Real-Time Metasurface Sensor for Monitoring Micropoisons in Aqueous Solutions Based on Gold Nanoparticles and Terahertz Spectroscopy
Abstract
1. Introduction
2. Metasurface Design, Simulations, and Experimental Setup
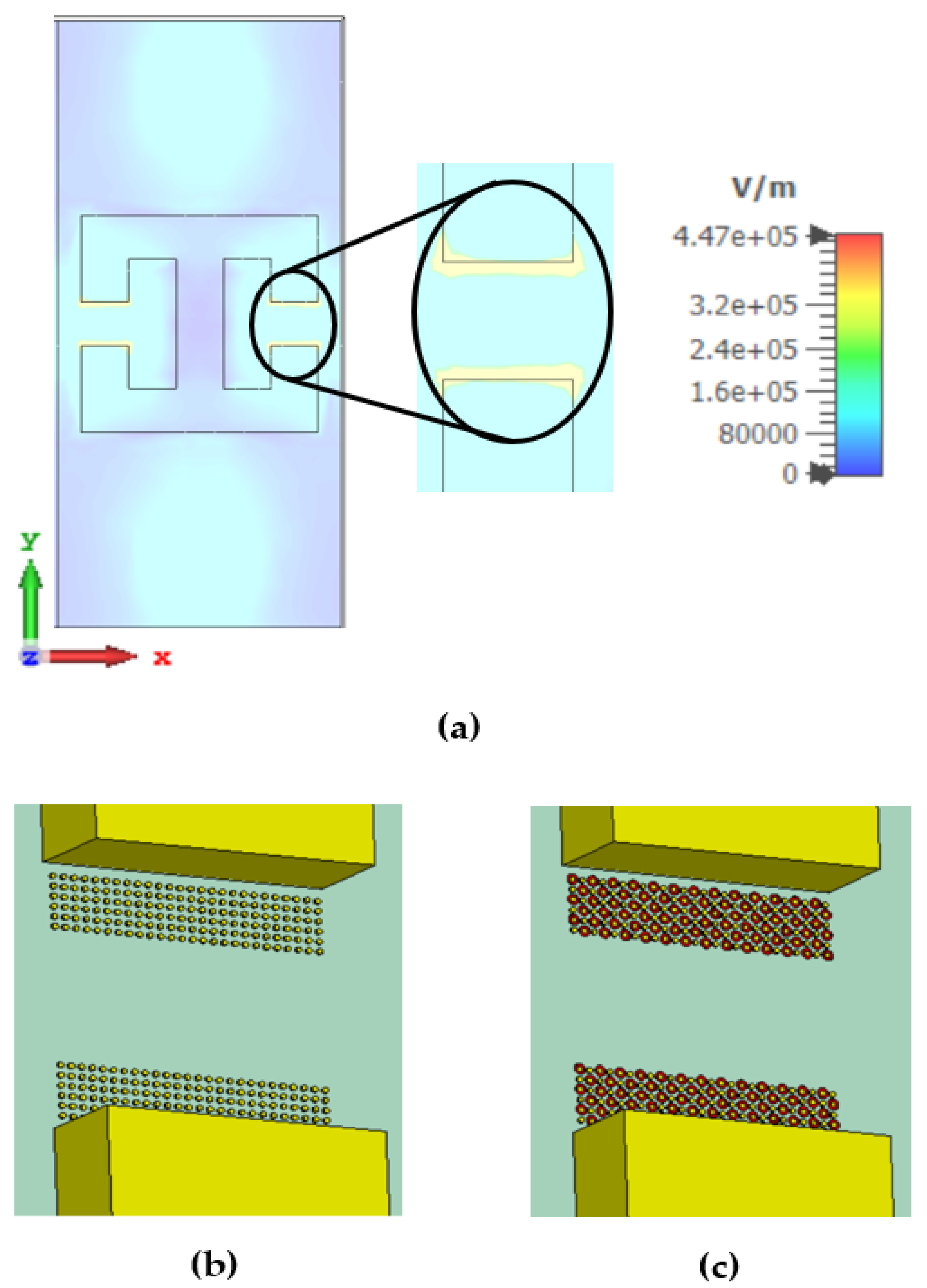
2.1. Metamaterial Design and Simulations
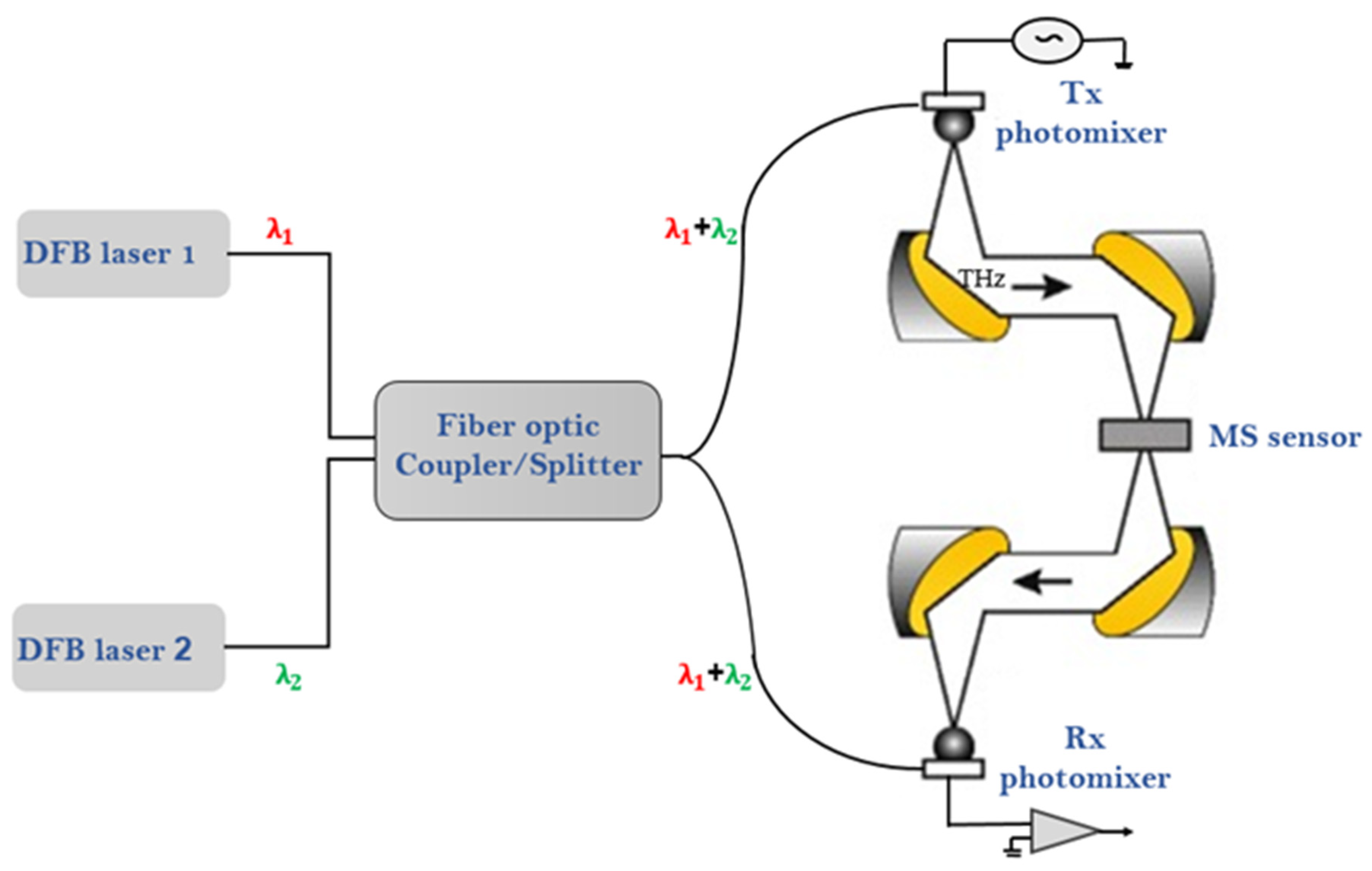
2.2. Experimental Setup
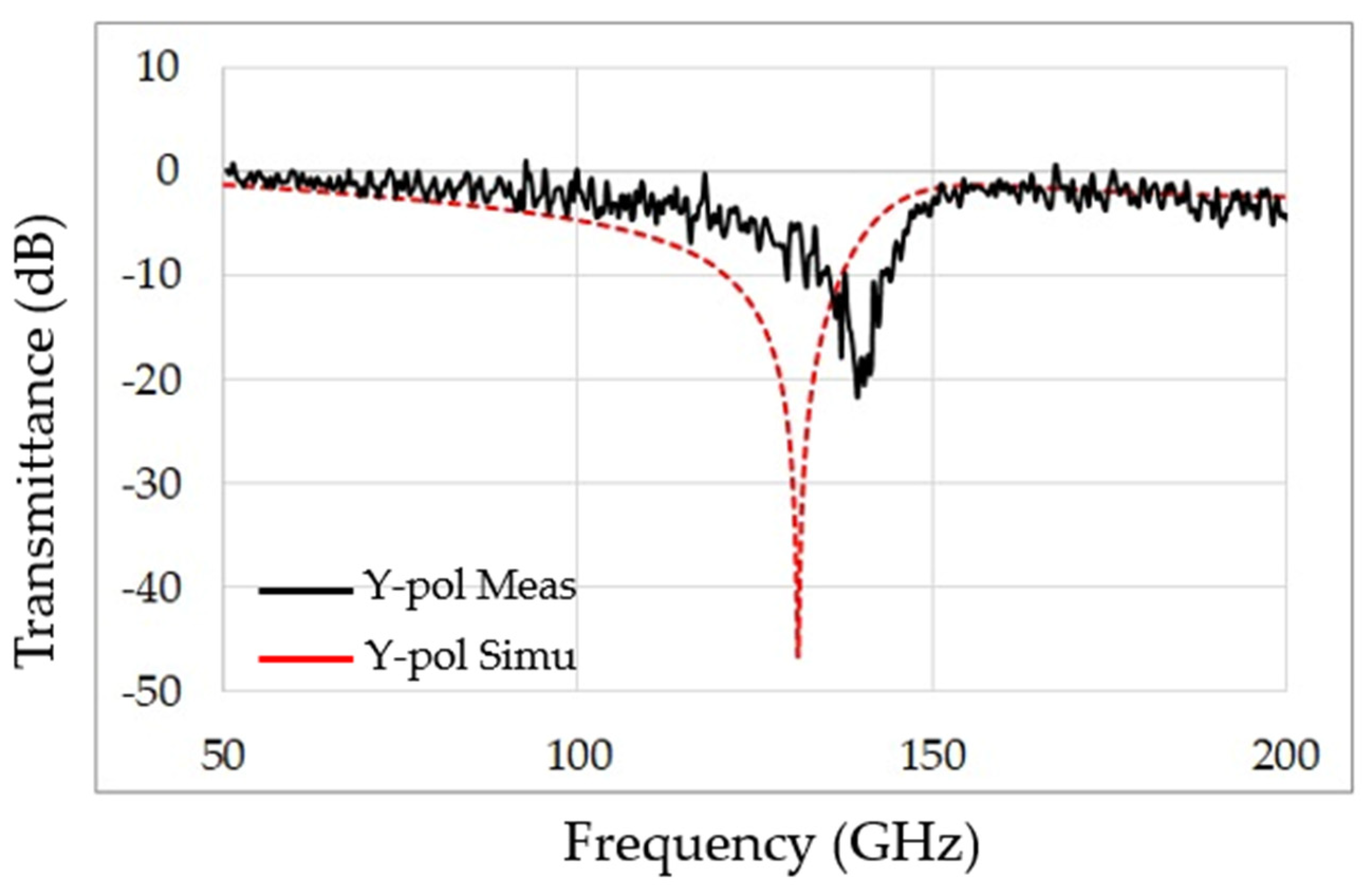
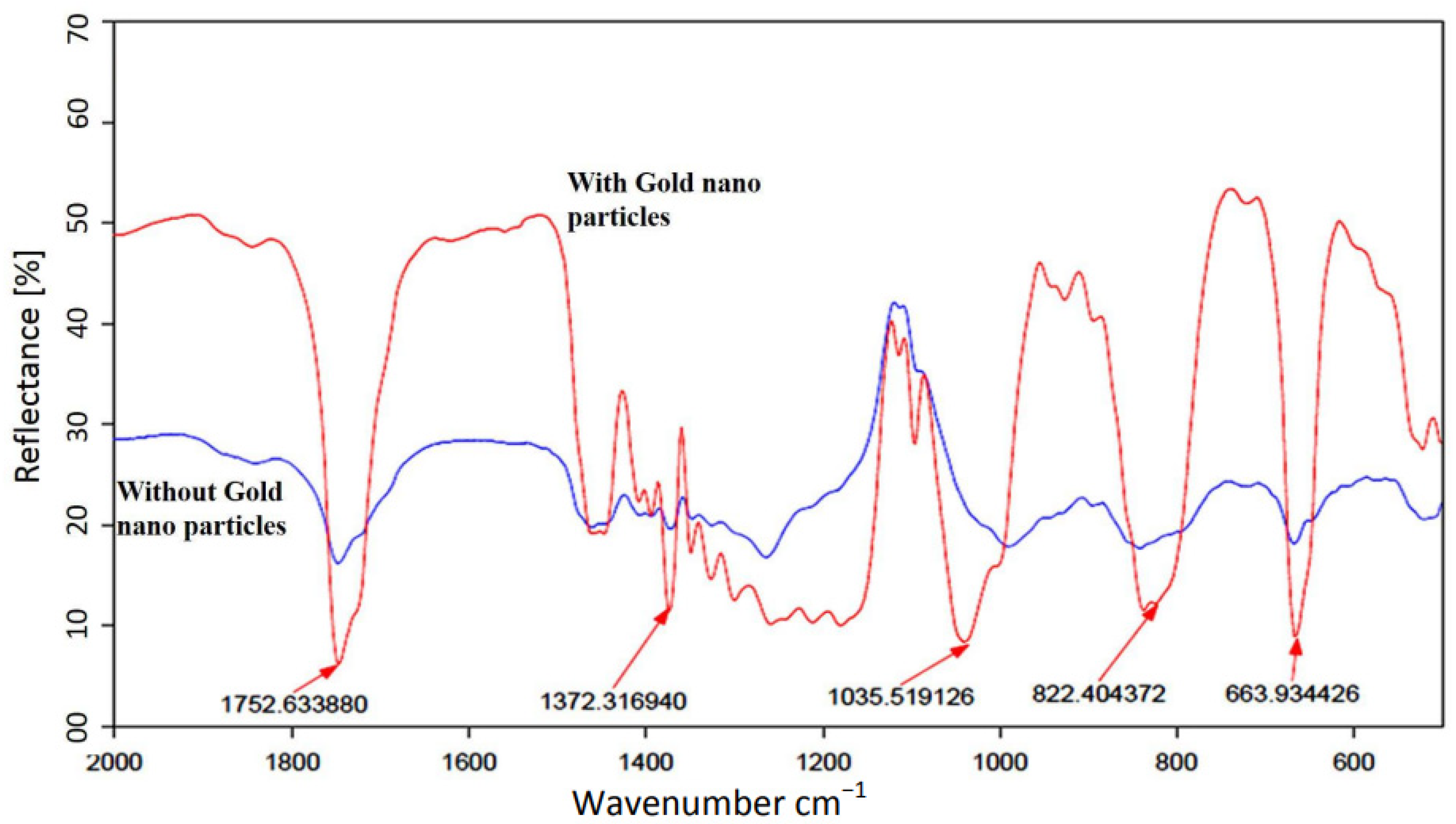
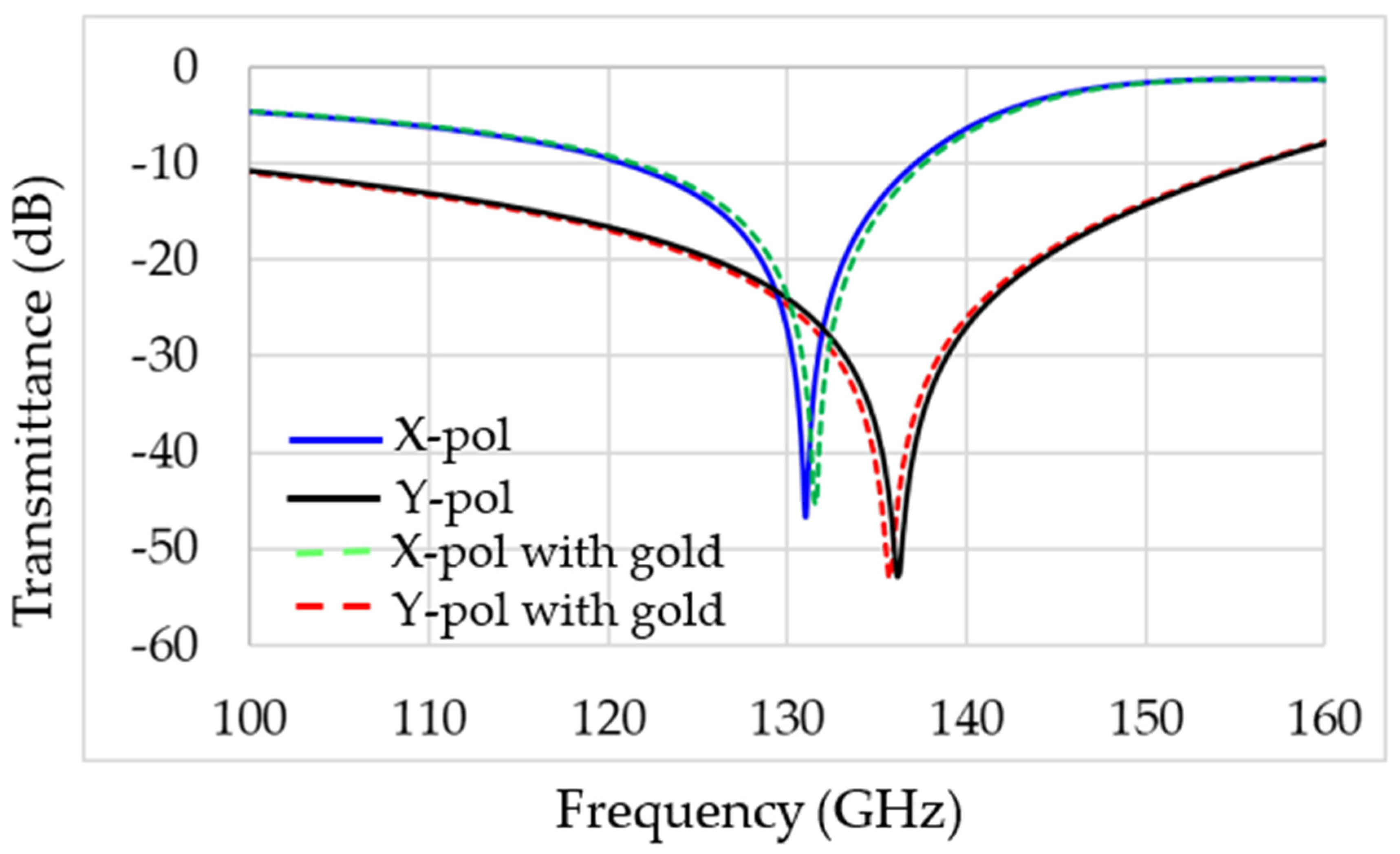
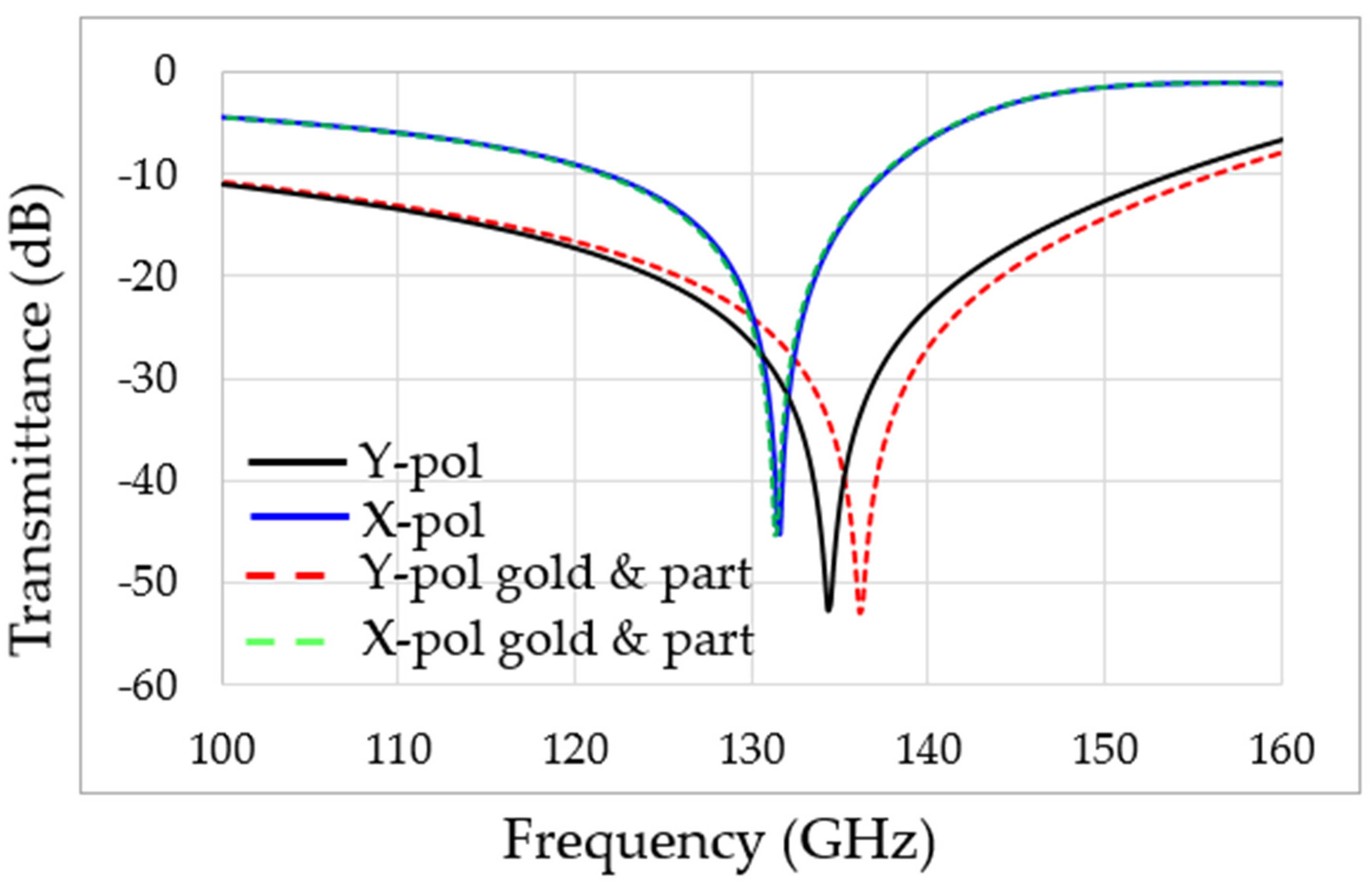
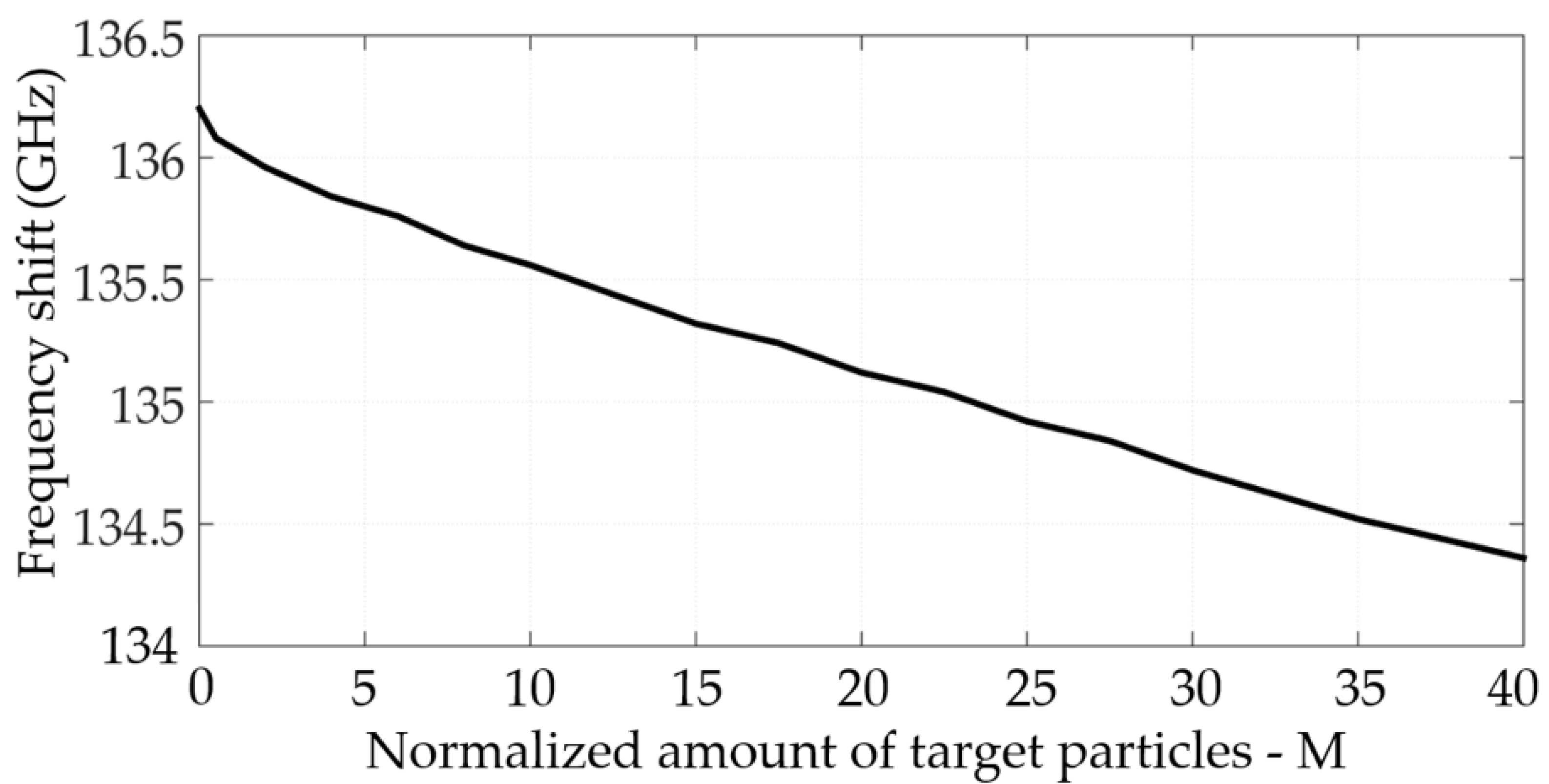
3. Experimental Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Richter, E.D.; Chuwers, P.; Levy, Y.; Gordon, M.; Grauer, F.; Marzouk, J.; Levy, S.; Barron, S.; Gruener, N. Health effects from exposure to organophosphate pesticides in workers and residents in Israel. Isr. J. Med. Sci. 1992, 28, 584–598. [Google Scholar] [PubMed]
- Fleet, B.; Little, C. Design and evaluation of electrochemical detectors for HPLC. J. Chromatogr. Sci. 1974, 12, 747–752. [Google Scholar] [CrossRef]
- Thurman, E.M.; Meyer, M.; Pomes, M.; Perry, C.A.; Schwab, A.P. Enzyme-linked immunosorbent assay compared with gas chromatography/mass spectrometry for the determination of triazine herbicides in water. Anal. Chem. 1990, 62, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Nagyová, S.; Tölgyessy, P. Validation including uncertainty estimation of a GC–MS/MS method for determination of selected halogenated priority substances in fish using rapid and efficient lipid removing sample preparation. Foods 2019, 8, 101. [Google Scholar] [CrossRef]
- Ndagire, C.T.; Muyonga, J.H.; Isabirye, D.; Odur, B.; Somda, S.M.A.; Bukenya, R.; Andrade, J.; Nakimbugwe, D. Assessing the reliability of FTIR spectroscopy measurements and validity of bioelectrical impedance analysis as a surrogate measure of body composition among children and adolescents aged 8–19 years attending schools in Kampala, Uganda. BMC Public Health 2018, 18, 687. [Google Scholar] [CrossRef]
- Shulzinger, A.; Abramovich, A.; Farber, E. Attenuated Total Reflectance (ATR)-FTIR spectral measurements in MIR and FIR (THz) range. In Proceedings of the 2009 IEEE International Conference on Microwaves, Communications, Antennas and Electronics Systems, Tel Aviv, Israel, 9–11 November 2009; pp. 1–3. [Google Scholar]
- Krasnok, A.; Baranov, D.; Li, H.; Miri, M.-A.; Monticone, F.; Alú, A. Anomalies in light scattering. Adv. Opt. Photonics 2019, 11, 892–951. [Google Scholar] [CrossRef]
- Asadchy, V.S.; Faniayeu, I.A.; Ra’di, Y.; Khakhomov, S.; Semchenko, I.; Tretyakov, S.A. Broadband reflectionless metasheets: Frequency-selective transmission and perfect absorption. Phys. Rev. X 2015, 5, 031005. [Google Scholar] [CrossRef]
- Janotta, M.; Karlowatz, M.; Vogt, F.; Mizaikoff, B. Sol–gel based mid-infrared evanescent wave sensors for detection of organophosphate pesticides in aqueous solution. Anal. Chim. Acta 2003, 496, 339–348. [Google Scholar] [CrossRef]
- Abramovich, A.; Shulzinger, A.; Ochana, M.; Rotshild, D. Metal Nano Layer Coating for Improving the Detection and Recognition of Micro-Poisons Using Reflection Spectroscopic Measurement. Opt. Photonics J. 2015, 5, 193. [Google Scholar] [CrossRef]
- Landy, N.I.; Sajuyigbe, S.; Mock, J.J.; Smith, D.R.; Padilla, W.J. Perfect metamaterial absorber. Phys. Rev. Lett. 2008, 100, 207402. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.F.; Smith, D.R.; del Hougne, P. Perfect absorption in a metasurface-programmable complex scattering enclosure. arXiv 2003, arXiv:2003.01766. [Google Scholar]
- Islam, K.M.R.; Choi, S. Compact Double-Layer FR4-Based Focusing Lens Using High-Efficiency Huygens’ Metasurface Unit Cells. Sensors 2020, 20, 6142. [Google Scholar] [CrossRef] [PubMed]
- Sol, J.; Smith, D.R.; del Hougne, P. Meta-Programmable Analog Differentiator Advanced Materials 24 September 2020. arXiv 2020, arXiv:2108.06178. [Google Scholar]
- Imani, M.F.; Smith, D.R.; del Hougne, P. Perfect Absorption in a Disordered Medium with Programmable Meta-Atom Inclusions. Adv. Funct. Mater. 2020, 30, 2005310. [Google Scholar] [CrossRef]
- Park, S.J.; Hong, J.T.; Choi, S.J.; Kim, H.S.; Park, W.K.; Han, S.T.; Park, J.Y.; Lee, S.; Kim, D.S.; Ahn, Y.H. Detection of microorganisms using terahertz metamaterials. Sci. Rep. 2014, 4, 4988. [Google Scholar] [CrossRef] [PubMed]
- Baena, J.D.; Bonache, J.; Martin, F.; Sillero, R.M.; Falcone, F.; Lopetegi, T.; Laso, M.; Garcia-Garcia, J.; Gil, I.; Portillo, M.; et al. Equivalent-circuit models for split-ring resonators and complementary split-ring resonators coupled to planar transmission lines. IEEE Trans. Microw. Theory Tech. 2005, 53, 1451–1461. [Google Scholar] [CrossRef]
- Dolling, G.; Enkrich, C.; Wegener, M.; Zhou, J.; Soukoulis, C.M.; Linden, S. Cut-wire pairs and plate pairs as magnetic atoms for optical metamaterials. Opt. Lett. 2005, 30, 3198–3200. [Google Scholar] [CrossRef]
- Sydoruk, O.; Tatartschuk, E.; Shamonina, E.A.; Solymar, L. Analytical formulation for the resonant frequency of split rings. J. Appl. Phys. 2009, 105, 014903. [Google Scholar] [CrossRef]
- Stanze, D.; Deninger, A.; Roggenbuck, A.; Schindler, S.; Schlak, M.; Sartorius, B. Compact cw terahertz spectrometer pumped at 1.5 μm wavelength. J. Infrared Millim. Terahertz Waves 2011, 32, 225–232. [Google Scholar] [CrossRef][Green Version]
- CST Studio Suite. CST of America. Available online: https://www.3ds.com/products-services/simulia/products/cst-studio-s (accessed on 1 February 2022).
- Huang, D.; Liao, F.; Molesa, S.; Redinger, D.; Subramanian, V. Plastic-compatible low resistance printable gold nanoparticle conductors for flexible electronics. J. Electrochem. Soc. 2003, 150, G412. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional gold nanoparticles: A novel nanomaterial for various medical applications and biological activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Allec, N.; Choi, M.; Yesupriya, N.; Szychowski, B.; White, M.; Kann, M.G.; Garcin, E.; Daniel, M.-C.; Badano, A. Small-angle X-ray scattering method to characterize molecular interactions: Proof of concept. Sci. Rep. 2015, 5, 12085. [Google Scholar] [CrossRef] [PubMed]
- Roggenbuck, A.; Schmitz, H.; Deninger, A.; Mayorga, I.C.; Hemberger, J.; Güsten, R.; Grüninger, M. Coherent broadband continuous-wave terahertz spectroscopy on solid-state samples. New J. Phys. 2010, 12, 043017. [Google Scholar] [CrossRef]
- Yang, K.; Yu, W.; Huang, G.; Zhou, J.; Yang, X.; Fu, W. Highly sensitive detection of Staphylococcus aureus by a THz metamaterial biosensor based on gold nanoparticles and rolling circle amplification. RSC Adv. 2020, 10, 26824–26833. [Google Scholar] [CrossRef]
- Martines-Arano, H.; García-Pérez, B.E.; Vidales-Hurtado, M.A.; Trejo-Valdez, M.; Hernández-Gómez, L.H.; Torres-Torres, C. Chaotic signatures exhibited by plasmonic effects in au nanoparticles with cells. Sensors 2019, 19, 4728. [Google Scholar] [CrossRef] [PubMed]
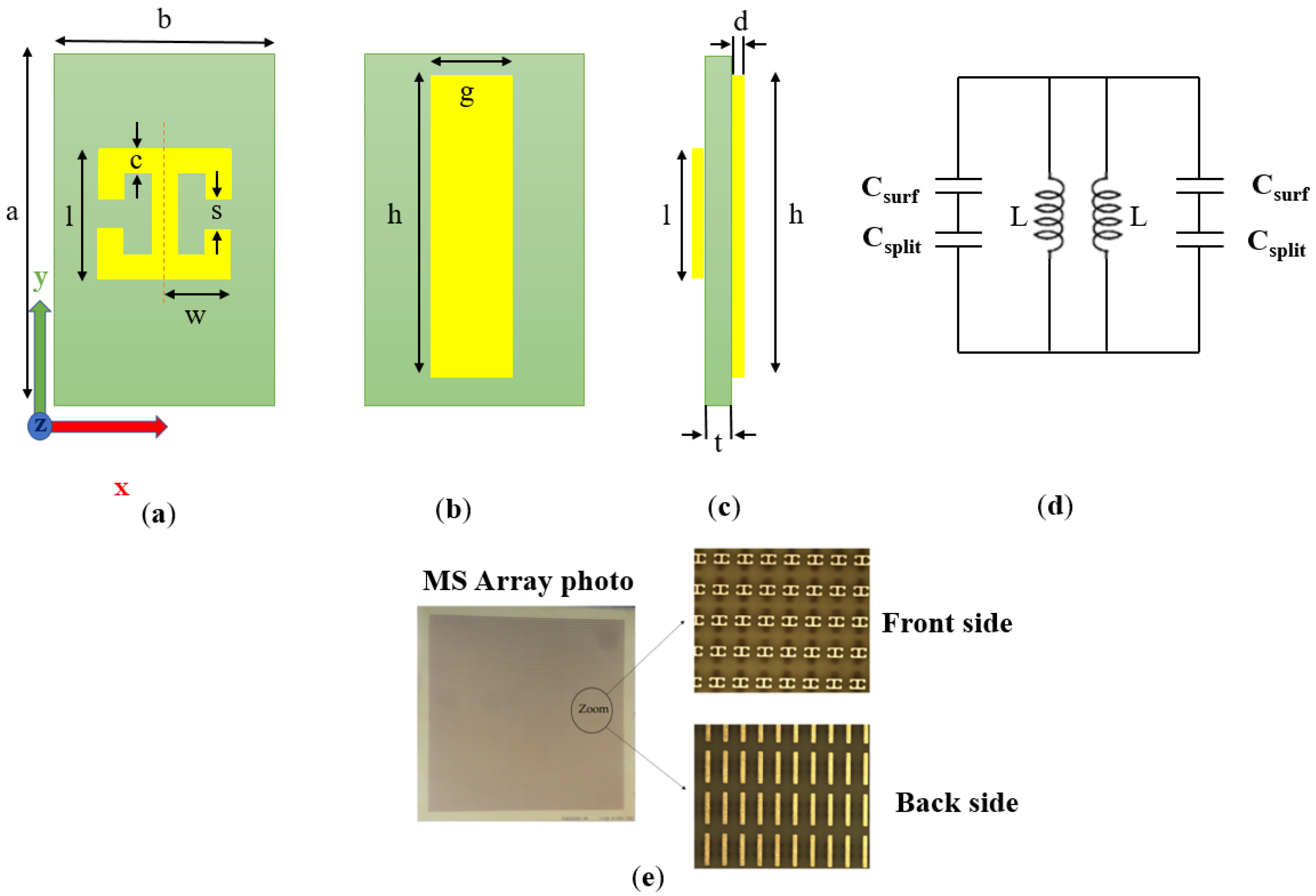

| Parameter | Description | Value (mm) |
|---|---|---|
| a | Unit cell length | 1.6 |
| b | Unit cell width | 0.6 |
| l | DSRR length | 0.5 |
| w | DSRR half width | 0.25 |
| c | DSRR strip width | 0.1 |
| s | DSRR gap | 0.1 |
| g | CW width | 0.2 |
| h | CW length | 1.2 |
| t | FR4 substrate thickness | 0.1 |
| d | Copper thickness | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramovich, A.; Azoulay, Y.; Rotshild, D. Real-Time Metasurface Sensor for Monitoring Micropoisons in Aqueous Solutions Based on Gold Nanoparticles and Terahertz Spectroscopy. Sensors 2022, 22, 1279. https://doi.org/10.3390/s22031279
Abramovich A, Azoulay Y, Rotshild D. Real-Time Metasurface Sensor for Monitoring Micropoisons in Aqueous Solutions Based on Gold Nanoparticles and Terahertz Spectroscopy. Sensors. 2022; 22(3):1279. https://doi.org/10.3390/s22031279
Chicago/Turabian StyleAbramovich, Amir, Yossi Azoulay, and David Rotshild. 2022. "Real-Time Metasurface Sensor for Monitoring Micropoisons in Aqueous Solutions Based on Gold Nanoparticles and Terahertz Spectroscopy" Sensors 22, no. 3: 1279. https://doi.org/10.3390/s22031279
APA StyleAbramovich, A., Azoulay, Y., & Rotshild, D. (2022). Real-Time Metasurface Sensor for Monitoring Micropoisons in Aqueous Solutions Based on Gold Nanoparticles and Terahertz Spectroscopy. Sensors, 22(3), 1279. https://doi.org/10.3390/s22031279






