A Three-Reagent “Green” Paper-Based Analytical Device for Solid-Phase Spectrometric and Colorimetric Determination of Dihydroquercetin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Procedures
3. Results and Discussion
3.1. Choice of the Reagents for Determination of Dihydroquercetin
3.2. Determination of Dihydroquercetin Using the Three-Reagent µPAD and Diffuse Reflectance Spectroscopy
3.2.1. Analytical Responses
3.2.2. Features of Merit
3.3. Determination of Dihydroquercetin Using the Three-Reagent µPAD and Digital Colorimetry
3.4. Selectivity and Analysis of Samples
3.5. Comparison with Other Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sriram, G.; Bhat, M.P.; Patil, P.; Uthappa, U.T.; Jung, H.Y.; Altalhi, T.; Kumeria, T.; Aminabhavi, T.M.; Pai, R.K.; Kurkuri, M.D. Paper-based microfluidic analytical devices for colorimetric detection of toxic ions: A review. Trends Anal. Chem. 2017, 93, 212–227. [Google Scholar] [CrossRef]
- Kung, C.-T.; Hou, C.-Y.; Wang, Y.-N.; Fu, L.-M. Microfluidic paper-based analytical devices for environmental analysis of soil, air, ecology and river water. Sens. Actuators B 2019, 301, 126855. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, M.; Kong, N.; Liu, J. Lab-on-paper micro- and nano-analytical devices: Fabrication, modification, detection and emerging applications. Microchim. Acta 2016, 183, 1521–1542. [Google Scholar] [CrossRef]
- Singhal, H.R.; Prabhu, A.; Giri Nandagopal, M.S.; Dheivasigamani, T.; Mani, N.K. One-dollar microfluidic paper-based analytical devices: Do-it-yourself approaches. Microchem. J. 2021, 165, 106126. [Google Scholar] [CrossRef]
- Murray, L.P.; Mace, C.R. Usability as a guiding principle for the design of paper-based, point-of-care devices–A review. Anal. Chim. Acta 2020, 1140, 236–249. [Google Scholar] [CrossRef]
- Bendicho, C.; Lavilla, I.; Pena-Pereira, F.; de la Calle, I.; Romero, V. Paper-based analytical devices for colorimetric and luminescent detection of mercury in waters: An overview. Sensors 2021, 21, 7571. [Google Scholar] [CrossRef]
- Alahmad, W.; Sahragard, A.; Varanusupakul, P. Online and offline preconcentration techniques on paper-based analytical devices for ultrasensitive chemical and biochemical analysis: A review. Biosens. Bioelectron. 2021, 194, 113574. [Google Scholar] [CrossRef]
- Giannoulas, G.; Tsogas, G.Z.; Giokas, D.L. Single-point calibration and standard addition assays on calibrant-loaded paper-based analytical devices. Talanta 2019, 201, 149–155. [Google Scholar] [CrossRef]
- Kappi, F.A.; Tsogas, G.Z.; Routsi, A.-M.; Christodouleas, D.C.; Giokas, D.L. Paper-based devices for biothiols sensing using the photochemical reduction of silver halides. Anal. Chim. Acta 2018, 1036, 89–96. [Google Scholar] [CrossRef]
- Duangdeewong, C.; Sitanurak, J.; Wilairat, P.; Nacapricha, D.; Teerasong, S. Microfluidic paper-based analytical device for convenient use in measurement of iodate in table salt and irrigation water. Microchem. J. 2020, 152, 104447. [Google Scholar] [CrossRef]
- Ungor, D.; Szilágyi, I.; Csapó, E. Yellow-emitting Au/Ag bimetallic nanoclusters with high photostability for detection of folic acid. J. Mol. Liq. 2021, 338, 116695. [Google Scholar] [CrossRef]
- Lee, W.-C.; Ng, H.-Y.; Hou, C.-Y.; Lee, C.-T.; Fu, L.-M. Recent advances in lab-on-paper diagnostic devices using blood samples. Lab A Chip 2021, 21, 1433–1453. [Google Scholar] [CrossRef] [PubMed]
- Subhi Sammani, M.; Clavijo, S.; Figuerola, A.; Cerdà, V. 3D printed structure coated with C18 particles in an online flow system coupled to HPLC-DAD for the determination of flavonoids in citrus external peel. Microchem. J. 2021, 168, 106421. [Google Scholar] [CrossRef]
- Campillo, N.; Viñas, P.; Férez-Melgarejo, G.; Hernández-Córdoba, M. Dispersive liquid–liquid microextraction for the determination of flavonoid aglycone compounds in honey using liquid chromatography with diode array detection and time-of-flight mass spectrometry. Talanta 2015, 131, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, H.; Wang, Q.; Liu, H.; Shen, H.; Xu, W.; Ge, J.; He, D. Rapid qualitative profiling and quantitative analysis of phenolics in Ribes meyeri leaves and their antioxidant and antidiabetic activities by HPLC-QTOF-MS/MS and UHPLC-MS/MS. J. Sep. Sci. 2021, 44, 1404–1420. [Google Scholar] [CrossRef] [PubMed]
- Di Masi, S.; De Benedetto, G.E.; Malitesta, C.; Saponari, M.; Citti, C.; Cannazza, G.; Ciccarella, G. HPLC-MS/MS method applied to an untargeted metabolomics approach for the diagnosis of “olive quick decline syndrome”. Anal. Bioanal. Chem. 2022, 414, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Raoof, J.B.; Ghani, M. Determination of quercetin: Via thin film microextraction using the in situ growth of Co-Al-layered double hydroxide nanosheets on an electrochemically anodized aluminum substrate followed by HPLC. Anal. Methods 2020, 12, 799–806. [Google Scholar] [CrossRef]
- Santos, W.N.L.D.; da Silva Sauthier, M.C.; dos Santos, A.M.P.; de Andrade Santana, D.; Almeida Azevedo, R.S.; da Cruz Caldas, J. Simultaneous determination of 13 phenolic bioactive compounds in guava (Psidium guajava L.) by HPLC-PAD with evaluation using PCA and Neural Network Analysis (NNA). Microchem. J. 2017, 133, 583–592. [Google Scholar] [CrossRef]
- Willie, P.; Uyoh, E.A.; Aikpokpodion, P.O. Gas chromatography-mass spectrometry (GC-MS) assay of bio-active compounds and phytochemical analyses in three species of apocynaceae. Pharmacogn. J. 2021, 13, 383–392. [Google Scholar] [CrossRef]
- Adeoye-Isijola, M.O.; Jonathan, S.G.; Coopoosamy, R.M.; Olajuyigbe, O.O. Molecular characterization, gas chromatography mass spectrometry analysis, phytochemical screening and insecticidal activities of ethanol extract of Lentinus squarrosulus against Aedes aegypti (Linnaeus). Mol. Biol. Rep. 2021, 48, 41–55. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Zhang, H.; Li, F.; Yang, F.-Q. Evaluation of xanthine oxidase inhibitory activity of flavonoids by an online capillary electrophoresis-based immobilized enzyme microreactor. Electrophoresis 2020, 41, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, H.; Qian, C.; Li, H.; Chen, D.D.Y. Characterization of interaction between Bcl-2 oncogene promoter I-Motif DNA and flavonoids using electrospray ionization mass spectrometry and pressure-assisted capillary electrophoresis frontal analysis. Talanta 2020, 215, 120885. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.F.; Palabiyik, I.M.; Solangi, A.R.; Memon, S.Q.; Mallah, A.B. Large volume sample stacking (LVSS) in capillary electrophoresis (CE) with response surface methodology (RSM) for the determination of phenolics in food samples. Anal. Lett. 2019, 52, 2853–2867. [Google Scholar] [CrossRef]
- Abdullah, A.A.; Yardım, Y.; Şentürk, Z. The performance of cathodically pretreated boron-doped diamond electrode in cationic surfactant media for enhancing the adsorptive stripping voltammetric determination of catechol-containing flavonoid quercetin in apple juice. Talanta 2018, 187, 156–164. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ibrahim, H.; Almandil, N.B.; Sayed, M.A.; Kawde, A.-N. A new hybrid nanocomposite electrode based on Au/CeO2-decorated functionalized glassy carbon microspheres for the voltammetric sensing of quercetin and its interaction with DNA. Anal. Methods 2020, 12, 2846–2857. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.-C.; Balram, D. Synthesis of amine-functionalized multi-walled carbon nanotube/3D rose flower-like zinc oxide nanocomposite for sensitive electrochemical detection of flavonoid morin. Anal. Chim. Acta 2020, 1095, 71–81. [Google Scholar] [CrossRef]
- Furletov, A.A.; Apyari, V.V.; Garshev, A.V.; Dmitrienko, S.G.; Zolotov, Y.A. Fast and sensitive determination of bioflavonoids using a new analytical system based on label-free silver triangular nanoplates. Sensors 2022, 22, 843. [Google Scholar] [CrossRef]
- Kurdyukov, E.E.; Vodop’yanova, O.A.; Moiseeva, I.Y.; Semenova, E.F. A method for the quantitative determination of the total flavonoid content of moringa leaves (Moringa oleifera). Mosc. Univ. Chem. Bull. 2021, 76, 224–226. [Google Scholar] [CrossRef]
- Khani, R.; Sheykhi, R.; Bagherzade, G. An environmentally friendly method based on micro-cloud point extraction for determination of trace amount of quercetin in food and fruit juice samples. Food Chem. 2019, 293, 220–225. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Clemente, A.; Summo, C.; Pasqualone, A.; Caponio, F. Towards green analysis of virgin olive oil phenolic compounds: Extraction by a natural deep eutectic solvent and direct spectrophotometric detection. Food Chem. 2016, 212, 43–47. [Google Scholar] [CrossRef]
- Dmitrienko, S.G.; Apyari, V.V.; Kudrinskaya, V.A.; Stepanova, A.V. Preconcentration of flavonoids on polyurethane foam and their direct determination by diffuse reflectance spectroscopy. Talanta 2012, 102, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Gutorova, S.V.; Apyari, V.V.; Kalinin, V.I.; Furletov, A.A.; Tolmacheva, V.V.; Gorbunova, M.V.; Dmitrienko, S.G. Composable paper-based analytical devices for determination of flavonoids. Sens. Actuators B 2021, 331, 129398. [Google Scholar] [CrossRef]
- Korenman, I.M. Photometric Analysis; Methods for the Determination of Organic Compounds (Fotometricheskii Analiz. Metody Opredeleniya Organicheskikh Soedinenii); Khimiya: Moscow, Russia, 1975. (In Russian) [Google Scholar]
- Kyei, S.K.; Akaranta, O.; Darko, G. Synthesis, characterization and antimicrobial activity of peanut skin extract-azo-compounds. Sci. Afr. 2020, 8, e00406. [Google Scholar] [CrossRef]
- Kudrinskaya, V.A.; Dmitrienko, S.G.; Zolotov, Y.A. Spectrophotometric determination of flavonoids using their diazotization with (4-nitrophenyl)diazonium tetrafluoroborate. Mosc. Univ. Chem. Bull. 2010, 65, 244–248. [Google Scholar] [CrossRef]
- Chernysh, V.V.; Proskurnin, M.A.; Kuznetsova, V.V.; Pakhomova, S.V. Determination of microamounts of phenols by thermal lens spectrometry. Anal. Commun. 1997, 34, 291–294. [Google Scholar] [CrossRef]
- Terenteva, E.A.; Apyari, V.V.; Dmitrienko, S.G.; Zolotov, Y.A. Formation of plasmonic silver nanoparticles by flavonoid reduction: A comparative study and application for determination of these substances. Spectrochim. Acta A 2015, 151, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Osonga, F.J.; Akgul, A.; Yazgan, I.; Akgul, A.; Ontman, R.; Kariuki, V.M.; Eshuna, G.B.; Sadik, O.A. Flavonoid-derived anisotropic silver nanoparticles inhibit growth and change the expression of virulence genes in Escherichia coli SM10. RSC Adv. 2018, 8, 4649–4661. [Google Scholar] [CrossRef] [Green Version]
- Apyari, V.V.; Dmitrienko, S.G. Using a digital camera and computer data processing for the determination of organic substances with diazotized polyurethane foams. J. Anal. Chem. 2008, 63, 530–537. [Google Scholar] [CrossRef]
- Monogarova, O.V.; Oskolok, K.V.; Apyari, V.V. Colorimetry in chemical analysis. J. Anal. Chem. 2018, 73, 1076–1084. [Google Scholar] [CrossRef]
- Al Lawati, H.A.J.; Hassanzadeh, J.; Bagheri, N.; Al Lawati, I. On paper synthesis of metal-organic framework as a chemiluminescence enhancer for estimating the total phenolic content of food samples using a smartphone readout. Talanta 2021, 234, 122648. [Google Scholar] [CrossRef]
- Scroccarello, A.; Della Pelle, F.; Rojas, D.; Ferraro, G.; Fratini, E.; Gaggiotti, S.; Cichelli, A.; Compagnone, D. Metal nanoparticles based lab-on-paper for phenolic compounds evaluation with no sample pretreatment. Application to extra virgin olive oil samples. Anal. Chim. Acta 2021, 1183, 338971. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Diduk, R.; Orozco, J.; Merkoçi, A. Paper strip-embedded graphene quantum dots: A screening device with a smartphone readout. Sci. Rep. 2017, 7, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarza, R.M.O.; Villarino, C.B.J.; Gregorio, C.G.C. Paper-based device for the detection and quantification of total polyphenols in plant-based beverages for potential use in quality assurance purposes. Philipp. J. Sci. 2022, 151, 193–204. [Google Scholar]
- Calabria, D.; Mirasoli, M.; Guardigli, M.; Simoni, P.; Zangheri, M.; Severi, P.; Caliceti, C.; Roda, A. Paper-based smartphone chemosensor for reflectometric on-site total polyphenols quantification in olive oil. Sens. Actuators B 2020, 305, 127522. [Google Scholar] [CrossRef]


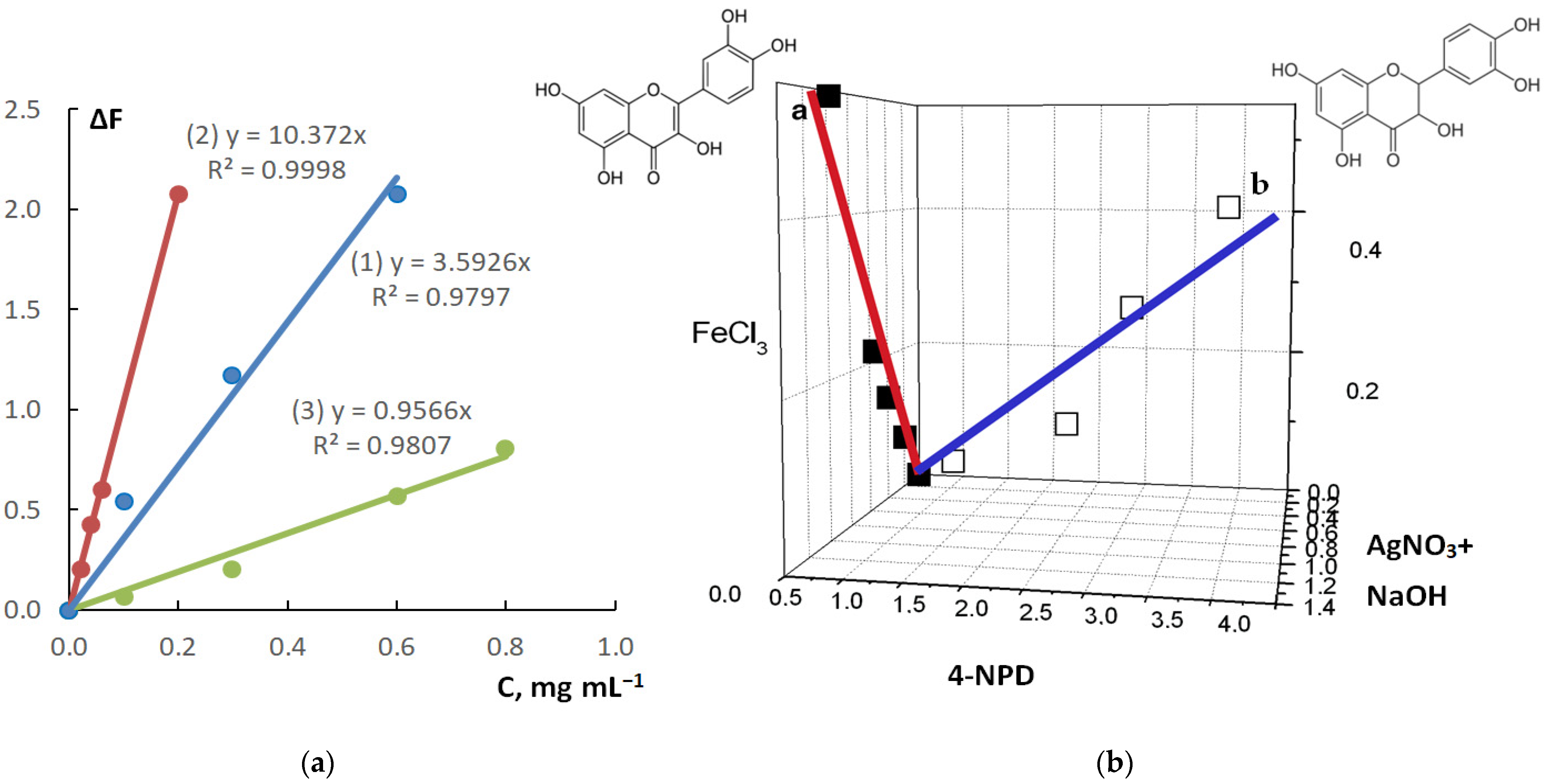
| Detection Zone | Calibration Graph Equation | R2 | Linearity Range, mg mL−1 | LOD, mg mL−1 | LOD, µg |
|---|---|---|---|---|---|
| 4-NPD | ΔF = 3.53·C | 0.978 | 0.026–1 | 0.0077 | 0.62 |
| AgNO3 + NaOH | ΔF = 0.83·C | 0.998 | 0.05–1 | 0.015 | 1.20 |
| FeCl3 | ΔF = 0.45·C | 0.973 | 0.13–1.1 | 0.039 | 3.12 |
| Detection Zone | Color Coordinate | Calibration Graph Equation | R2 | Linearity Range, mg mL−1 | LOD, mg mL−1 | LOD, µg |
|---|---|---|---|---|---|---|
| 4-NPD | Red | R = 190.3 + 35.4·exp(−c/0.27) | 0.978 | 0.43–1 | 0.13 | 10 |
| Green | G = 163.8 + 47.5·exp(−c/0.32) | 0.991 | 0.34–1 | 0.1 | 8.0 | |
| Blue | B = 80.6 + 86·exp(−c/0.28) | 0.999 | 0.11–1 | 0.03 | 2.4 | |
| AgNO3 + NaOH | Red | R = 153 + 45.4·exp(−c/0.19) | 0.986 | 0.22–1 | 0.07 | 5.2 |
| Blue | B = 73.1 + 71.1·exp(−c/0.37) | 0.971 | 0.34–1 | 0.1 | 8.0 | |
| FeCl3 | Red | R = 160.3 + 70.3·exp(−c/0.44) | 0.987 | 0.43–1 | 0.13 | 10 |
| Green | G = 140.1 + 78.2·exp(−c/0.60) | 0.996 | 0.44–1 | 0.13 | 10 |
| Labeled Content, mg | µPAD | HPLC | |||
|---|---|---|---|---|---|
| Detection Zone | Found ± tP,f∙s/√n, mg | RSD, % | Found ± tP,f∙s/√n, mg | RSD, % | |
| 25 | AgNO3 | 17 ± 11 | 26 | 29 ± 2 | 3 |
| 4-NPD | 37 ± 11 | 12 | |||
| FeCl3 | 38 ± 8 | 9 | |||
| µPAD | Analytical Instrument | Analyte | Linearity Range, µg mL−1 | LOD, µg mL−1 | Reference |
|---|---|---|---|---|---|
| µPAD based on chemiluminescence of luminol/H2O2 system enhanced with cobalt-imidazole metal-organic framework | Smartphone | Gallic acid Quercetin Catechin Kaempferol Caffeic acid | 0.5–50 1–100 1–100 2–100 2–120 | 0.12 0.28 0.46 0.85 1.23 | [41] |
| µPAD based on producing silver nanoparticles | Mini-spectrophotometer | Quercetin Morin Dihydroquercetin | 7–100 16–100 43–100 | 2.3 5.2 14 | [32] |
| µPAD based on growth of gold and silver nanoparticles | Digital camera | Total polyphenol (hydroxytyrosol) | 25–500 | 5 (Au) 6 (Ag) | [42] |
| µPAD based on luminescent graphene quantum dots embedded into nitrocellulose matrix | Smartphone | Quercetin | 5–75 | 7.1; 20 | [43] |
| Three reagent µPAD | Mini-spectro- photometer | Dihydroquercetin | 26–1000 | 7.7; 15; 39 | This study |
| µPAD based on iron tartrate | Table-top scanner | Total polyphenol (gallic acid) | 0–1200 | 20 | [44] |
| µPAD based on the Folin–Ciocalteu reagent | Smartphone | Total polyphenol | 0–800 | 30 (µg g−1) | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apyari, V.V.; Furletov, A.A.; Kalinin, V.I.; Dmitrienko, S.G.; Zolotov, Y.A. A Three-Reagent “Green” Paper-Based Analytical Device for Solid-Phase Spectrometric and Colorimetric Determination of Dihydroquercetin. Sensors 2022, 22, 2893. https://doi.org/10.3390/s22082893
Apyari VV, Furletov AA, Kalinin VI, Dmitrienko SG, Zolotov YA. A Three-Reagent “Green” Paper-Based Analytical Device for Solid-Phase Spectrometric and Colorimetric Determination of Dihydroquercetin. Sensors. 2022; 22(8):2893. https://doi.org/10.3390/s22082893
Chicago/Turabian StyleApyari, Vladimir V., Aleksei A. Furletov, Vyacheslav I. Kalinin, Stanislava G. Dmitrienko, and Yury A. Zolotov. 2022. "A Three-Reagent “Green” Paper-Based Analytical Device for Solid-Phase Spectrometric and Colorimetric Determination of Dihydroquercetin" Sensors 22, no. 8: 2893. https://doi.org/10.3390/s22082893
APA StyleApyari, V. V., Furletov, A. A., Kalinin, V. I., Dmitrienko, S. G., & Zolotov, Y. A. (2022). A Three-Reagent “Green” Paper-Based Analytical Device for Solid-Phase Spectrometric and Colorimetric Determination of Dihydroquercetin. Sensors, 22(8), 2893. https://doi.org/10.3390/s22082893







