Identification of the Principle of Taste Sensors to Detect Non-Charged Bitter Substances by 1H-NMR Measurement
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Measuring Caffeine in Taste Sensors Using Various HBAs
2.3. 1H NMR Measurement of Caffeine and Each Substance
3. Results and Discussion
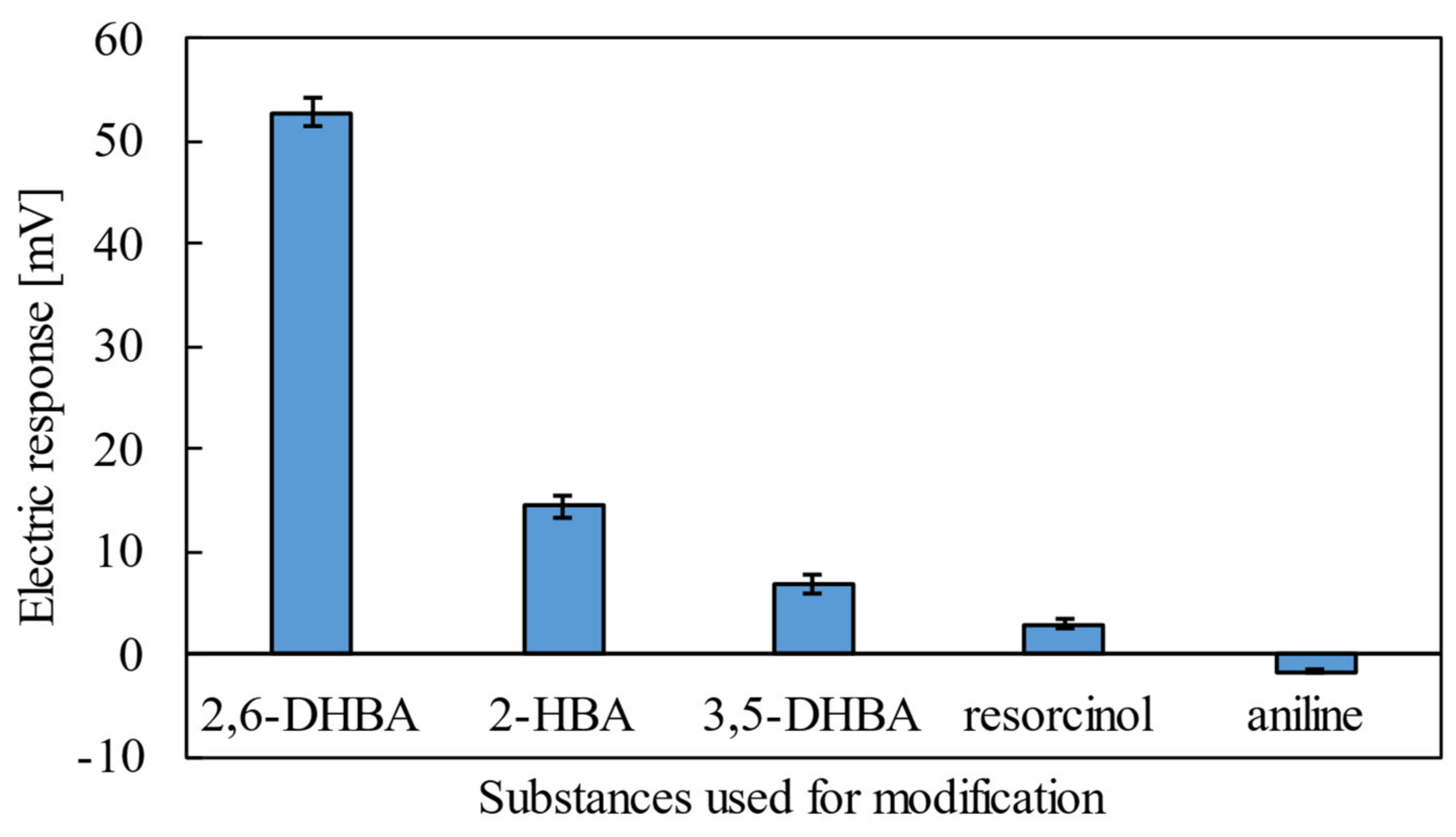
3.1. Response of Taste Sensor
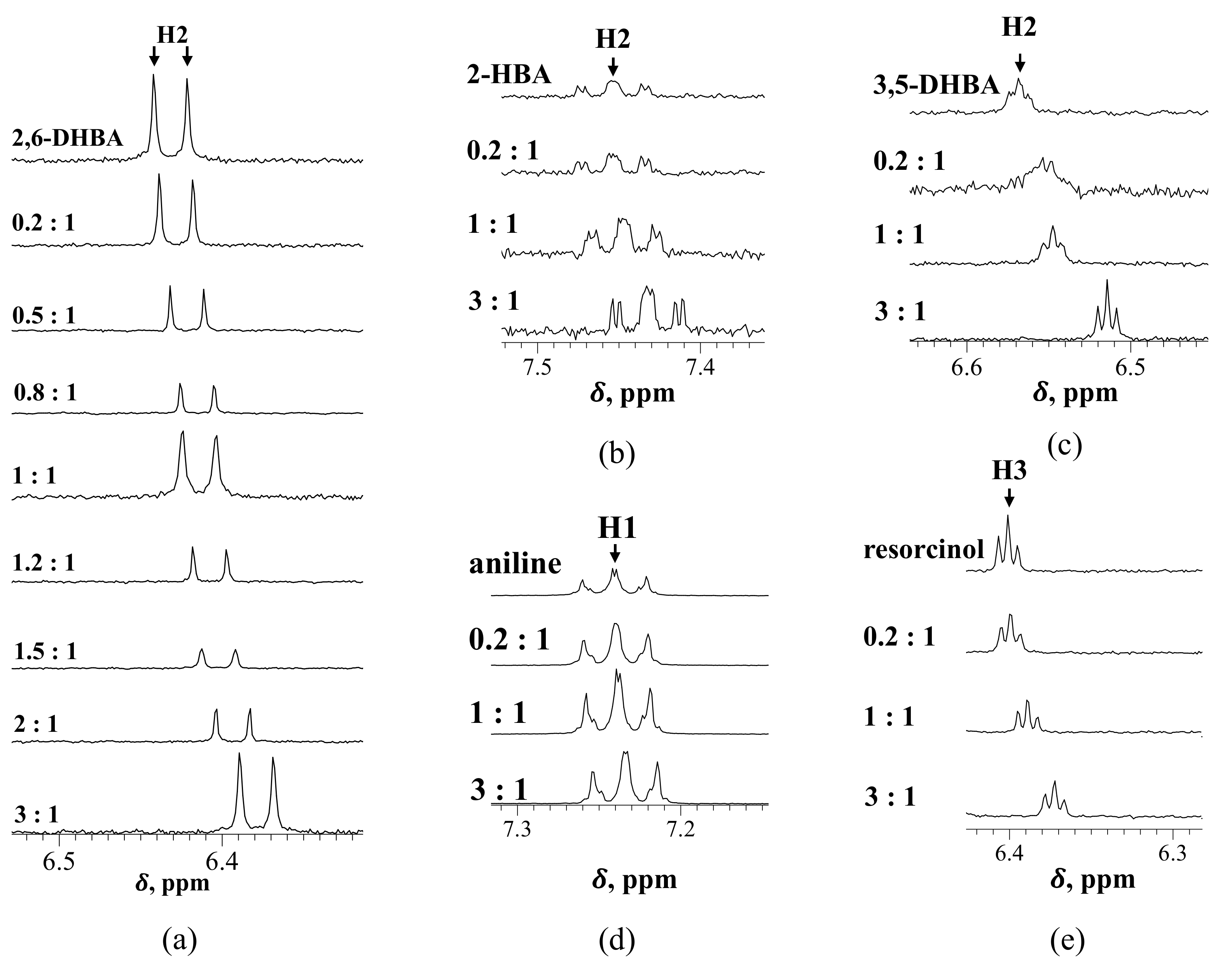
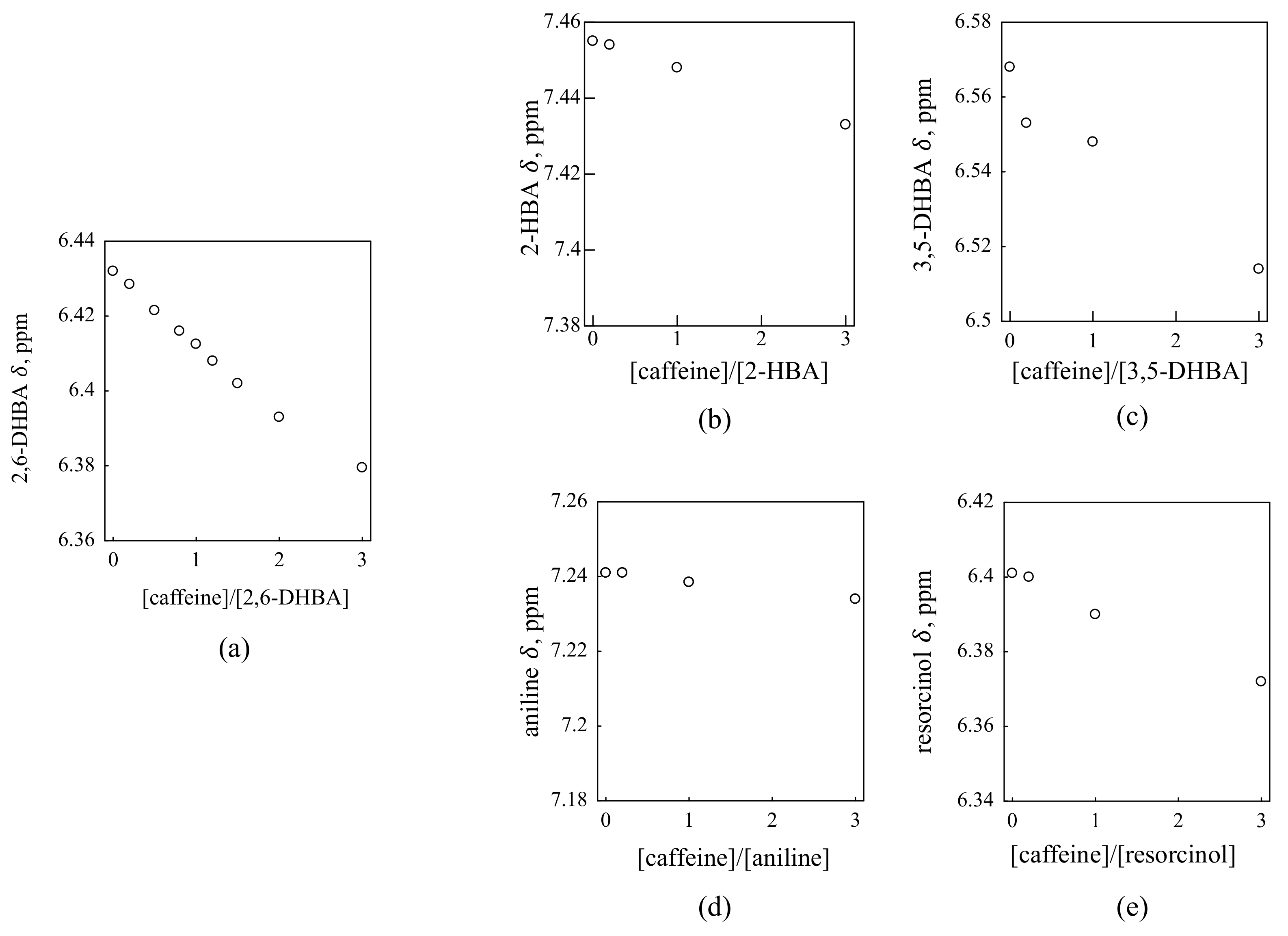
3.2. Investigation of the Interaction between HBA and Caffeine by 1H NMR
3.3. Comparison of Taste Sensor Results and 1H NMR Results
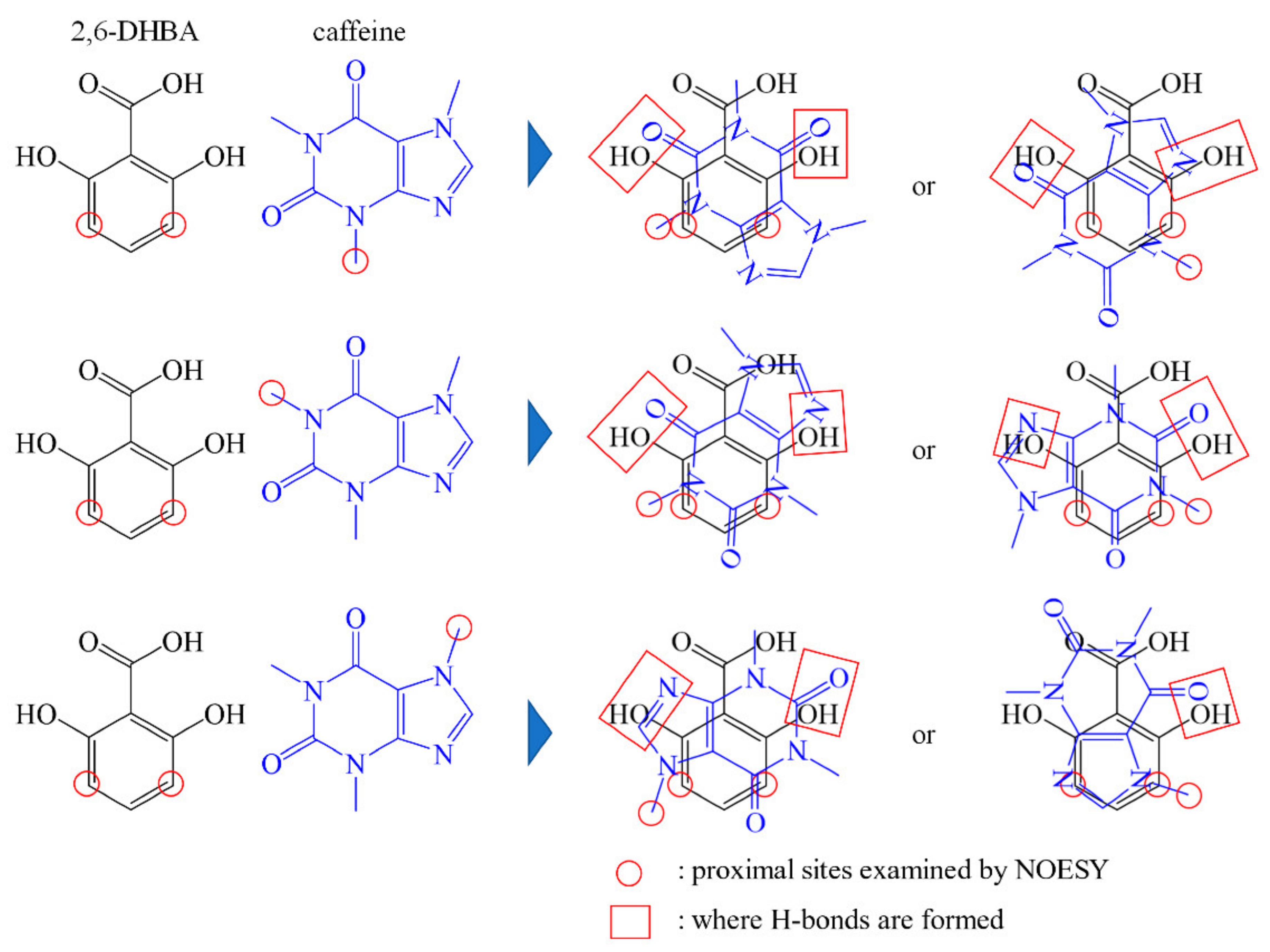
3.4. Prediction of the Binding form of 2,6-DHBA to Caffeine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Riul, A.; Correa, D.S. A First Taste to Electronic Tongues. In Electronic Tongues; Flavio, M.S., Maria, L.B.A.R., Jr., Eds.; IOP: Bristol, UK, 2021; pp. 1-1–1-4. ISBN 978-0-7503-3687-1. [Google Scholar]
- Wu, X.; Tahara, Y.; Yatabe, R.; Toko, K. Taste Sensor: Electronic Tongue with Lipid Membranes. Anal. Sci. 2020, 36, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Toko, K.; Tahara, Y.; Habara, M.; Kobayashi, Y.; Ikezaki, H. Taste Sensor: Electronic Tongue with Global Selectivity. In Essentials of Machine Olfaction and Taste; Wiley: Hoboken, NJ, USA, 2016; pp. 87–174. ISBN 9781118768495. [Google Scholar]
- Nuñez, L.; Cetó, X.; Pividori, M.I.; Zanoni, M.V.B.; del Valle, M. Development and Application of an Electronic Tongue for Detection and Monitoring of Nitrate, Nitrite and Ammonium Levels in Waters. Microchem. J. 2013, 110, 273–279. [Google Scholar] [CrossRef]
- Tahara, Y.; Toko, K. Electronic Tongues—A Review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A Comparative Study on Two Electronic Tongues for Pharmaceutical Formulation Development. J. Pharm. Biomed. Anal. 2011, 55, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, P.; Wróblewski, W. Sensor Arrays for Liquid Sensing—Electronic Tongue Systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Toko, K. Taste Sensor. Sens. Actuators B Chem. 2000, 64, 205–215. [Google Scholar] [CrossRef]
- Fujimoto, H.; Narita, Y.; Iwai, K.; Hanzawa, T.; Kobayashi, T.; Kakiuchi, M.; Ariki, S.; Wu, X.; Miyake, K.; Tahara, Y.; et al. Bitterness Compounds in Coffee Brew Measured by Analytical Instruments and Taste Sensing System. Food Chem. 2021, 342, 128228. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Miyake, K.; Tahara, Y.; Fujimoto, H.; Iwai, K.; Narita, Y.; Hanzawa, T.; Kobayashi, T.; Kakiuchi, M.; Ariki, S.; et al. Quantification of Bitterness of Coffee in the Presence of High-Potency Sweeteners Using Taste Sensors. Sens. Actuators B Chem. 2020, 309, 127784. [Google Scholar] [CrossRef]
- Yoshimatsu, J.; Toko, K.; Tahara, Y.; Ishida, M.; Habara, M.; Ikezaki, H.; Kojima, H.; Ikegami, S.; Yoshida, M.; Uchida, T. Development of Taste Sensor to Detect Non-Charged Bitter Substances. Sensors 2020, 20, 3455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ishida, M.; Onodera, T.; Toko, K. Effect of Hydroxybenzoic Acids on Caffeine Detection Using Taste Sensor with Lipid/polymer Membranes. Sensors 2022, 22, 1607. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Habara, M.; Toko, K. Development of Caffeine Detection Using Taste Sensor with Lipid/Polymer Membranes. Sens. Mater. 2008, 20, 171–178. [Google Scholar] [CrossRef]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Nagana Gowda, G.A.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Bak, J.; Miyazaki, Y.; Nakano, H.; Matsui, T. Ligand-aided 1H Nuclear Magnetic Resonance Spectroscopy for Non-destructive Estimation of Sulfate Content in Sulfated Saccharides. Anal. Sci. 2020, 36, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Ihm, H.; Yun, S.; Kim, H.G.; Kim, J.K.; Kim, K.S. Tripodal Nitro-Imidazolium Receptor for Anion Binding Driven by (C-H) + X-Hydrogen Bonds. Org. Lett. 2002, 4, 2897–2900. [Google Scholar] [CrossRef] [PubMed]
- Bodenhausen, G.; Kogler, H.; Ernst, R.R. Selection of Coherence-Transfer Pathways in NMR Pulse Experiments. J. Magn. Reson. 1984, 58, 370–388. [Google Scholar] [CrossRef][Green Version]
- States, D.J.; Haberkorn, R.A.; Ruben, D.J. A 2D Nuclear Overhauser Experiment with Pure Absorption Phase in Four Quadrants. J. Magn. Reson. 1982, 48, 286–292. [Google Scholar] [CrossRef]
- Jeener, J.; Meier, B.H.; Bachmann, P.; Ernst, R.R. Investigation of Exchange Processes by Two-Dimensional NMR Spectroscopy. J. Chem. Phys. 1979, 71, 4546–4553. [Google Scholar] [CrossRef]
- Liao, X.; Gautam, M.; Grill, A.; Zhu, H.J. Effect of Position Isomerism on the Formation and Physicochemical Properties of Pharmaceutical Co-crystals. J. Pharm. Sci. 2010, 99, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Bučar, D.K.; Henry, R.F.; Lou, X.; Duerst, R.W.; MacGillivray, L.R.; Zhang, G.G.Z. Cocrystals of Caffeine and Hydroxybenzoic Acids Composed of Multiple Supramolecular Heterosynthons: Screening via Solution-Mediated Phase Transformation and Structural Characterization. Cryst. Growth Des. 2009, 9, 1932–1943. [Google Scholar] [CrossRef]

| Taste Sensor Response | Interactions Investigated by 1H NMR | |
|---|---|---|
| 2,6-DHBA | 52 mV | Yes |
| 2-HBA | 15 mV | Yes |
| 3,5-DHBA | 7 mV | Yes |
| Resorcinol | 3 mV | Yes |
| Aniline | −2 mV | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, M.; Ide, H.; Arima, K.; Zhao, Z.; Matsui, T.; Toko, K. Identification of the Principle of Taste Sensors to Detect Non-Charged Bitter Substances by 1H-NMR Measurement. Sensors 2022, 22, 2592. https://doi.org/10.3390/s22072592
Ishida M, Ide H, Arima K, Zhao Z, Matsui T, Toko K. Identification of the Principle of Taste Sensors to Detect Non-Charged Bitter Substances by 1H-NMR Measurement. Sensors. 2022; 22(7):2592. https://doi.org/10.3390/s22072592
Chicago/Turabian StyleIshida, Misaki, Haruna Ide, Keishiro Arima, Zeyu Zhao, Toshiro Matsui, and Kiyoshi Toko. 2022. "Identification of the Principle of Taste Sensors to Detect Non-Charged Bitter Substances by 1H-NMR Measurement" Sensors 22, no. 7: 2592. https://doi.org/10.3390/s22072592
APA StyleIshida, M., Ide, H., Arima, K., Zhao, Z., Matsui, T., & Toko, K. (2022). Identification of the Principle of Taste Sensors to Detect Non-Charged Bitter Substances by 1H-NMR Measurement. Sensors, 22(7), 2592. https://doi.org/10.3390/s22072592







