High Specificity of Single Inertial Sensor-Supplemented Timed Up and Go Test for Assessing Fall Risk in Elderly Nursing Home Residents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Protocol
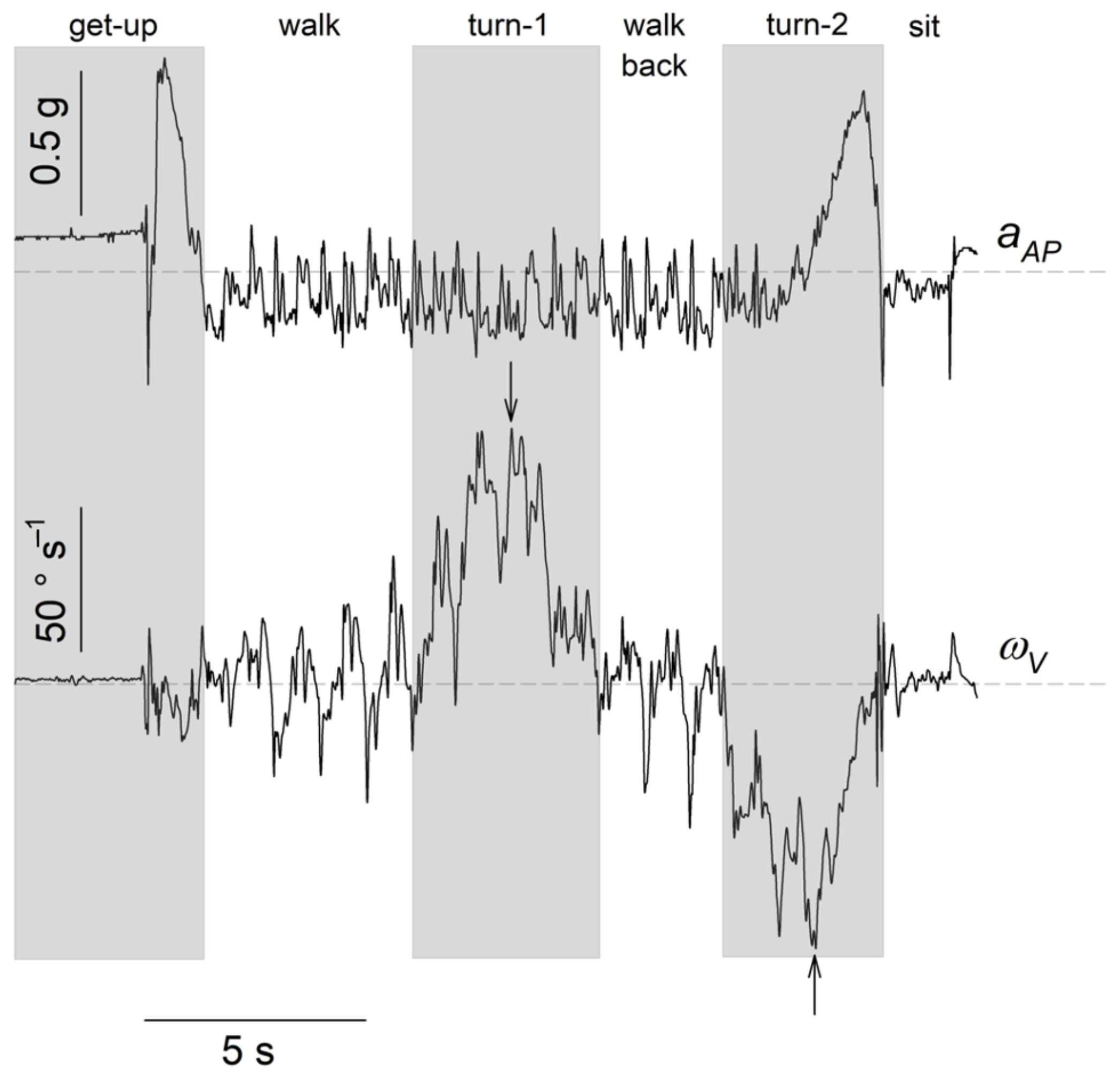
2.3. Division of the TUG into Subtasks and Selected Kinematic Parameters
2.4. Statistical Analysis
- (M0) only parameter used when comparing F and NF groups (TUG in Table 2);
- (M1) all parameters with used when comparing F and NF groups (kinTUG in Table 2): ;
- (M2) all parameters with used when comparing F and NF groups (iTUG in Table 2): and = ;
- (M3) all parameters with used when comparing F and NF groups (i + TUG in Table 2): , , = Walking aid required (Yes = 1, No = 0) and = Gender (M = 1, F = 0);
- (M4) all parameters with when comparing F and NF groups (i + TUG2 in Table 2): , = , = Walking aid required, = Gender, = and = .
3. Results
3.1. Population
3.2. F versus NF Comparison
3.3. Multiple Logistic Regressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Falls. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/falls (accessed on 2 January 2022).
- Rubenstein, L.Z. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age Ageing 2006, 35, ii37–ii41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [PubMed] [Green Version]
- Schoene, D.; Wu, S.M.S.; Mikolaizak, A.S.; Menant, J.C.; Smith, S.T.; Delbaere, K.; Lord, S.R. Discriminative Ability and Predictive Validity of the Timed Up and Go Test in Identifying Older People Who Fall: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2013, 61, 202–208. [Google Scholar] [CrossRef]
- Pettersson, B.; Nordin, E.; Ramnemark, A.; Lundin-Olsson, L. Neither Timed Up and Go test nor Short Physical Performance Battery predict future falls among independent adults aged ≥75 years living in the community. J. Frailty Sarcopenia Falls 2020, 5, 24–30. [Google Scholar] [CrossRef]
- Buisseret, F.; Catinus, L.; Grenard, R.; Jojczyk, L.; Fievez, D.; Barvaux, V.; Dierick, F. Timed Up and Go and Six-Minute Walking Tests with Wearable Inertial Sensor: One Step Further for the Prediction of the Risk of Fall in Elderly Nursing Home People. Sensors 2020, 20, 3207. [Google Scholar] [CrossRef]
- Kurosawa, C.; Shimadu, N.; Yamamoto, S. Where do healthy older adults take more time during the Timed Up and Go test? J. Phys. Ther. Sci. 2020, 32, 663–668. [Google Scholar] [CrossRef]
- Salarian, A.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Aminian, K. iTUG, a Sensitive and Reliable Measure of Mobility. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Vervoort, D.; Vuillerme, N.; Kosse, N.; Hortobágyi, T.; Lamoth, C.J.C. Multivariate Analyses and Classification of Inertial Sensor Data to Identify Aging Effects on the Timed-Up-and-Go Test. PLoS ONE 2016, 11, e0155984. [Google Scholar] [CrossRef] [Green Version]
- Bergquist, R.; Nerz, C.; Taraldsen, K.; Mellone, S.; Ihlen, E.A.; Vereijken, B.; Helbostad, J.L.; Becker, C.; Mikolaizak, A.S. Predicting Advanced Balance Ability and Mobility with an Instrumented Timed Up and Go Test. Sensors 2020, 20, 4987. [Google Scholar] [CrossRef]
- Beyea, J.; McGibbon, C.A.; Sexton, A.; Noble, J.; O’Connell, C. Convergent Validity of a Wearable Sensor System for Measuring Sub-Task Performance during the Timed Up-and-Go Test. Sensors 2017, 17, 934. [Google Scholar] [CrossRef] [Green Version]
- Mangano, G.R.; Valle, M.S.; Casabona, A.; Vagnini, A.; Cioni, M. Age-Related Changes in Mobility Evaluated by the Timed Up and Go Test Instrumented through a Single Sensor. Sensors 2020, 20, 719. [Google Scholar] [CrossRef] [Green Version]
- Cimolin, V.; Cau, N.; Malchiodi Albedi, G.; Aspesi, V.; Merenda, V.; Galli, M.; Capodaglio, P. Do wearable sensors add meaningful information to the Timed Up and Go test? A study on obese women. J. Electromyogr. Kinesiol. 2019, 44, 78–85. [Google Scholar] [CrossRef]
- Newman, M.A.; Hirsch, M.A.; Peindl, R.D.; Habet, N.A.; Tsai, T.J.; Runyon, M.S.; Huynh, T.; Phillips, C.; Zheng, N. Use of an instrumented dual-task timed up and go test in children with traumatic brain injury. Gait Posture 2020, 76, 193–197. [Google Scholar] [CrossRef]
- Kim, K.J.; Gimmon, Y.; Millar, J.; Brewer, K.; Serrador, J.; Schubert, M.C. The Instrumented Timed “Up and Go” Test Distinguishes Turning Characteristics in Vestibular Hypofunction. Phys. Ther. 2021, 101, pzab103. [Google Scholar] [CrossRef]
- Caronni, A.; Sterpi, I.; Antoniotti, P.; Aristidou, E.; Nicolaci, F.; Picardi, M.; Pintavalle, G.; Redaelli, V.; Achille, G.; Sciumè, L.; et al. Criterion validity of the instrumented Timed Up and Go test: A partial least square regression study. Gait Posture 2018, 61, 287–293. [Google Scholar] [CrossRef]
- Dite, W.; Temple, V.A. Development of a Clinical Measure of Turning for Older Adults. Am. J. Phys. Med. Rehabil. 2002, 81, 857–866. [Google Scholar] [CrossRef]
- Leach, J.M.; Mellone, S.; Palumbo, P.; Bandinelli, S.; Chiari, L. Natural turn measures predict recurrent falls in community-dwelling older adults: A longitudinal cohort study. Sci. Rep. 2018, 8, 4316. [Google Scholar] [CrossRef] [Green Version]
- Mancini, M.; Schlueter, H.; El-Gohary, M.; Mattek, N.; Duncan, C.; Kaye, J.; Horak, F.B. Continuous Monitoring of Turning Mobility and Its Association to Falls and Cognitive Function: A Pilot Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Skrba, Z.; O’Mullane, B.; Greene, B.; Scanaill, C.; Fan, C.W.; Quigley, A.; Nixon, P. Objective real-time assessment of walking and turning in elderly adults. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Berlin, Germany, 23–27 July 2009. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Melendez-Calderon, A.; Burdet, E. A Robust and Sensitive Metric for Quantifying Movement Smoothness. IEEE Trans. Biomed. Eng. 2012, 59, 2126–2136. [Google Scholar] [CrossRef]
- Hodkinson, H.M. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972, 1, 233–238. [Google Scholar] [CrossRef]
- Stapleton, C.; Hough, P.; Oldmeadow, L.; Bull, K.; Hill, K.; Greenwood, K. Four-item fall risk screening tool for subacute and residential aged care: The first step in fall prevention. Australas. J. Ageing 2009, 28, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Incalzi, R.A.; Cesari, M.; Pedone, C.; Carosella, L.; Carbonin, P. Construct Validity of the Abbreviated Mental Test in Older Medical Inpatients. Dement. Geriatr. Cogn. Disord. 2003, 15, 199–206. [Google Scholar] [CrossRef]
- Steffen, T.M.; Hacker, T.A.; Mollinger, L. Age- and Gender-Related Test Performance in Community-Dwelling Elderly People: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and Gait Speeds. Phys. Ther. 2002, 82, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Zarzeczny, R.; Nawrat-Szołtysik, A.; Polak, A.; Maliszewski, J.; Kiełtyka, A.; Matyja, B.; Dudek, M.; Zborowska, J.; Wajdman, A. Aging effect on the instrumented Timed-Up-and-Go test variables in nursing home women aged 80–93 years. Biogerontology 2017, 18, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.; Galvin, R.; Keogh, C.; Horgan, F.; Fahey, T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta- analysis. BMC Geriatr. 2014, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Soto-Varela, A.; Rossi-Izquierdo, M.; del Río-Valeiras, M.; Faraldo-García, A.; Vaamonde-Sánchez-Andrade, I.; Lirola-Delgado, A.; Santos-Pérez, S. Modified Timed Up and Go Test for Tendency to Fall and Balance Assessment in Elderly Patients with Gait Instability. Front. Neurol. 2020, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chou, L.S. Effects of Muscle Strength and Balance Control on Sit-to-Walk and Turn Durations in the Timed Up and Go Test. Arch. Phys. Med. Rehabil. 2017, 98, 2471–2476. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Weiss, A.; Buchman, A.S.; Bennett, D.A.; Giladi, N.; Hausdorff, J.M. Association Between Performance on Timed Up and Go Subtasks and Mild Cognitive Impairment: Further Insights into the Links Between Cognitive and Motor Function. J. Am. Geriatr. Soc. 2014, 62, 673–678. [Google Scholar] [CrossRef]
- Ambrose, A.F.; Paul, G.; Hausdorff, J.M. Risk factors for falls among older adults: A review of the literature. Maturitas 2013, 75, 51–61. [Google Scholar] [CrossRef]
- Oliver, D. Risk factors and risk assessment tools for falls in hospital in-patients: A systematic review. Age Ageing 2004, 33, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Martinez, B.; Gomes, I.; Oliveira, C.; Ramos, I.; Rocha, M.; Forgiarini, L.A., Jr.; Camelier, F.; Camelier, A. Accuracy of the Timed Up and Go test for predicting sarcopenia in elderly hospitalized patients. Clinics 2015, 70, 369–372. [Google Scholar] [CrossRef]
- Pijnappels, M.; van der Burg, P.J.C.E.; Reeves, N.D.; van Dieën, J.H. Identification of elderly fallers by muscle strength measures. Eur. J. Appl. Physiol. 2007, 102, 585–592. [Google Scholar] [CrossRef] [Green Version]
| Parameter | F | NF | p |
|---|---|---|---|
| Residents (n) | 24 | 49 | |
| Age (years) | 0.646 | ||
| 66–96 | 65–96 | ||
| Medication | 4 [2–5] | 3 [2–5] | |
| FRAT | 11 [10–14] | 10 [8–12] | |
| (s) | 24.5 ± 8.5 | 21.5 ± 8.1 | 0.096 |
| Gender (M/F) | 6/18 | 22/27 | 0.128 |
| Walking aid required (Yes/No) | 15/9 | 21/28 | 0.140 |
| Post-stroke hemiparesis (Yes/No) | 2/21 | 4/45 | 0.677 |
| Possibility of dementia (Yes/No) | 3/21 | 9/40 | 0.739 |
| Alzheimer disease (Yes/No) | 5/19 | 14/35 | 0.577 |
| Previous heart surgery (Yes/No) | 8/16 | 9/40 | 0.238 |
| Diabetic polyneuropathy (Yes/No) | 3/21 | 8/41 | 1 |
| Hip or knee replacement (Yes/No) | 3/21 | 9/40 | 0.739 |
| Parameters | M0 | M1 | M2 | M3 | M4 |
|---|---|---|---|---|---|
| (TUG) | (kinTUG) | (iTUG) | (i + TUG) | (i + TUG2) | |
| −1.709 | 1.423 | 0.822 | 1.207 | 1.553 | |
| −0.0245 | −0.0213 | −0.0208 | −0.0231 | ||
| 0.0434 | 0.0139 | −0.0046 | 0.0197 | ||
| Walking aid required | 0.403 | 0.333 | |||
| Gender | −0.637 | −0.649 | |||
| −0.0027 | |||||
| − 0.124 | |||||
| Performance Indicators | |||||
| (%) | 8.3 | 8.3 | 12.5 | 29.2 | 20.8 |
| (%) | 95.9 | 91.8 | 91.8 | 95.9 | 91.8 |
| (%) | 67.1 | 64.4 | 65.7 | 74.0 | 68.5 |
| Parameters | F | NF | p |
|---|---|---|---|
| (° s) | 0.031 | ||
| 0.203 | |||
| (s) | 0.293 | ||
| 0.304 | |||
| (° s) | 0.315 | ||
| (s) | 0.338 | ||
| (s) | 0.545 | ||
| (s) | 0.569 | ||
| (s) | 0.634 | ||
| (s) | 0.773 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dierick, F.; Stoffel, P.-L.; Schütz, G.; Buisseret, F. High Specificity of Single Inertial Sensor-Supplemented Timed Up and Go Test for Assessing Fall Risk in Elderly Nursing Home Residents. Sensors 2022, 22, 2339. https://doi.org/10.3390/s22062339
Dierick F, Stoffel P-L, Schütz G, Buisseret F. High Specificity of Single Inertial Sensor-Supplemented Timed Up and Go Test for Assessing Fall Risk in Elderly Nursing Home Residents. Sensors. 2022; 22(6):2339. https://doi.org/10.3390/s22062339
Chicago/Turabian StyleDierick, Frédéric, Pierre-Loup Stoffel, Gaston Schütz, and Fabien Buisseret. 2022. "High Specificity of Single Inertial Sensor-Supplemented Timed Up and Go Test for Assessing Fall Risk in Elderly Nursing Home Residents" Sensors 22, no. 6: 2339. https://doi.org/10.3390/s22062339
APA StyleDierick, F., Stoffel, P.-L., Schütz, G., & Buisseret, F. (2022). High Specificity of Single Inertial Sensor-Supplemented Timed Up and Go Test for Assessing Fall Risk in Elderly Nursing Home Residents. Sensors, 22(6), 2339. https://doi.org/10.3390/s22062339







