Near-Infrared Time-Resolved Spectroscopy Shows Anterior Prefrontal Blood Volume Reduction in Schizophrenia but Not in Major Depressive Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measurement Protocol
2.3. Near-Infrared Time-Resolved Spectroscopy
- Light Speed = 0.29979 mm/ps
- Refractive Index in the brain = 1.37
- Mean TOF = ∫ t f(t)dt/∫ f(t)dt
- Distribution of TOF = f(t)
2.4. Calculation of Absolute Value of Cerebral Hemoglobin Concentrations
2.5. Ratios to Systemic Blood Hemoglobin Concentration
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Prefrontal Hemoglobin Concentration in the Healthy Control Assessed by NIR-TRS
4.2. Prefrontal Hemoglobin Concentration in Schizophrenia
4.3. Prefrontal Hemoglobin Concentration in Depression
4.4. Assessment of Prefrontal Blood Volume in Schizophrenia and Depression Using NIR-TRS
4.5. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuster, J.M. The Prefrontal Cortex; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 978-0-1237-3644-4. [Google Scholar]
- Ingvar, D.H.; Franzén, G. Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta Psychiatr. Scand. 1974, 50, 425–462. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, M.S.; DeLisi, L.E.; Holcomb, H.H.; Cappelletti, J.; King, A.C.; Johnson, J.; Erin Hazlett, E.; Dowling-Zimmerman, S.; Post, R.M.; Morihisa, J.; et al. Anteroposterior Gradients in Cerebral Glucose Use in Schizophrenia and Affective Disorders. Arch. Gen. Psychiatry 1984, 41, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Franzén, G.; Ingvar, D.H. Absence of activation in frontal structures during psychological testing of chronic schizophrenics. J. Neurol. Neurosurg. Psychiatry 1975, 38, 1027–1032. [Google Scholar] [CrossRef][Green Version]
- Catafau, A.M.; Parellada, E.; Lomeña, F.J.; Bernardo, M.; Pavía, J.; Ros, D.; Setoain, J.; Gonzalez-Monclús, E. Prefrontal and temporal blood flow in schizophrenia: Resting and activation technetium-99m-HMPAO SPECT patterns in young neuroleptic-naive patients with acute disease. J. Nucl. Med. 1994, 35, 935–941. [Google Scholar]
- Kawasaki, Y.; Maeda, Y.; Suzuki, M.; Urata, K.; Higashima, M.; Kiba, K.; Yamaguchi, N.; Matsuda, H.; Hisada, K. SPECT analysis of regional cerebral blood flow changes in patients with schizophrenia during the Wisconsin Card Sorting Test. Schizophr. Res. 1993, 10, 109–116. [Google Scholar] [CrossRef]
- Fu, C.H.F.; Suckling, J.; Williams, S.C.R.; Andrew, C.M.; Vythelingum, G.N.; McGuire, P.K. Effects of psychotic state and task demand on prefrontal function in schizophrenia: An fMRI study of overt verbal fluency. Am. J. Psychiatry 2005, 162, 485–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krug, A.; Cabanis, M.; Pyka, M.; Pauly, K.; Kellermann, T.; Walter, H.; Wagner, M.; Landsberg, M.; Shah, N.J.; Winterer, G.; et al. Attenuated prefrontal activation during decision-making under uncertainty in schizophrenia: A multi-center fMRI study. Schizophr. Res. 2014, 152, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Stip, E.; Fahim, C.; Mancini-Marïe, A.; Bentaleb, L.A.; Mensour, B.; Mendrek, A.; Beauregard, M. Restoration of frontal activation during a treatment with quetiapine: An fMRI study of blunted affect in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 21–26. [Google Scholar] [CrossRef]
- Egashira, K.; Matsuo, K.; Nakashima, M.; Watanuki, T.; Harada, K.; Nakano, M.; Matsubara, T.; Takahashi, K.; Watanabe, Y. Blunted brain activation in patients with schizophrenia in response to emotional cognitive inhibition: A functional near-infrared spectroscopy study. Schizophr. Res. 2015, 162, 196–204. [Google Scholar] [CrossRef]
- Quan, W.; Wu, T.; Li, Z.; Wang, Y.; Dong, W.; Lv, B. Reduced prefrontal activation during a verbal fluency task in Chinese-speaking patients with schizophrenia as measured by near-infrared spectroscopy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 58, 51–58. [Google Scholar] [CrossRef]
- Shinba, T.; Nagano, M.; Kariya, N.; Ogawa, K.; Shinozaki, T.; Shimosato, S.; Hoshi, Y. Near-infrared spectroscopy analysis of frontal lobe dysfunction in schizophrenia. Biol. Psychiatry 2004, 55, 154–164. [Google Scholar] [CrossRef]
- Takizawa, R.; Kasai, K.; Kawakubo, Y.; Marumo, K.; Kawasaki, S.; Yamasue, H.; Fukuda, M. Reduced frontopolar activation during verbal fluency task in schizophrenia: A multi-channel near-infrared spectroscopy study. Schizophr. Res. 2008, 99, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Sumitani, S.; Watanabe, Y.; Akiyama, M.; Ohmori, T. Multi-channel near-infrared spectroscopy reveals reduced prefrontal activation in schizophrenia patients during performance of the kana Stroop task. J. Med. Investig. 2012, 59, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Watanuki, T.; Matsuo, K.; Egashira, K.; Nakashima, M.; Harada, K.; Nakano, M.; Matsubara, T.; Takahashi, K.; Watanabe, Y. Precentral and inferior prefrontal hypoactivation during facial emotion recognition in patients with schizophrenia: A functional near-infrared spectroscopy study. Schizophr. Res. 2016, 170, 109–114. [Google Scholar] [CrossRef]
- Kurachi, M.; Kobayashi, K.; Matsubara, R.; Hiramatsu, H.; Yamaguchi, N.; Matsuda, H.; Maeda, T.; Hisada, K. Regional cerebral blood flow in schizophrenic disorders. Eur. Neurol. 1985, 24, 176–181. [Google Scholar] [CrossRef]
- Soyka, M.; Koch, W.; Möller, H.J.; Rüther, T.; Tatsch, K. Hypermetabolic pattern in frontal cortex and other brain regions in unmedicated schizophrenia patients. Results from a FDG-PET study. Eur. Arch. Psychiatry Clin. Neurosci. 2005, 255, 308–312. [Google Scholar] [CrossRef]
- Berman, K.F.; Doran, A.R.; Pickar, D.; Weinberger, D.R. Is the mechanism of prefrontal hypofunction in depression the same as in schizophrenia? Regional cerebral blood flow during cognitive activation. Br. J. Psychiatry 1993, 162, 183–192. [Google Scholar] [CrossRef]
- Akiyama, T.; Koeda, M.; Okubo, Y.; Kimura, M. Hypofunction of left dorsolateral prefrontal cortex in depression during verbal fluency task: A multi-channel near-infrared spectroscopy study. J. Affect. Disord. 2018, 231, 83–90. [Google Scholar] [CrossRef]
- Chou, P.H.; Yao, Y.H.; Zheng, R.X.; Liou, Y.L.; Liu, T.T.; Lane, H.Y.; Yang, A.C.; Wang, S.C. Deep Neural Network to Differentiate Brain Activity Between Patients with First-Episode Schizophrenia and Healthy Individuals: A Multi-Channel Near Infrared Spectroscopy Study. Front. Psychiatry 2021, 12, 655292. [Google Scholar] [CrossRef]
- Gündüz, H.; Baran, Z.; Kır, Y.; Baskak, N.S.; Baskak, B. Investigation of the cortical activity during episodic future thinking in schizophrenia: A functional near-infrared spectroscopy study. Behav. Neurosci. 2020, 134, 344–357. [Google Scholar] [CrossRef]
- Kawano, M.; Kanazawa, T.; Kikuyama, H.; Tsutsumi, A.; Kinoshita, S.; Kawabata, Y.; Yamauchi, S.; Uenishi, H.; Kawashige, S.; Imazu, S.; et al. Correlation between frontal lobe oxy-hemoglobin and severity of depression assessed using near-infrared spectroscopy. J. Affect. Disord. 2016, 205, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Matsumura, H.; Yamada, T.; Ikezawa, S.; Mitani, H.; Adachi, A.; Nakagome, K. Reduced frontopolar activation during verbal fluency task associated with poor social functioning in late-onset major depression: Multi-channel near-infrared spectroscopy study. Psychiatry Clin. Neurosci. 2008, 62, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.; Wolf, R.C.; Spitzer, M.; Vasic, N. Increased left prefrontal activation in patients with unipolar depression: An event-related, parametric, performance-controlled fMRI study. J. Affect. Disord. 2007, 101, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Kawashima, R.; Awata, S.; Ono, S.; Sato, K.; Goto, R.; Koyama, M.; Sato, M.; Fukuda, H. Hypoperfusion in the limbic system and prefrontal cortex in depression: SPECT with anatomic standardization technique. J. Nucl. Med. 1996, 37, 410–414. [Google Scholar]
- Navarro, V.; Gastó, C.; Lomeña, F.; Mateos, J.J.; Marcos, T.; Portella, M.J. Normalization of frontal cerebral perfusion in remitted elderly major depression: A 12-month follow-up SPECT study. Neuroimage 2002, 16, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Suto, T.; Fukuda, M.; Ito, M.; Uehara, T.; Mikuni, M. Multichannel near-infrared spectroscopy in depression and schizophrenia: Cognitive brain activation study. Biol. Psychiatry 2004, 55, 501–511. [Google Scholar] [CrossRef]
- Hoshi, Y. Hemodynamic signals in fNIRS. Prog. Brain Res. 2016, 225, 153–179. [Google Scholar]
- Hoshi, Y.; Shinba, T.; Sato, C.; Doi, N. Resting hypofrontality in schizophrenia: A study using near-infrared time-resolved spectroscopy. Schizophr. Res. 2006, 84, 411–420. [Google Scholar] [CrossRef]
- Inagaki, A.; Inada, T. Dose equivalence of psychotropic drugs. Part 18: Dose equivalence of psychotropic drugs: 2006-version. Jpn. J. Clin. Psychopharmacol. 2006, 9, 1443–1447. (In Japanese) [Google Scholar]
- Oda, M.; Yamashita, Y.; Nakano, T.; Suzuki, A.; Shimizu, K.; Hirano, I.; Shimomura, F.; Ohmae, E.; Suzuki, T.; Tsuchiya, Y. Near-infrared time-resolved spectroscopy system for tissue oxygenation monitor. Proc. SPIE 1999, 3597, 611–617. [Google Scholar]
- Shinba, T.; Kariya, N.; Matsuda, S.; Matsuda, H.; Obara, Y. Increase of frontal cerebral blood volume during transcranial magnetic stimulation in depression is related to treatment effectiveness: A pilot study with near-infrared spectroscopy. Psychiatry Clin. Neurosci. 2018, 72, 602–610. [Google Scholar] [CrossRef]
- Patterson, M.S.; Madsen, S.J.; Moulton, J.D.; Wilson, B.C. Diffusion equation representation of photon migration in tissue. In Proceedings of the IEEE 1991 MTT-S International Microwave Symposium Digest, Boston, MA, USA, 10–14 July 1991; pp. 905–908. [Google Scholar]
- Fusch, C.; Scharrer, B.; Hungerland, E.; Moeller, H. Body water, lean bodyand fat mass of healthy children as measured by deuterium oxide dilution. Isot. Environ. Health Stud. 1993, 29, 125–131. [Google Scholar]
- Oshiro, I.; Takenaka, T.; Maeda, J. New method for hemoglobin determination by using sodium lauryl sulfate (SLS). Clin. Biochem. 1982, 15, 83–88. [Google Scholar] [CrossRef]
- Tanida, M.; Sakatani, K.; Tsujii, T. Relation between working memory performance and evoked cerebral blood oxygenation changes in the prefrontal cortex evaluated by quantitative time-resolved near-infrared spectroscopy. Neurol. Res. 2012, 34, 114–119. [Google Scholar] [CrossRef]
- Oyama, K.; Hu, L.; Sakatani, K. Prediction of MMSE Score Using Time-Resolved Near-Infrared Spectroscopy. Adv. Exp. Med. Biol. 2018, 1072, 145–150. [Google Scholar]
- Matsuda, H.; Tsuji, S.; Shuke, N.; Sumiya, H.; Tonami, N.; Hisada, K. A quantitative approach to technetium-99m hexamethylpropylene amine oxime. Eur. J. Nucl. Med. 1992, 19, 195–200. [Google Scholar] [CrossRef]
- Hirayasu, Y.; Tanaka, S.; Shenton, M.E.; Salisbury, D.F.; DeSantis, M.A.; Levitt, J.J.; Wible, C.; Yurgelun-Todd, D.; Kikinis, R.; Jolesz, F.A.; et al. Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb. Cortex. 2001, 11, 374–381. [Google Scholar] [CrossRef]
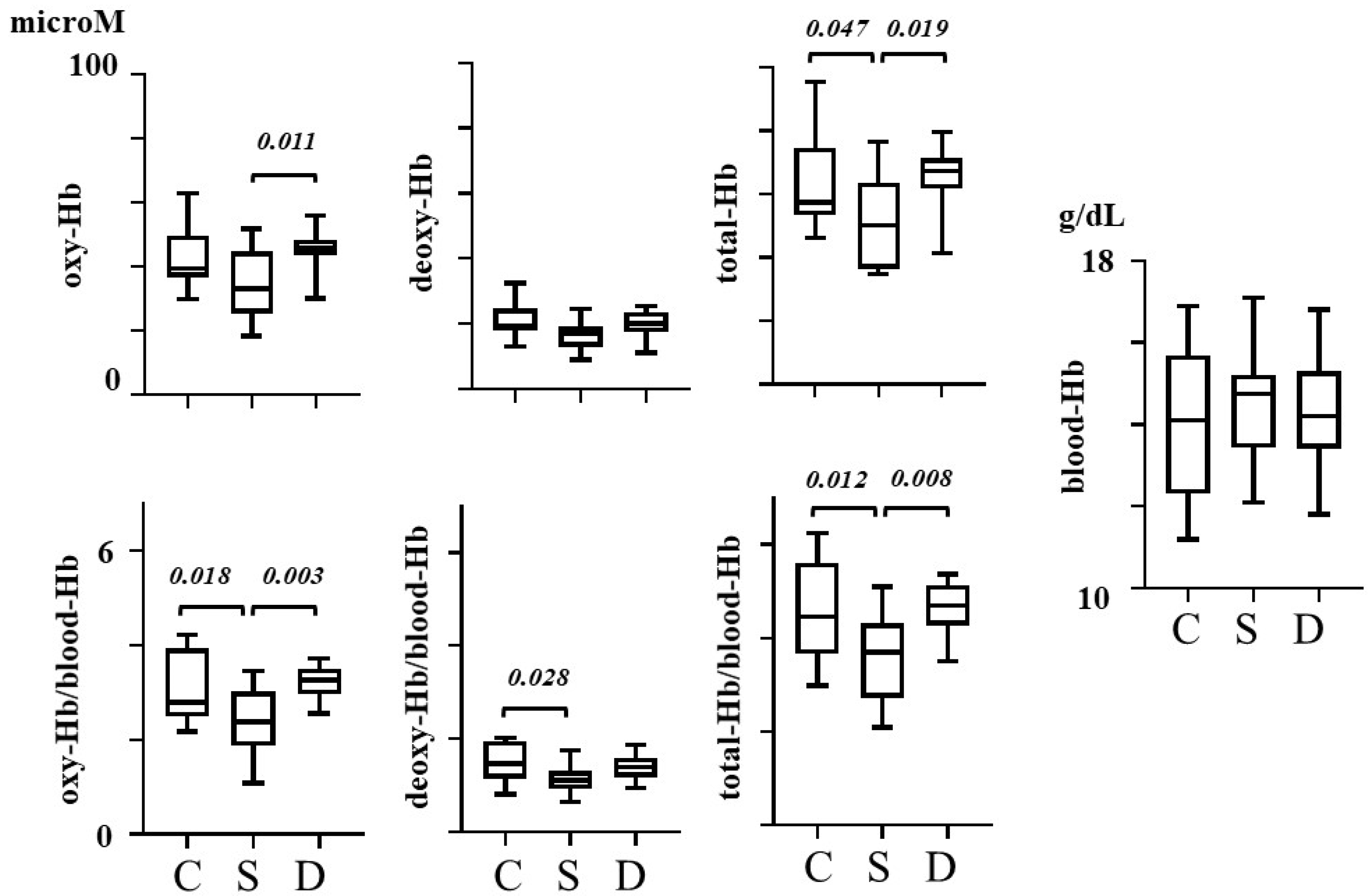
| Control | Schizophrenia | Depression | One-Way ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (s.d.) | Mean | (s.d.) | Mean | (s.d.) | F(2,39) | p | ||
| oxy-Hb | microM | 42.8 | (10.0) | 34.7 | (10.4) | 45.6 | (7.1) | 1.84 | 0.013 |
| deoxy-Hb | microM | 20.7 | (5.2) | 16.4 | (4.6) | 19.8 | (4.1) | 1.06 | 0.058 |
| total-Hb | microM | 63.5 | (14.1) | 51.1 | (13.9) | 65.4 | (10.7) | 2.76 | 0.016 |
| oxy-Hb/total-Hb (SO2) | % | 67.4 | (4.8) | 67.6 | (6.5) | 69.9 | (2.6) | 0.68 | 0.289 |
| blood-Hb | g/dL | 14.1 | (1.7) | 14.6 | (1.6) | 14.1 | (1.3) | 0.32 | 0.691 |
| oxy-Hb/blood-Hb | 3.06 | (0.70) | 2.40 | (0.69) | 3.21 | (0.35) | 0.09 | 0.003 | |
| deoxy-Hb/blood-Hb | 1.50 | (0.42) | 1.14 | (0.34) | 1.40 | (0.27) | 0.07 | 0.032 | |
| total-Hb/blood-Hb | 4.56 | (1.03) | 3.54 | (0.95) | 4.61 | (0.58) | 0.15 | 0.005 | |
| pathlength (759 nm) | cm | 20.4 | (2.3) | 21.4 | (2.9) | 20.8 | (1.9) | 0.48 | 0.585 |
| pathlength (796 nm) | cm | 20.3 | (2.2) | 21.4 | (3.0) | 20.8 | (1.6) | 0.41 | 0.483 |
| pathlength (835 nm) | cm | 19.2 | (2.0) | 20.0 | (2.8) | 19.5 | (1.5) | 0.39 | 0.598 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinba, T.; Kariya, N.; Matsuda, S.; Arai, M.; Itokawa, M.; Hoshi, Y. Near-Infrared Time-Resolved Spectroscopy Shows Anterior Prefrontal Blood Volume Reduction in Schizophrenia but Not in Major Depressive Disorder. Sensors 2022, 22, 1594. https://doi.org/10.3390/s22041594
Shinba T, Kariya N, Matsuda S, Arai M, Itokawa M, Hoshi Y. Near-Infrared Time-Resolved Spectroscopy Shows Anterior Prefrontal Blood Volume Reduction in Schizophrenia but Not in Major Depressive Disorder. Sensors. 2022; 22(4):1594. https://doi.org/10.3390/s22041594
Chicago/Turabian StyleShinba, Toshikazu, Nobutoshi Kariya, Saori Matsuda, Makoto Arai, Masanari Itokawa, and Yoko Hoshi. 2022. "Near-Infrared Time-Resolved Spectroscopy Shows Anterior Prefrontal Blood Volume Reduction in Schizophrenia but Not in Major Depressive Disorder" Sensors 22, no. 4: 1594. https://doi.org/10.3390/s22041594
APA StyleShinba, T., Kariya, N., Matsuda, S., Arai, M., Itokawa, M., & Hoshi, Y. (2022). Near-Infrared Time-Resolved Spectroscopy Shows Anterior Prefrontal Blood Volume Reduction in Schizophrenia but Not in Major Depressive Disorder. Sensors, 22(4), 1594. https://doi.org/10.3390/s22041594








