxide. Nippon Kagaku Kaishi 1991, 240, 1677–1683. [Google Scholar] [CrossRef]
- Grenoble, D.C.; Estadt, M.M.; Ollis, D.F. The chemistry and catalysis of the water gas shift reaction. 1. The kinetics over supported metal catalysts. J. Catal. 1981, 67, 90–102. [Google Scholar] [CrossRef]
- Bunluesin, T.; Gorte, R.J.; Graham, G.W. Studies of the water-gas-shift reaction on ceria-supported Pt, Pd, and Rh: Implications for oxygen-storage properties. Appl. Catal. B 1998, 15, 107–114. [Google Scholar] [CrossRef]
- Hahn, S.H.; Barsan, N.; Weimar, U.; Ehakov, S.G.; Visser, J.H.; Soltis, R.E. CO sensing with SnO2 thick film sensors: Role of oxygen and water vapour. Thin Solid Films 2003, 436, 17–24. [Google Scholar] [CrossRef]
- Ma, N.; Suematsu, K.; Yuasa, M.; Shimanoe, K. Pd Size Effect on the Gas Sensing Properties of Pd-Loaded SnO2 in Humid Atmosphere. ACS Appl. Mater. Interfaces 2015, 7, 15618–15625. [Google Scholar] [CrossRef]
- Liu, C.; Suematsu, K.; Uchiyama, A.; Watanabe, K.; Guo, Y.; Wand, D.; Shimanoe, K. Impact of Pd nanoparticle loading method on SnO2 surface for natural gas detection in humid atmosphere. J. Mater. Sci. 2021, 56, 13975–13988. [Google Scholar] [CrossRef]
- Teranishi, T.; Miyake, M. Size Control of Palladium Nanoparticles and Their Crystal Structures. Chem. Mater. 1998, 10, 594–600. [Google Scholar] [CrossRef]
- Niu, S.; Guo, W.; Lin, T.-W.; Yu, W.; Wu, Y.; Ji, X.; Shao, L. Nanoscale Pd supported on 3D porous carbon for enhanced selective oxidation of benzyl alcohol. RSC Adv. 2017, 7, 25885. [Google Scholar] [CrossRef] [Green Version]
- Sakai, G.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N. Theory of Gas-Diffusion Controlled Sensitivity for Thin Film Semiconductor Gas Sensor. Sens. Actuators B 2001, 80, 125–131. [Google Scholar] [CrossRef]
- Suematsu, K.; Shin, Y.; Hua, Z.; Yoshida, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Nanoparticle Cluster Gas Sensor: Controlled Clustering of SnO2 Nanoparticles for Highly Sensitive Toluene Detection. ACS Appl. Mater. Interfaces 2014, 6, 5319–5326. [Google Scholar] [CrossRef]
- Suematsu, K.; Watanabe, K.; Tou, A.; Sun, Y.; Shimanoe, K. Ultraselective Toluene-Gas Sensor: Nanosized Gold Loaded on Zinc Oxide Nanoparticles. Anal. Chem. 2018, 90, 1959–1966. [Google Scholar] [CrossRef]
- Kutukov, P.; Rumyantseva, M.; Krivetskiy, V.; Filatova, D.; Batuk, M.; Hadermann, J.; Khmelevsky, N.; Aksenenko, A.; Gaskov, A. Influence of Mono- and Bimetallic PtOx, PdOx, PtPdOx Clusters on CO Sensing by SnO2 Based Gas Sensors. Nanomaterials 2018, 8, 917. [Google Scholar] [CrossRef] [Green Version]
- Sayegh, S.; Lee, J.-H.; Yang, D.-H.; Weber, M.; Latsunskyi, I.; Coy, E.; Razzouk, A.; Kim, S.S.; Bechelany, M. Humidity-resistant gas sensors based on SnO2 nanowires coated with a porous alumina nanomembrane by molecular layer deposition. Sens. Actuator B 2021, 344, 130302. [Google Scholar] [CrossRef]
- Li, G.; Cheng, Z.; Xiang, Q.; Yan, L.; Wang, X.; Xu, J. Bimetal PdAu decorated SnO2 nanosheets based gas sensor with temperature-dependent dual selectivity for detecting formaldehyde and acetone. Sens. Actuator B 2019, 283, 590–601. [Google Scholar] [CrossRef]
- Zito, C.A.; Oerfecto, T.M.; Volanti, D.P. Palladium-Loaded Hierarchical Flower-like Tin Dioxide Structure as Chemosensor Exhibiting High Ethanol Response in Humid Conditions. Adv. Mater. Interfaces 2017, 4, 1700847. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Zhou, R.; Li, P.; Zhang, W.; Suematsu, K.; Hu, J. Synthesis of In2O3 nanocubes, nanocube clusters, and nanocubes-embedded Au nanoparticles for conductometric CO sensors. Sens. Actuator B 2021, 345, 130433. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Suematsu, K.; Zhang, W.; Zhang, W.; Zhuiykov, S.; Shimanoe, K.; Hui, J. MOF-derived Au-NiO/In2O3 for selective and fast detection of toluene at ppb-level in high humid environments. Sens. Actuator B 2022, 360, 131631. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suematsu, K.; Uchiyama, A.; Watanabe, K.; Shimanoe, K. Highly Sensitive Carbon Monoxide Sensor Element with Wide-Range Humidity Resistance by Loading Pd Nanoparticles on SnO2 Surface. Sensors 2022, 22, 2934. https://doi.org/10.3390/s22082934
Suematsu K, Uchiyama A, Watanabe K, Shimanoe K. Highly Sensitive Carbon Monoxide Sensor Element with Wide-Range Humidity Resistance by Loading Pd Nanoparticles on SnO2 Surface. Sensors. 2022; 22(8):2934. https://doi.org/10.3390/s22082934
Chicago/Turabian StyleSuematsu, Koichi, Akihito Uchiyama, Ken Watanabe, and Kengo Shimanoe. 2022. "Highly Sensitive Carbon Monoxide Sensor Element with Wide-Range Humidity Resistance by Loading Pd Nanoparticles on SnO2 Surface" Sensors 22, no. 8: 2934. https://doi.org/10.3390/s22082934
APA StyleSuematsu, K., Uchiyama, A., Watanabe, K., & Shimanoe, K. (2022). Highly Sensitive Carbon Monoxide Sensor Element with Wide-Range Humidity Resistance by Loading Pd Nanoparticles on SnO2 Surface. Sensors, 22(8), 2934. https://doi.org/10.3390/s22082934






