Electrochemical Cytosensor Based on a Gold Nanostar-Decorated Graphene Oxide Platform for Gastric Cancer Cell Detection
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Apparatus
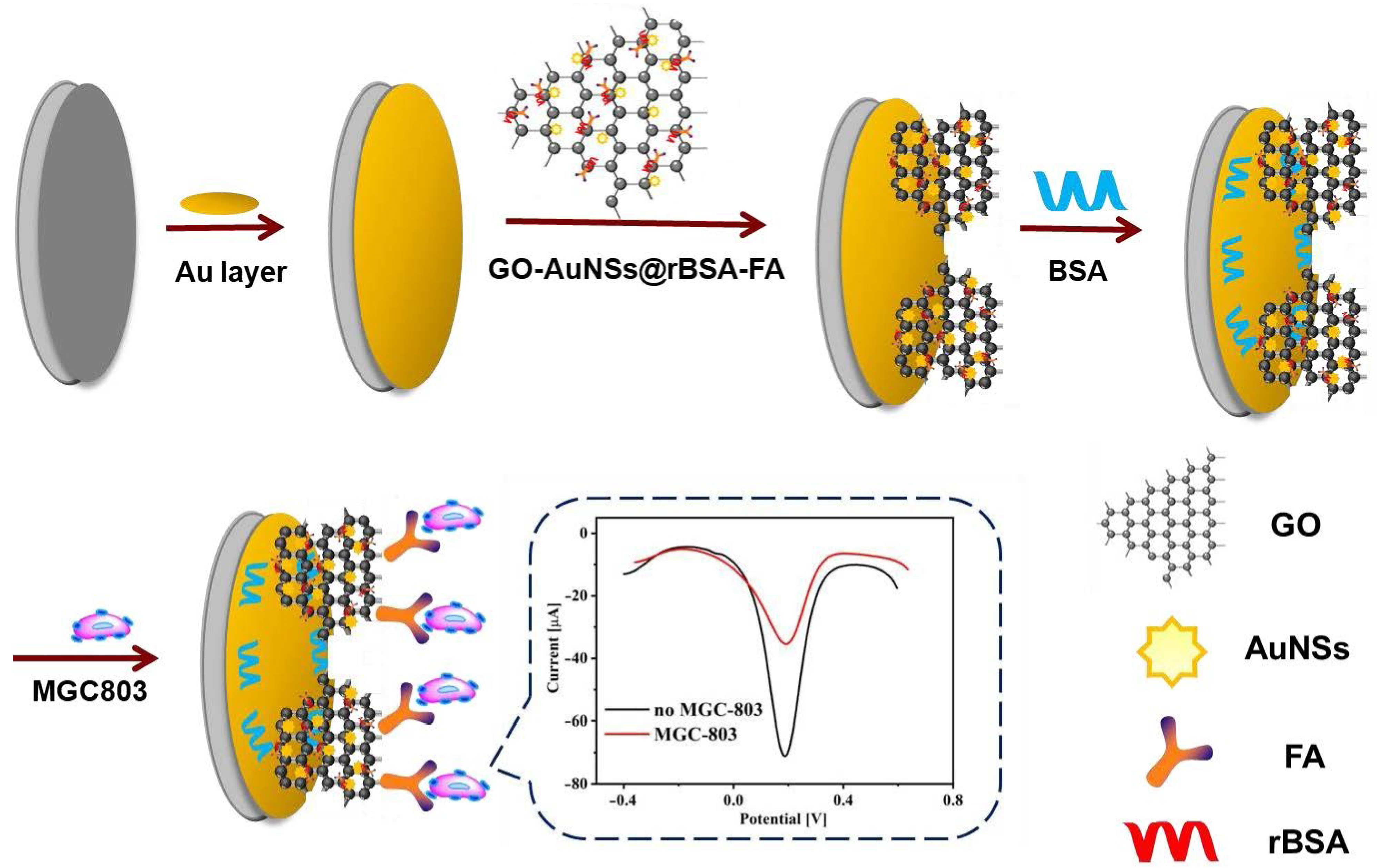
2.3. Preparation of GO-AuNSs@rBSA-FA Nanocomposites
2.3.1. Preparation of GO-AuNS Nanocomposites
2.3.2. Preparation of rBSA-FA Nanocomposites
2.3.3. Preparation of GO-AuNSs@rBSA-FA Nanocomposites
2.4. Assembly of Electrochemical Cytosensors Based on GO-AuNSs@rBSA-FA Nanocomposites
2.5. Electrochemical Detection of MGC-803 Cells
3. Results and Discussion
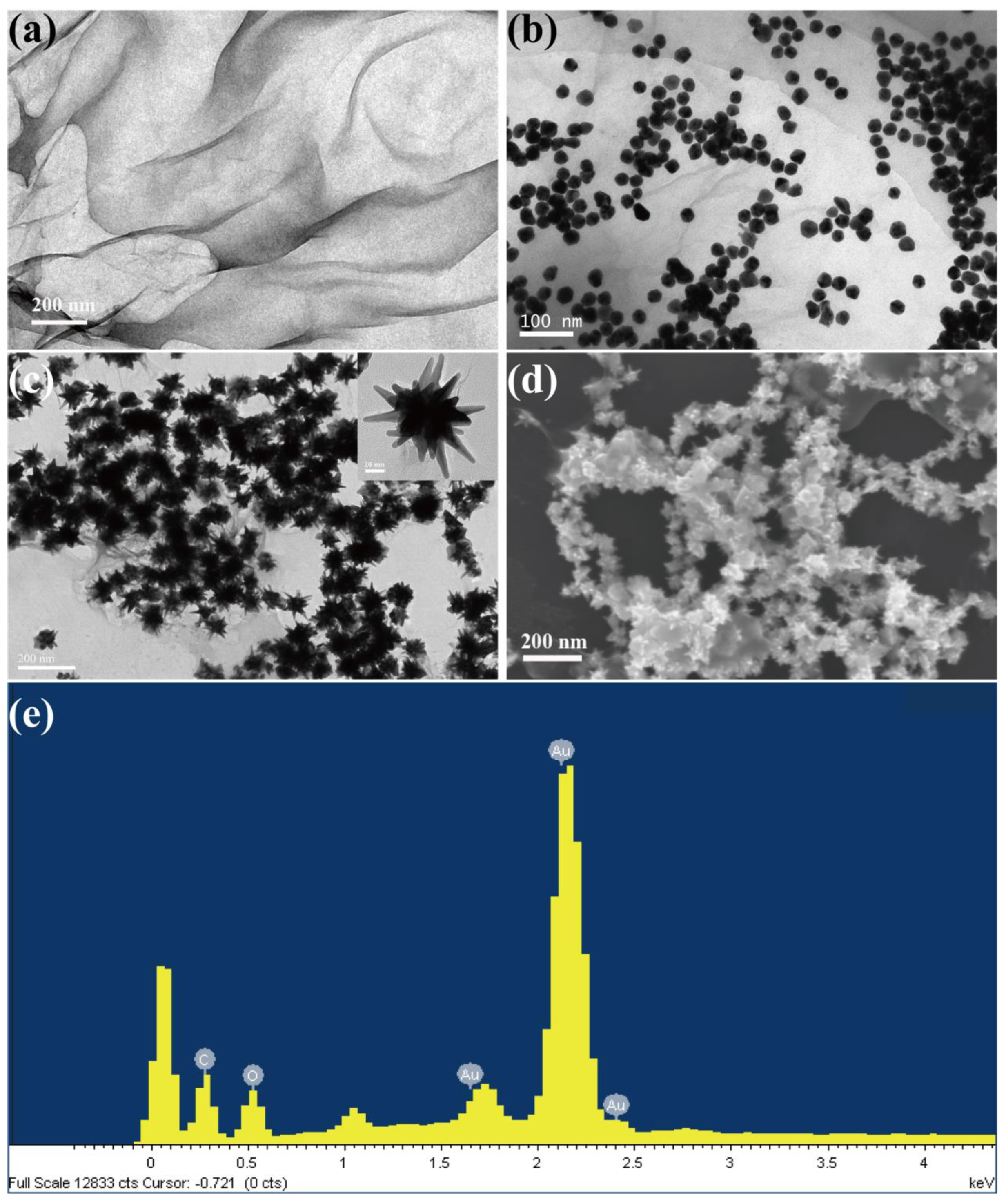
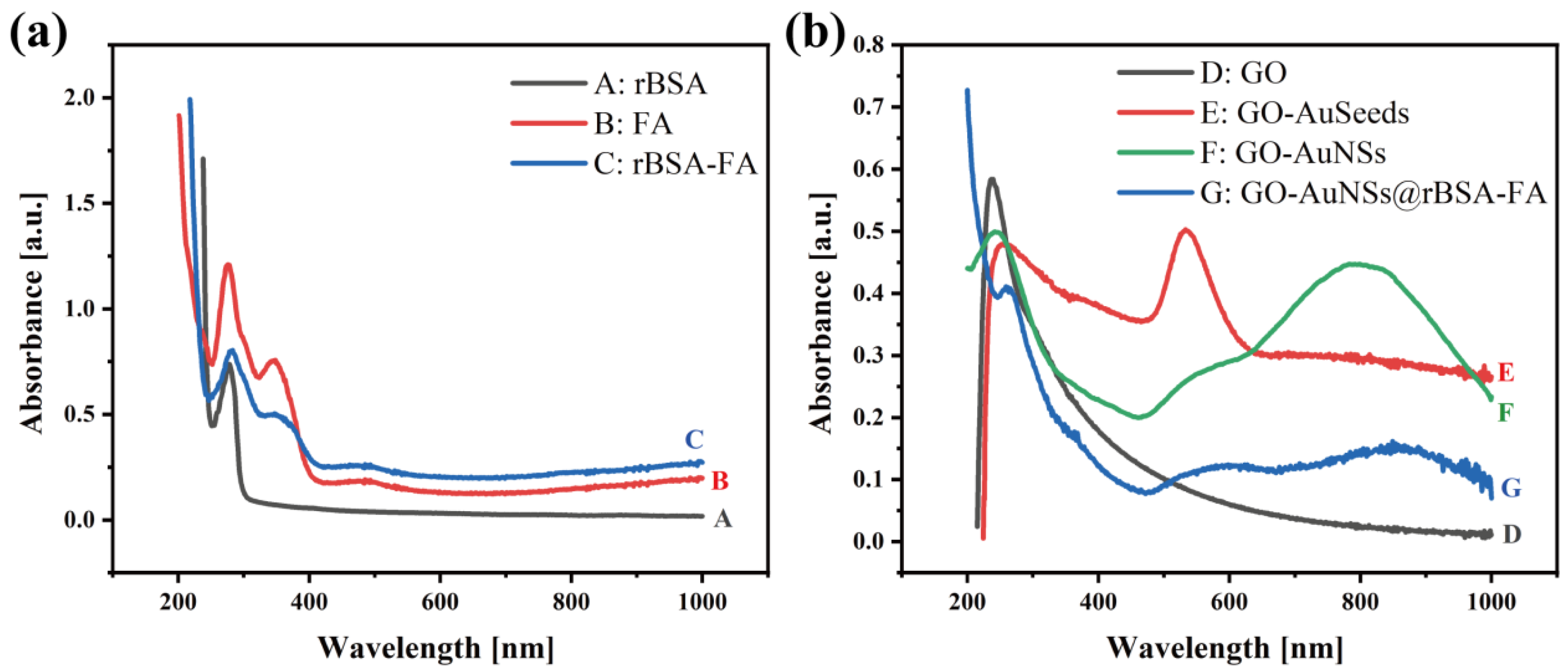
3.1. Characterization of GO-AuNSs@rBSA-FA
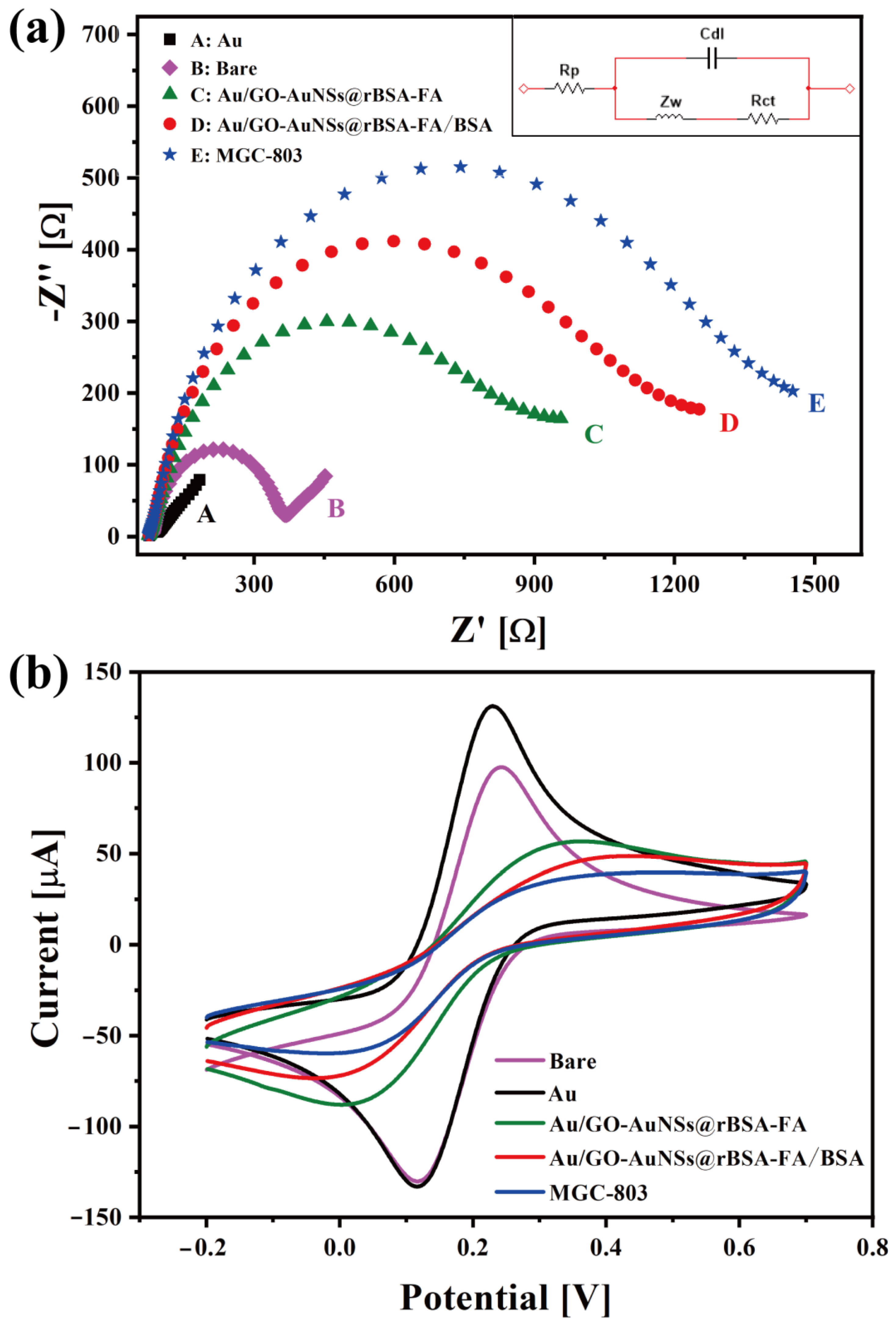
3.2. Electrochemical Performances of GO-AuNSs@rBSA-FA
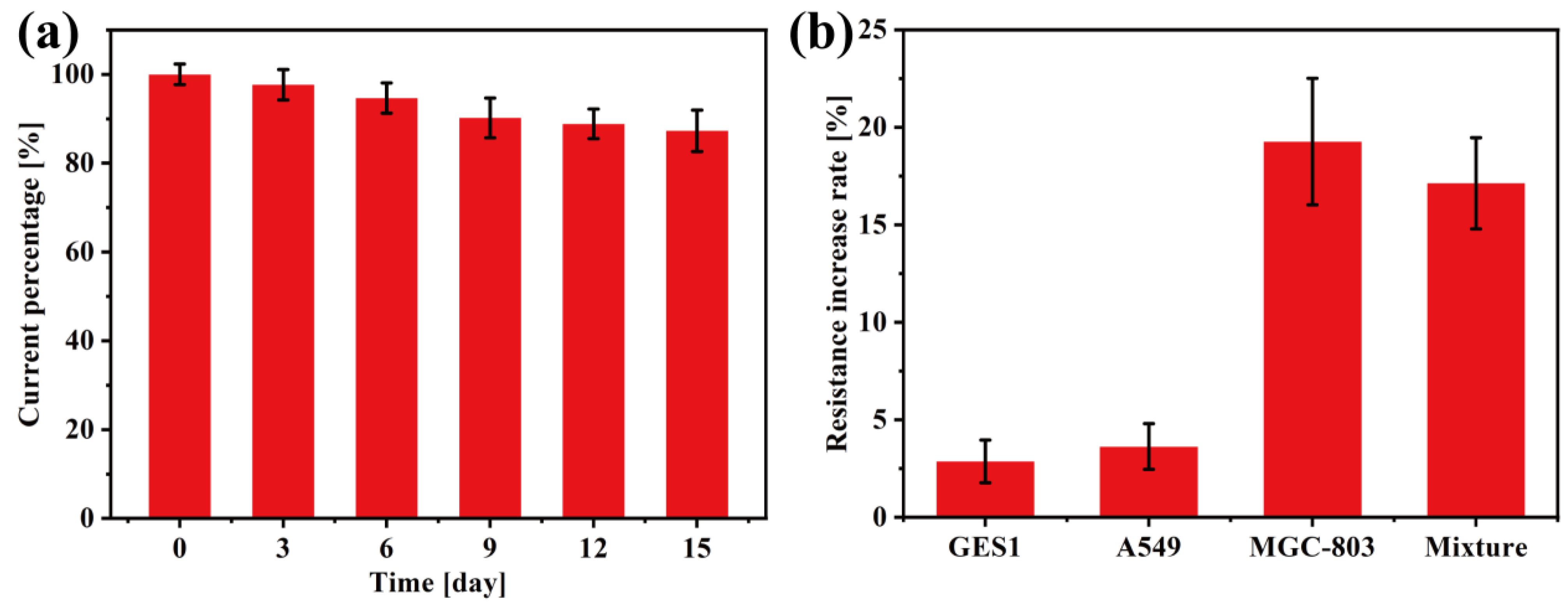
3.3. Electrochemical Detection of Gastric Cancer Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.; Jemal, A. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020, 70, 313. [Google Scholar]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Chen, W.; Wang, J.; Cai, W.; Kong, S.; Wu, C. Folic acid-functionalized zirconium metal-organic frameworks based electrochemical impedance biosensor for the cancer cell detection. Sens. Actuators B Chem. 2019, 301, 127073. [Google Scholar] [CrossRef]
- Ruiyi, L.; Tinling, P.; Hongxia, C.; Jinsong, S.; Zaijun, L. Electrochemical detection of cancer cells in human blood using folic acid and glutamic acid-functionalized graphene quantum dot-palladium@gold as redox probe with excellent electrocatalytic activity and target recognition. Sens. Actuators B Chem. 2020, 309, 127709. [Google Scholar] [CrossRef]
- Weng, J.; Zhang, Z.; Sun, L.; Wang, J.A. High sensitive detection of cancer cell with a folic acid-based boron-doped diamond electrode using an AC impedimetric approach. Biosens. Bioelectron. 2011, 26, 1847–1852. [Google Scholar] [CrossRef]
- Wang, Y.; Song, C.; Wang, M.; Xie, Y.; Mi, L.; Wang, G. Rapid, Label-Free, and Highly Sensitive Detection of Cervical Cancer With Fluorescence Lifetime Imaging Microscopy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 228–234. [Google Scholar] [CrossRef]
- Liu, J.; Qin, Y.; Li, D.; Wang, T.; Liu, Y.; Wang, J.; Wang, E. Highly sensitive and selective detection of cancer cell with a label-free electrochemical cytosensor. Biosens. Bioelectron. 2013, 41, 436–441. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Du, X.; Liu, M. Application of electrochemical biosensors in tumor cell detection. Thorac. Cancer 2020, 11, 840–850. [Google Scholar] [CrossRef]
- Sun, D.; Lu, J.; Zhang, L.; Chen, Z. Aptamer-based electrochemical cytosensors for tumor cell detection in cancer diagnosis: A review. Anal. Chim. Acta 2019, 1082, 1–17. [Google Scholar] [CrossRef]
- Soleymani, J.; Hasanzadeh, M.; Somi, M.H.; Shadjou, N.; Jouyban, A. Highly sensitive and specific cytosensing of HT 29 colorectal cancer cells using folic acid functionalized-KCC-1 nanoparticles. Biosens. Bioelectron. 2019, 132, 122–131. [Google Scholar] [CrossRef]
- Castillo, J.J.; Svendsen, W.E.; Rozlosnik, N.; Escobar, P.; Martínez, F.; Castillo-León, J. Detection of cancer cells using a peptide nanotube–folic acid modified graphene electrode. Analyst 2013, 138, 1026–1031. [Google Scholar] [CrossRef]
- Guo, Y.; Shu, Y.; Li, A.; Li, B.; Pi, J.; Cai, J.; Cai, H.-H.; Gao, Q. Efficient electrochemical detection of cancer cells on in situ surface-functionalized MoS2 nanosheets. J. Mat. Chem. B 2017, 5, 5532–5538. [Google Scholar] [CrossRef]
- Cao, Y.; Dai, Y.; Chen, H.; Tang, Y.; Chen, X.; Wang, Y.; Zhao, J.; Zhu, X. Integration of fluorescence imaging and electrochemical biosensing for both qualitative location and quantitative detection of cancer cells. Biosens. Bioelectron. 2019, 130, 132–138. [Google Scholar] [CrossRef]
- Alizadeh, N.; Salimi, A.; Hallaj, R.; Fathi, F.; Soleimani, F. CuO/WO3 nanoparticles decorated graphene oxide nanosheets with enhanced peroxidase-like activity for electrochemical cancer cell detection and targeted therapeutics. Mater. Sci. Eng. C 2019, 99, 1374–1383. [Google Scholar] [CrossRef]
- Tian, L.; Qi, J.; Qian, K.; Oderinde, O.; Liu, Q.; Yao, C.; Song, W.; Wang, Y. Copper (II) oxide nanozyme based electrochemical cytosensor for high sensitive detection of circulating tumor cells in breast cancer. J. Electroanal. Chem. 2018, 812, 1–9. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W.; Gholami, A. Gold nanostars-diagnosis, bioimaging and biomedical applications. Drug Metabol. Rev. 2020, 52, 299–318. [Google Scholar] [CrossRef]
- Tran, H.L.; Darmanto, W.; Doong, R.-A. Electrochemical immunosensor for ultra-sensitive detection of attomolar prostate specific antigen with sulfur-doped graphene quantum dot@gold nanostar as the probe. Electrochim. Acta 2021, 389, 138700. [Google Scholar] [CrossRef]
- Cui, X.; Wei, T.; Hao, M.; Qi, Q.; Wang, H.; Dai, Z. Highly sensitive and selective colorimetric sensor for thiocyanate based on electrochemical oxidation-assisted complexation reaction with Gold nanostars etching. J. Hazard. Mater. 2020, 391, 122217. [Google Scholar] [CrossRef]
- Zhang, A.; Guo, W.; Ke, H.; Zhang, X.; Zhang, H.; Huang, C.; Yang, D.; Jia, N.; Cui, D. Sandwich-format ECL immunosensor based on Au star@BSA-Luminol nanocomposites for determination of human chorionic gonadotropin. Biosens. Bioelectron. 2018, 101, 219–226. [Google Scholar] [CrossRef]
- Jin, G.H.; Ko, E.; Kim, M.K.; Tran, V.-K.; Son, S.E.; Geng, Y.; Hur, W.; Seong, G.H. Graphene oxide-gold nanozyme for highly sensitive electrochemical detection of hydrogen peroxide. Sens. Actuators B Chem. 2018, 274, 201–209. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, N.; Yu, W.-C.; Balram, D. Electrochemical detection of an antibiotic drug chloramphenicol based on a graphene oxide/hierarchical zinc oxide nanocomposite. Inorg. Chem. Front. 2019, 6, 82–93. [Google Scholar] [CrossRef]
- Wu, C.; Sun, D.; Li, Q.; Wu, K. Electrochemical sensor for toxic ractopamine and clenbuterol based on the enhancement effect of graphene oxide. Sens. Actuators B Chem. 2012, 168, 178–184. [Google Scholar] [CrossRef]
- Manavalan, S.; Ganesamurthi, J.; Chen, S.-M.; Veerakumar, P.; Murugan, K. A robust Mn@FeNi-S/graphene oxide nanocomposite as a high-efficiency catalyst for the non-enzymatic electrochemical detection of hydrogen peroxide. Nanoscale 2020, 12, 5961–5972. [Google Scholar] [CrossRef]
- Vinodha, G.; Shima, P.D.; Cindrella, L. Mesoporous magnetite nanoparticle-decorated graphene oxide nanosheets for efficient electrochemical detection of hydrazine. J. Mater. Sci. 2019, 54, 4073–4088. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Rao, Z.; Dhanjai; Wu, J.; Shen, Y.; Boddula, R.; Huang, Y. Electrochemical (bio) sensors go green. Biosens. Bioelectron. 2020, 163, 112270. [Google Scholar] [CrossRef]
- Arfin, T.; Rangari, S.N. Graphene oxide–ZnO nanocomposite modified electrode for the detection of phenol. Anal. Methods 2018, 10, 347–358. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Atty, S.A.; Merey, H.A.; Fattah, T.A.; Foster, C.W.; Banks, C.E. Titanium nanoparticles (TiO2)/graphene oxide nanosheets (GO): An electrochemical sensing platform for the sensitive and simultaneous determination of benzocaine in the presence of antipyrine. Analyst 2017, 142, 3674–3679. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Xu, L.; Zhou, C.; Xing, R.; Dai, Q.; Liu, D.; Song, H. Synthesis of Graphene Oxide Based CuO Nanoparticles Composite Electrode for Highly Enhanced Nonenzymatic Glucose Detection. ACS Appl. Mater. Interfaces 2013, 5, 12928–12934. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Meng, C. Template Fabrication of Amorphous Co2SiO4 Nanobelts/Graphene Oxide Composites with Enhanced Electrochemical Performances for Hybrid Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 3830–3839. [Google Scholar] [CrossRef]
- Ding, Y.-Z.; Zhang, Y.-D.; Shi, Y.-P. Polyaniline spinel particles with ultrahigh-performance liquid chromatography tandem mass spectrometry for rapid vitamin B9 determination in rice. Talanta 2022, 241, 123278. [Google Scholar] [CrossRef] [PubMed]
- Winiarski, J.P.; Rampanelli, R.; Bassani, J.C.; Mezalira, D.Z.; Jost, C.L. Multi-walled carbon nanotubes/nickel hydroxide composite applied as electrochemical sensor for folic acid (vitamin B9) in food samples. J. Food Compos. Anal. 2020, 92, 103511. [Google Scholar] [CrossRef]
- Ye, H.; Song, L.; Zhang, F.; Li, J.; Su, Z.; Zhang, Y. Highly Sensitive Electrochemical Detection of Folic Acid by Using a Hollow Carbon Nanospheres@molybdenum Disulfide Modified Electrode. Anal. Sci. 2021, 37, 575–580. [Google Scholar] [CrossRef]
- Marchetti, C.; Palaia, I.; Giorgini, M.; De Medici, C.; Iadarola, R.; Vertechy, L.; Domenici, L.; Di Donato, V.; Tomao, F.; Muzii, L.; et al. Targeted drug delivery via folate receptors in recurrent ovarian cancer: A review. Onco Targets Ther. 2014, 7, 1223–1236. [Google Scholar] [CrossRef] [Green Version]
- Correia, A.R.; Sampaio, I.; Comparetti, E.J.; Vieira, N.C.S.; Zucolotto, V. Optimized PAH/Folic acid layer-by-layer films as an electrochemical biosensor for the detection of folate receptors. Bioelectrochemistry 2021, 137, 107685. [Google Scholar] [CrossRef]
- Ruiyi, L.; Fangchao, C.; Haiyan, Z.; Xiulan, S.; Zaijun, L. Electrochemical sensor for detection of cancer cell based on folic acid and octadecylamine-functionalized graphene aerogel microspheres. Biosens. Bioelectron. 2018, 119, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Pan, F.; Liu, J.; Wang, K.; Zhang, C.; Cheng, S.; Lu, L.; Zhang, W.; Zhang, Z. Breath Analysis Based on Surface-Enhanced Raman Scattering Sensors Distinguishes Early and Advanced Gastric Cancer Patients from Healthy Persons. ACS Nano 2016, 10, 8169–8179. [Google Scholar] [CrossRef]
- Xue, T.; Wang, S.; Ou, G.; Li, Y.; Ruan, H.; Li, Z.; Ma, Y.; Zou, R.; Qiu, J.; Shen, Z.; et al. Detection of circulating tumor cells based on improved SERS-active magnetic nanoparticles. Anal. Methods 2019, 11, 2918–2928. [Google Scholar] [CrossRef]
- Zhang, A.; Huang, C.; Shi, H.; Guo, W.; Zhang, X.; Xiang, H.; Jia, T.; Miao, F.; Jia, N. Electrochemiluminescence immunosensor for sensitive determination of tumor biomarker CEA based on multifunctionalized Flower-like Au@BSA nanoparticles. Sens. Actuators B Chem. 2017, 238, 24–31. [Google Scholar] [CrossRef]
- Zhang, A.; Pan, S.; Zhang, Y.; Chang, J.; Cheng, J.; Huang, Z.; Li, T.; Zhang, C.; de la Fuentea, J.M.; Zhang, Q.; et al. Carbon-gold hybrid nanoprobes for real-time imaging, photothermal/photodynamic and nanozyme oxidative therapy. Theranostics 2019, 9, 3443–3458. [Google Scholar] [CrossRef]
- Chaiyakun, S.; Witit-anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Khoury, C.G.; Vo-Dinh, T. Gold Nanostars for Surface-Enhanced Raman Scattering: Synthesis, Characterization and Optimization. J. Phys. Chem. C 2008, 112, 18849–18859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esenturk, E.N.; Hight Walker, A.R. Gold nanostar @ iron oxide core–shell nanostructures: Synthesis, characterization, and demonstrated surface-enhanced Raman scattering properties. J. Nanopart. Res. 2012, 15, 1364. [Google Scholar] [CrossRef]
- Cheng, W.; Ding, L.; Lei, J.; Ding, S.; Ju, H. Effective Cell Capture with Tetrapeptide-Functionalized Carbon Nanotubes and Dual Signal Amplification for Cytosensing and Evaluation of Cell Surface Carbohydrate. Anal. Chem. 2008, 80, 3867–3872. [Google Scholar] [CrossRef]
- Ding, L.; Hao, C.; Xue, Y.; Ju, H. A Bio-Inspired Support of Gold Nanoparticles-Chitosan Nanocomposites Gel for Immobilization and Electrochemical Study of K562 Leukemia Cells. Biomacromolecules 2007, 8, 1341–1346. [Google Scholar] [CrossRef]
- Zheng, T.-T.; Zhang, R.; Zou, L.; Zhu, J.-J. A label-free cytosensor for the enhanced electrochemical detection of cancer cells using polydopamine-coated carbon nanotubes. Analyst 2012, 137, 1316–1318. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Zheng, T.-T.; Cheng, F.-F.; Zhang, J.-R.; Zhu, J.-J. Toward the Early Evaluation of Therapeutic Effects: An Electrochemical Platform for Ultrasensitive Detection of Apoptotic Cells. Anal. Chem. 2011, 83, 7902–7909. [Google Scholar] [CrossRef]
- Nie, G.; Bai, Z.; Yu, W.; Chen, J. Electrochemiluminescence Biosensor Based on Conducting Poly(5-formylindole) for Sensitive Detection of Ramos Cells. Biomacromolecules 2013, 14, 834–840. [Google Scholar] [CrossRef]
- Tao, Y.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Incorporating Graphene Oxide and Gold Nanoclusters: A Synergistic Catalyst with Surprisingly High Peroxidase-Like Activity Over a Broad pH Range and its Application for Cancer Cell Detection. Adv. Mater. 2013, 25, 2594–2599. [Google Scholar] [CrossRef]
- Yang, G.; Cao, J.; Li, L.; Rana, R.K.; Zhu, J.-J. Carboxymethyl chitosan-functionalized graphene for label-free electrochemical cytosensing. Carbon 2013, 51, 124–133. [Google Scholar] [CrossRef]
- Jing, A.; Zhang, C.; Liang, G.; Feng, W.; Tian, Z.; Jing, C. Hyaluronate-Functionalized Graphene for Label-Free Electrochemical Cytosensing. Micromachines 2018, 9, 669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
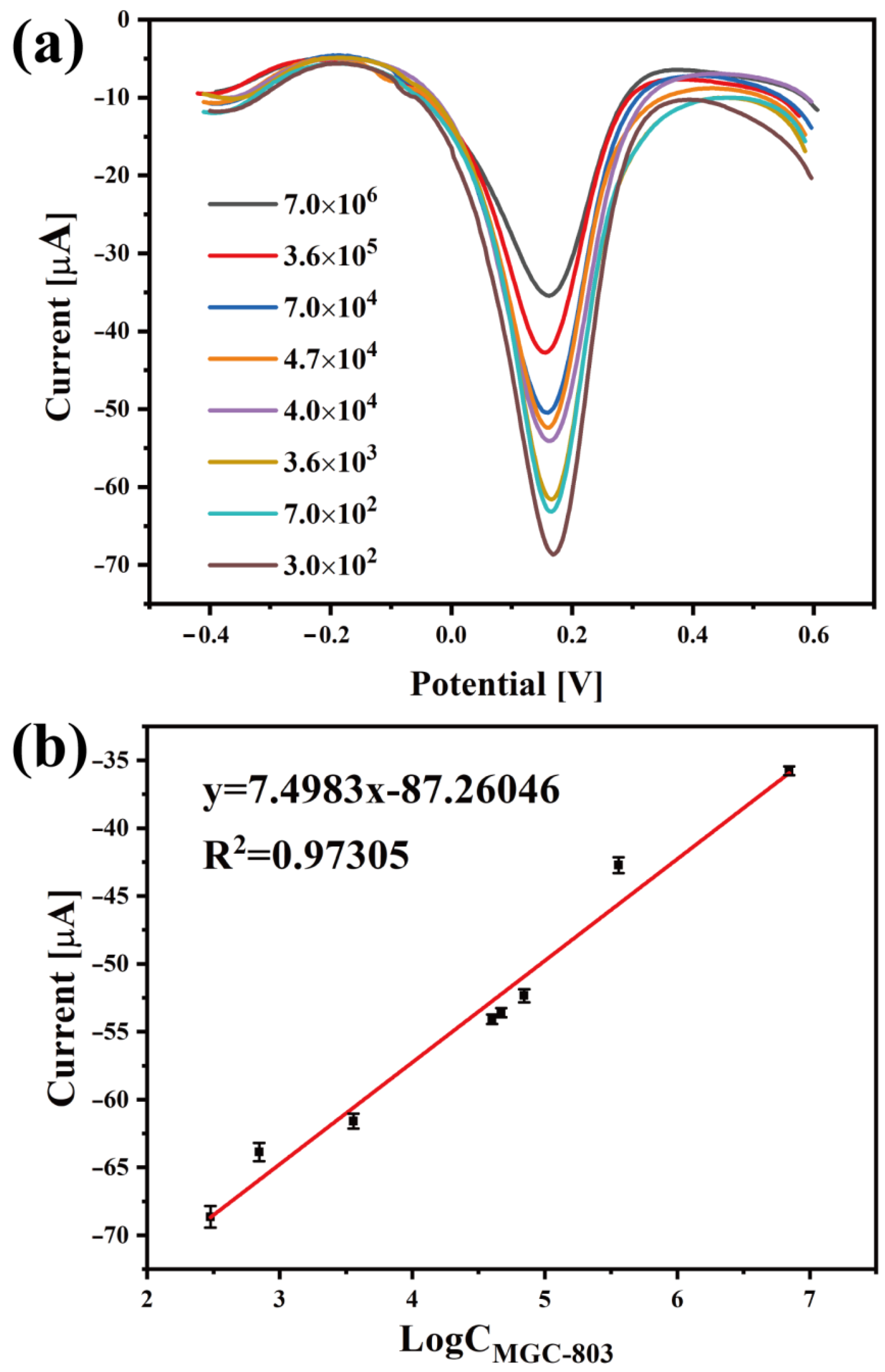
| Concentration of MGC-803 Cells (Cell/mL) | 3 × 102 | 7 × 102 | 3.6 × 103 | 4 × 104 | 4.7 × 104 | 7 × 104 | 3.6 × 105 | 7 × 106 |
|---|---|---|---|---|---|---|---|---|
| The maximum value (μA) | 68.65 | 63.87 | 61.59 | 54.09 | 53.6 | 52.35 | 42.72 | 35.78 |
| Detection Technique | Nanomaterials | Linear Range (Cell/mL) | Detection Limit (Cell/mL) | References |
|---|---|---|---|---|
| DPV | Peptides–single-walled carbon nanotubes | 1.0 × 103~1.0 × 107 | 620 | [44] |
| EIS, CV | AuNPs–chitosan | 1.34 × 104~1.34 × 108 | 871 | [45] |
| EIS | CNTs@PDA-FA | 5 × 102~5 × 106 | 500 | [46] |
| ASV | SiO2@QDs–ConA | 1 × 103~1 × 107 | 1000 | [47] |
| ECL | Ru–DNA–AuNPs | 5 × 102~1 × 105 | 300 | [48] |
| Colorimetric | GO-AuNCs | 1 × 103~2 × 105 | - | [49] |
| EIS | FA/PEI/CMC-G | 5 × 102~5 × 106 | 500 | [50] |
| EIS | Hyaluronate/graphene | 5 × 102~5 × 106 | 100 | [51] |
| DPV | GO-AuNSs@ rBSA-FA | 3 × 102~7 × 106 | 100 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Liu, Q.; Huang, Z.; Zhang, Q.; Wang, R.; Cui, D. Electrochemical Cytosensor Based on a Gold Nanostar-Decorated Graphene Oxide Platform for Gastric Cancer Cell Detection. Sensors 2022, 22, 2783. https://doi.org/10.3390/s22072783
Zhang A, Liu Q, Huang Z, Zhang Q, Wang R, Cui D. Electrochemical Cytosensor Based on a Gold Nanostar-Decorated Graphene Oxide Platform for Gastric Cancer Cell Detection. Sensors. 2022; 22(7):2783. https://doi.org/10.3390/s22072783
Chicago/Turabian StyleZhang, Amin, Qianwen Liu, Zhicheng Huang, Qian Zhang, Ruhao Wang, and Daxiang Cui. 2022. "Electrochemical Cytosensor Based on a Gold Nanostar-Decorated Graphene Oxide Platform for Gastric Cancer Cell Detection" Sensors 22, no. 7: 2783. https://doi.org/10.3390/s22072783
APA StyleZhang, A., Liu, Q., Huang, Z., Zhang, Q., Wang, R., & Cui, D. (2022). Electrochemical Cytosensor Based on a Gold Nanostar-Decorated Graphene Oxide Platform for Gastric Cancer Cell Detection. Sensors, 22(7), 2783. https://doi.org/10.3390/s22072783






