The Immediate Carryover Effects of Peroneal Functional Electrical Stimulation Differ between People with and without Chronic Ankle Instability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Ankle Kinematics
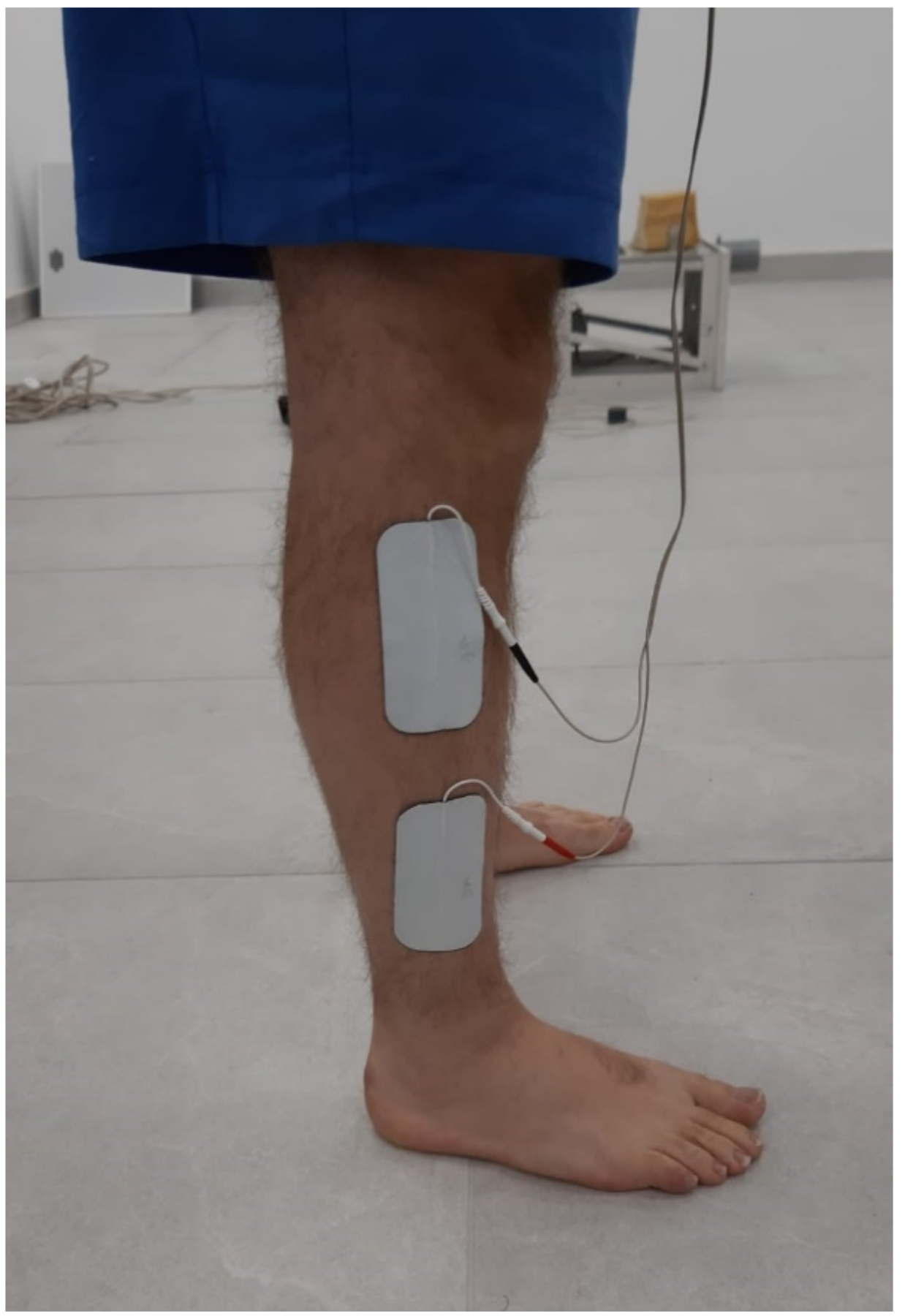
2.4. Peroneal Activity
2.5. Peroneal FES
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Ankle Kinematics
3.2. Peroneal Activity
3.3. Secondary Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hertel, J.; Corbett, R.O. An Updated Model of Chronic Ankle Instability. J. Athl. Train. 2019, 54, 572–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, M.S.; Chan, K.M.; So, C.H.; Yuan, W.Y. An Epidemiological Survey on Ankle Sprain. Br. J. Sports Med. 1994, 28, 112–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, C.; Bleakley, C.; Hertel, J.; Caulfield, B.; Ryan, J.; Delahunt, E. Recovery From a First-Time Lateral Ankle Sprain and the Predictors of Chronic Ankle Instability: A Prospective Cohort Analysis. Am. J. Sports Med. 2016, 44, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Arnold, B.L.; Wright, C.J.; Ross, S.E. Functional Ankle Instability and Health-Related Quality of Life. J. Athl. Train. 2011, 46, 634–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houston, M.N.; Van Lunen, B.L.; Hoch, M.C. Health-Related Quality of Life in Individuals with Chronic Ankle Instability. J. Athl. Train. 2014, 49, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Houston, M.N.; Hoch, J.M.; Gabriner, M.L.; Kirby, J.L.; Hoch, M.C. Clinical and Laboratory Measures Associated with Health-Related Quality of Life in Individuals with Chronic Ankle Instability. Phys. Ther. Sport Off. J. Assoc. Chart. Physiother. Sports Med. 2015, 16, 169–175. [Google Scholar] [CrossRef]
- Houston, M.N.; Hoch, J.M.; Hoch, M.C. Patient-Reported Outcome Measures in Individuals With Chronic Ankle Instability: A Systematic Review. J. Athl. Train. 2015, 50, 1019–1033. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.E.; Docherty, C.L. Current Health-Related Quality of Life Is Lower in Former Division I Collegiate Athletes than in Non-Collegiate Athletes. Am. J. Sports Med. 2014, 42, 423–429. [Google Scholar] [CrossRef]
- Konradsen, L.; Bech, L.; Ehrenbjerg, M.; Nickelsen, T. Seven Years Follow-up after Ankle Inversion Trauma. Scand. J. Med. Sci. Sports 2002, 12, 129–135. [Google Scholar] [CrossRef]
- Hubbard-Turner, T.; Turner, M.J. Physical Activity Levels in College Students With Chronic Ankle Instability. J. Athl. Train. 2015, 50, 742–747. [Google Scholar] [CrossRef] [Green Version]
- Wikstrom, E.A.; Hubbard-Turner, T.; Woods, S.; Guderian, S.; Turner, M.J. Developing a Mouse Model of Chronic Ankle Instability. Med. Sci. Sports Exerc. 2015, 47, 866–872. [Google Scholar] [CrossRef]
- Verhagen, R.A.; de Keizer, G.; van Dijk, C.N. Long-Term Follow-up of Inversion Trauma of the Ankle. Arch. Orthop. Trauma Surg. 1995, 114, 92–96. [Google Scholar] [CrossRef]
- Valderrabano, V.; Horisberger, M.; Russell, I.; Dougall, H.; Hintermann, B. Etiology of Ankle Osteoarthritis. Clin. Orthop. 2009, 467, 1800–1806. [Google Scholar] [CrossRef] [Green Version]
- Valderrabano, V.; Pagenstert, G.; Horisberger, M.; Knupp, M.; Hintermann, B. Sports and Recreation Activity of Ankle Arthritis Patients before and after Total Ankle Replacement. Am. J. Sports Med. 2006, 34, 993–999. [Google Scholar] [CrossRef]
- Valderrabano, V.; Hintermann, B.; Horisberger, M.; Fung, T.S. Ligamentous Posttraumatic Ankle Osteoarthritis. Am. J. Sports Med. 2006, 34, 612–620. [Google Scholar] [CrossRef]
- Hirose, K.; Murakami, G.; Minowa, T.; Kura, H.; Yamashita, T. Lateral Ligament Injury of the Ankle and Associated Articular Cartilage Degeneration in the Talocrural Joint: Anatomic Study Using Elderly Cadavers. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2004, 9, 37–43. [Google Scholar] [CrossRef]
- Taga, I.; Shino, K.; Inoue, M.; Nakata, K.; Maeda, A. Articular Cartilage Lesions in Ankles with Lateral Ligament Injury. An Arthroscopic Study. Am. J. Sports Med. 1993, 21, 120–126, discussion 126–127. [Google Scholar] [CrossRef]
- Bischof, J.E.; Spritzer, C.E.; Caputo, A.M.; Easley, M.E.; DeOrio, J.K.; Nunley, J.A.; DeFrate, L.E. In Vivo Cartilage Contact Strains in Patients with Lateral Ankle Instability. J. Biomech. 2010, 43, 2561–2566. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Kwon, J.W.; Choi, W.J.; Lee, J.W. Comparison of Outcomes for Osteochondral Lesions of the Talus With and Without Chronic Lateral Ankle Instability. Foot Ankle Int. 2015, 36, 1050–1057. [Google Scholar] [CrossRef]
- McCann, R.S.; Crossett, I.D.; Terada, M.; Kosik, K.B.; Bolding, B.A.; Gribble, P.A. Hip Strength and Star Excursion Balance Test Deficits of Patients with Chronic Ankle Instability. J. Sci. Med. Sport 2017, 20, 992–996. [Google Scholar] [CrossRef]
- Olmsted, L.C.; Carcia, C.R.; Hertel, J.; Shultz, S.J. Efficacy of the Star Excursion Balance Tests in Detecting Reach Deficits in Subjects With Chronic Ankle Instability. J. Athl. Train. 2002, 37, 501–506. [Google Scholar]
- Khalaj, N.; Vicenzino, B.; Heales, L.J.; Smith, M.D. Is Chronic Ankle Instability Associated with Impaired Muscle Strength? Ankle, Knee and Hip Muscle Strength in Individuals with Chronic Ankle Instability: A Systematic Review with Meta-Analysis. Br. J. Sports Med. 2020, 54, 839–847. [Google Scholar] [CrossRef]
- Moisan, G.; Descarreaux, M.; Cantin, V. Effects of Chronic Ankle Instability on Kinetics, Kinematics and Muscle Activity during Walking and Running: A Systematic Review. Gait Posture 2017, 52, 381–399. [Google Scholar] [CrossRef]
- Drewes, L.K.; McKeon, P.O.; Paolini, G.; Riley, P.; Kerrigan, D.C.; Ingersoll, C.D.; Hertel, J. Altered Ankle Kinematics and Shank-Rear-Foot Coupling in Those with Chronic Ankle Instability. J. Sport Rehabil. 2009, 18, 375–388. [Google Scholar] [CrossRef]
- Monaghan, K.; Delahunt, E.; Caulfield, B. Ankle Function during Gait in Patients with Chronic Ankle Instability Compared to Controls. Clin. Biomech. Bristol Avon 2006, 21, 168–174. [Google Scholar] [CrossRef]
- Wright, C.J.; Arnold, B.L.; Ross, S.E.; Pidcoe, P.E. Individuals With Functional Ankle Instability, but Not Copers, Have Increased Forefoot Inversion During Walking Gait. Athl. Train. Sports Health Care 2013, 5, 201–209. [Google Scholar] [CrossRef] [Green Version]
- De Ridder, R.; Willems, T.; Vanrenterghem, J.; Robinson, M.; Pataky, T.; Roosen, P. Gait Kinematics of Subjects with Ankle Instability Using a Multisegmented Foot Model. Med. Sci. Sports Exerc. 2013, 45, 2129–2136. [Google Scholar] [CrossRef]
- Delahunt, E.; Monaghan, K.; Caulfield, B. Altered Neuromuscular Control and Ankle Joint Kinematics during Walking in Subjects with Functional Instability of the Ankle Joint. Am. J. Sports Med. 2006, 34, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Feger, M.A.; Donovan, L.; Hart, J.M.; Hertel, J. Lower Extremity Muscle Activation in Patients with or without Chronic Ankle Instability during Walking. J. Athl. Train. 2015, 50, 350–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ty Hopkins, J.; Coglianese, M.; Glasgow, P.; Reese, S.; Seeley, M.K. Alterations in Evertor/Invertor Muscle Activation and Center of Pressure Trajectory in Participants with Functional Ankle Instability. J. Electromyogr. Kinesiol. 2012, 22, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.T.; Ingersoll, C.D. Arthrogenic Muscle Inhibition: A Limiting Factor in Joint Rehabilitation. J. Sport Rehabil. 2000, 9, 135–159. [Google Scholar] [CrossRef]
- Kim, K.-M.; Kim, J.-S.; Cruz-Díaz, D.; Ryu, S.; Kang, M.; Taube, W. Changes in Spinal and Corticospinal Excitability in Patients with Chronic Ankle Instability: A Systematic Review with Meta-Analysis. J. Clin. Med. 2019, 8, 1037. [Google Scholar] [CrossRef] [Green Version]
- Sonnery-Cottet, B.; Saithna, A.; Quelard, B.; Daggett, M.; Borade, A.; Ouanezar, H.; Thaunat, M.; Blakeney, W.G. Arthrogenic Muscle Inhibition after ACL Reconstruction: A Scoping Review of the Efficacy of Interventions. Br. J. Sports Med. 2019, 53, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Charlton, C.S.; Ridding, M.C.; Thompson, P.D.; Miles, T.S. Prolonged Peripheral Nerve Stimulation Induces Persistent Changes in Excitability of Human Motor Cortex. J. Neurol. Sci. 2003, 208, 79–85. [Google Scholar] [CrossRef]
- Kaelin-Lang, A.; Luft, A.R.; Sawaki, L.; Burstein, A.H.; Sohn, Y.H.; Cohen, L.G. Modulation of Human Corticomotor Excitability by Somatosensory Input. J. Physiol. 2002, 540, 623–633. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Rothwell, J.C. The Effect of Sensory Input and Attention on the Sensorimotor Organization of the Hand Area of the Human Motor Cortex. J. Physiol. 2004, 561, 307–320. [Google Scholar] [CrossRef]
- Lagerquist, O.; Mang, C.S.; Collins, D.F. Changes in Spinal but Not Cortical Excitability Following Combined Electrical Stimulation of the Tibial Nerve and Voluntary Plantar-Flexion. Exp. Brain Res. 2012, 222, 41–53. [Google Scholar] [CrossRef]
- Kato, T.; Sasaki, A.; Yokoyama, H.; Milosevic, M.; Nakazawa, K. Effects of Neuromuscular Electrical Stimulation and Voluntary Commands on the Spinal Reflex Excitability of Remote Limb Muscles. Exp. Brain Res. 2019, 237, 3195–3205. [Google Scholar] [CrossRef] [Green Version]
- Training and Orthotic Effects Related to Functional Electrical Stimulation of the Peroneal Nerve in Stroke*. Available online: http://www.medicaljournals.se/jrm/content/html/10.2340/16501977-2181 (accessed on 25 January 2022).
- Kafri, M.; Laufer, Y. Therapeutic Effects of Functional Electrical Stimulation on Gait in Individuals Post-Stroke. Ann. Biomed. Eng. 2015, 43, 451–466. [Google Scholar] [CrossRef]
- Gregory, C.M.; Bickel, C.S. Recruitment Patterns in Human Skeletal Muscle during Electrical Stimulation. Phys. Ther. 2005, 85, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Borzuola, R.; Labanca, L.; Macaluso, A.; Laudani, L. Modulation of Spinal Excitability Following Neuromuscular Electrical Stimulation Superimposed to Voluntary Contraction. Eur. J. Appl. Physiol. 2020, 120, 2105–2113. [Google Scholar] [CrossRef]
- Thompson, A.K.; Doran, B.; Stein, R.B. Short-Term Effects of Functional Electrical Stimulation on Spinal Excitatory and Inhibitory Reflexes in Ankle Extensor and Flexor Muscles. Exp. Brain Res. 2006, 170, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Khaslavskaia, S.; Sinkjaer, T. Motor Cortex Excitability Following Repetitive Electrical Stimulation of the Common Peroneal Nerve Depends on the Voluntary Drive. Exp. Brain Res. 2005, 162, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Wang, J.-S. The Immediate Effect of FES and TENS on Gait Parameters in Patients after Stroke. J. Phys. Ther. Sci. 2017, 29, 2212–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, J.A.; Hsiao, H.; Wright, T.; Binder-Macleod, S.A. Single Session of Functional Electrical Stimulation-Assisted Walking Produces Corticomotor Symmetry Changes Related to Changes in Poststroke Walking Mechanics. Phys. Ther. 2017, 97, 550–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isakov, E.; Bowker, P. Influence of a Single FES Treatment on Hemiparetic Legs. Physiotherapy 2002, 88, 269–272. [Google Scholar] [CrossRef]
- Koski, L.; Mernar, T.J.; Dobkin, B.H. Immediate and Long-Term Changes in Corticomotor Output in Response to Rehabilitation: Correlation with Functional Improvements in Chronic Stroke. Neurorehabil. Neural Repair 2004, 18, 230–249. [Google Scholar] [CrossRef]
- Moran, U.; Gottlieb, U.; Gam, A.; Springer, S. Functional Electrical Stimulation Following Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Pilot Study. J. Neuroeng. Rehabil. 2019, 16, 89. [Google Scholar] [CrossRef] [Green Version]
- Bamber, Z.A.; Sun, W.; Menon, R.S.; Wheeler, P.C.; Swain, I.D.; Fong, D.T.P. Functional Electrical Stimulation of Peroneal Muscles on Balance in Healthy Females. Cyborg Bionic Syst. 2021, 2021, 9801097. [Google Scholar] [CrossRef]
- Pataky, T.C. Power1D: A Python Toolbox for Numerical Power Estimates in Experiments Involving One-Dimensional Continua. PeerJ Comput. Sci. 2017, 3, e125. [Google Scholar] [CrossRef] [Green Version]
- Gribble, P.A.; Delahunt, E.; Bleakley, C.; Caulfield, B.; Docherty, C.L.; Fourchet, F.; Fong, D.; Hertel, J.; Hiller, C.; Kaminski, T.W.; et al. Selection Criteria for Patients with Chronic Ankle Instability in Controlled Research: A Position Statement of the International Ankle Consortium. J. Orthop. Sports Phys. Ther. 2013, 43, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Malliaropoulos, N.; Papacostas, E.; Papalada, A.; Maffulli, N. Acute Lateral Ankle Sprains in Track and Field Athletes: An Expanded Classification. Foot Ankle Clin. 2006, 11, 497–507. [Google Scholar] [CrossRef]
- Hiller, C.E.; Refshauge, K.M.; Bundy, A.C.; Herbert, R.D.; Kilbreath, S.L. The Cumberland Ankle Instability Tool: A Report of Validity and Reliability Testing. Arch. Phys. Med. Rehabil. 2006, 87, 1235–1241. [Google Scholar] [CrossRef]
- Martin, R.L.; Irrgang, J.J.; Burdett, R.G.; Conti, S.F.; Van Swearingen, J.M. Evidence of Validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005, 26, 968–983. [Google Scholar] [CrossRef]
- Koldenhoven, R.M.; Hart, J.; Saliba, S.; Abel, M.F.; Hertel, J. Gait Kinematics & Kinetics at Three Walking Speeds in Individuals with Chronic Ankle Instability and Ankle Sprain Copers. Gait Posture 2019, 74, 169–175. [Google Scholar] [CrossRef]
- Terada, M.; Morgan, K.D.; Gribble, P.A. Altered Movement Strategy of Chronic Ankle Instability Individuals with Postural Instability Classified Based on Nyquist and Bode Analyses. Clin. Biomech. Bristol Avon 2019, 69, 39–43. [Google Scholar] [CrossRef]
- Gottlieb, U.; Balasukumaran, T.; Hoffman, J.R.; Springer, S. Agreement of Gait Events Detection during Treadmill Backward Walking by Kinematic Data and Inertial Motion Units. Sensors 2020, 20, 6331. [Google Scholar] [CrossRef]
- Zeni, J.A.; Richards, J.G.; Higginson, J.S. Two Simple Methods for Determining Gait Events during Treadmill and Overground Walking Using Kinematic Data. Gait Posture 2008, 27, 710–714. [Google Scholar] [CrossRef] [Green Version]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.F.; Blok, J.H.; Rau, G.; Klug, C.; Hägg, G.; Blok, W.J. European Recommendations for Surface Electromyography. Roessingh Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Hausdorff, J.M.; Ring, H. Effects of a New Radio Frequency-Controlled Neuroprosthesis on Gait Symmetry and Rhythmicity in Patients with Chronic Hemiparesis. Am. J. Phys. Med. Rehabil. 2008, 87, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Balasukumaran, T.; Gottlieb, U.; Springer, S. Spatiotemporal Gait Characteristics and Ankle Kinematics of Backward Walking in People with Chronic Ankle Instability. Sci. Rep. 2020, 10, 11515. [Google Scholar] [CrossRef] [PubMed]
- Koldenhoven, R.M.; Feger, M.A.; Fraser, J.J.; Hertel, J. Variability in Center of Pressure Position and Muscle Activation during Walking with Chronic Ankle Instability. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2018, 38, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Pataky, T.C. One-Dimensional Statistical Parametric Mapping in Python. Comput. Methods Biomech. Biomed. Engin. 2012, 15, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Yousif, H.A.; Zakaria, A.; Rahim, N.A.; Salleh, A.F.B.; Mahmood, M.; Alfarhan, K.A.; Kamarudin, L.M.; Mamduh, S.M.; Hasan, A.M.; Hussain, M.K. Assessment of Muscles Fatigue Based on Surface EMG Signals Using Machine Learning and Statistical Approaches: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 705, 012010. [Google Scholar] [CrossRef]
- Delahunt, E. Neuromuscular Contributions to Functional Instability of the Ankle Joint. J. Bodyw. Mov. Ther. 2007, 11, 203–213. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.W.; Jung, T.S.; Jang, W.Y. Reliability and Usefulness of the Single Leg Heel Raise Balance Test in Patients with Chronic Ankle Instability. Sci. Rep. 2021, 11, 20369. [Google Scholar] [CrossRef]
- Ibitoye, M.O.; Hamzaid, N.A.; Hasnan, N.; Abdul Wahab, A.K.; Davis, G.M. Strategies for Rapid Muscle Fatigue Reduction during FES Exercise in Individuals with Spinal Cord Injury: A Systematic Review. PLoS ONE 2016, 11, e0149024. [Google Scholar] [CrossRef] [Green Version]
- Kesar, T.M.; Perumal, R.; Jancosko, A.; Reisman, D.S.; Rudolph, K.S.; Higginson, J.S.; Binder-Macleod, S.A. Novel Patterns of Functional Electrical Stimulation Have an Immediate Effect on Dorsiflexor Muscle Function During Gait for People Poststroke. Phys. Ther. 2010, 90, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Enoka, R.M.; Amiridis, I.G.; Duchateau, J. Electrical Stimulation of Muscle: Electrophysiology and Rehabilitation. Physiology 2020, 35, 40–56. [Google Scholar] [CrossRef]
- Burcal, C.J.; Wikstrom, E.A. Plantar Cutaneous Sensitivity With and Without Cognitive Loading in People With Chronic Ankle Instability, Copers, and Uninjured Controls. J. Orthop. Sports Phys. Ther. 2016, 46, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Springer, S.; Gottlieb, U. Effects of Dual-Task and Walking Speed on Gait Variability in People with Chronic Ankle Instability: A Cross-Sectional Study. BMC Musculoskelet. Disord. 2017, 18, 316. [Google Scholar] [CrossRef] [Green Version]


| CAI (n = 24) | Controls (n = 24) | U | p | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |||
| Age | 30.5 (6.3) | 30.0 (8.8) | 30.0 (6.6) | 28.0 (8.0) | 307 | 0.702 |
| Stature (m) | 1.74 (0.1) | 1.74 (0.1) | 1.71 (0.1) | 1.7 (0.2) | 340 | 0.287 |
| Body mass (kg) | 76.4 (12.6) | 78.0 (18.8) | 70.7 (12.4) | 72.5 (18.8) | 359 | 0.146 |
| BMI (kg/m2) | 25.1 (3.6) | 25.2 (5.1) | 24.1 (3.1) | 23.5 (4.3) | 338 | 0.307 |
| CAIT (intervention) | 14.0 (6.1) | 15.5 (10.2) | 29.0 (1.4) | 30.0 (2.2) | 0 | <0.001 |
| CAIT (control) | 21.2 (7.0) | 22.5 (10.0) | 29.2 (1.2) | 30.0 (2.0) | 84.5 | <0.001 |
| FAAM (ADL) | 90.4 (10.4) | 94.0 (14.1) | 99.4 (1.6) | 100.0 (0.0) | 64 | <0.001 |
| FAAM (sport) | 62.7 (15.0) | 67.9 (19.6) | 98.1 (3.5) | 100.0 (3.6) | 0 | <0.001 |
| SSP + 20% (m/s) | 1.6 (0.19) | 1.6 (0.22) | 1.5 (0.2) | 1.5 (0.29) | 342.5 | 0.264 |
| n | % | n | % | X2(1) | p | |
| Gender (women) | 7 | 29.1% | 11 | 45.8% | 0.8 | 0.371 |
| Previously sprained an ankle | 24 | 100% | 5 | 20.80% | 28.2 | <0.001 |
| Intervention leg (Right) | 15 | 62.5% | 13 | 54.2% | 0.08 | 0.769 |
| Bilateral CAI | 15 | 62.5% | ||||
| CAI | Controls | Between Groups | Between Measurements | Group × Measurement Interaction | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |||||
| MDF (Hz) | Pre | 101.9 (30.8) | 92.3 (58.7) | 105.9 (33.9) | 109.1 (50.3) | F(1,46) = 0.68, p = 0.41 | F(1,46) = 16.64, p < 0.001 | F(1,46) = 0.07, p = 0.79 |
| Post | 90.3 (35.7) | 100.7 (42.0) | 94.2 (32.2) | 92.3 (50.3) | ||||
| RMS (%MVIC × 103) | Pre | 7.3 (7.6) | 5.3 (5.1) | 4.6 (3.1) | 3.9 (4.7) | F(1,46) = 4.53, p = 0.04 | F(1,46) = 3.04, p = 0.08 | F(1,46) = 4.3, p = 0.04 |
| Post | 8.4 (7.2) | 6.5 (7.7) | 4.6 (3.1) | 3.7 (4.4) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gottlieb, U.; Hoffman, J.R.; Springer, S. The Immediate Carryover Effects of Peroneal Functional Electrical Stimulation Differ between People with and without Chronic Ankle Instability. Sensors 2022, 22, 1622. https://doi.org/10.3390/s22041622
Gottlieb U, Hoffman JR, Springer S. The Immediate Carryover Effects of Peroneal Functional Electrical Stimulation Differ between People with and without Chronic Ankle Instability. Sensors. 2022; 22(4):1622. https://doi.org/10.3390/s22041622
Chicago/Turabian StyleGottlieb, Uri, Jay R. Hoffman, and Shmuel Springer. 2022. "The Immediate Carryover Effects of Peroneal Functional Electrical Stimulation Differ between People with and without Chronic Ankle Instability" Sensors 22, no. 4: 1622. https://doi.org/10.3390/s22041622
APA StyleGottlieb, U., Hoffman, J. R., & Springer, S. (2022). The Immediate Carryover Effects of Peroneal Functional Electrical Stimulation Differ between People with and without Chronic Ankle Instability. Sensors, 22(4), 1622. https://doi.org/10.3390/s22041622







