High-Throughput Continuous-Flow Separation in a Micro Free-Flow Electrophoresis Glass Chip Based on Laser Microfabrication
Abstract
:1. Introduction
2. Materials and Methods
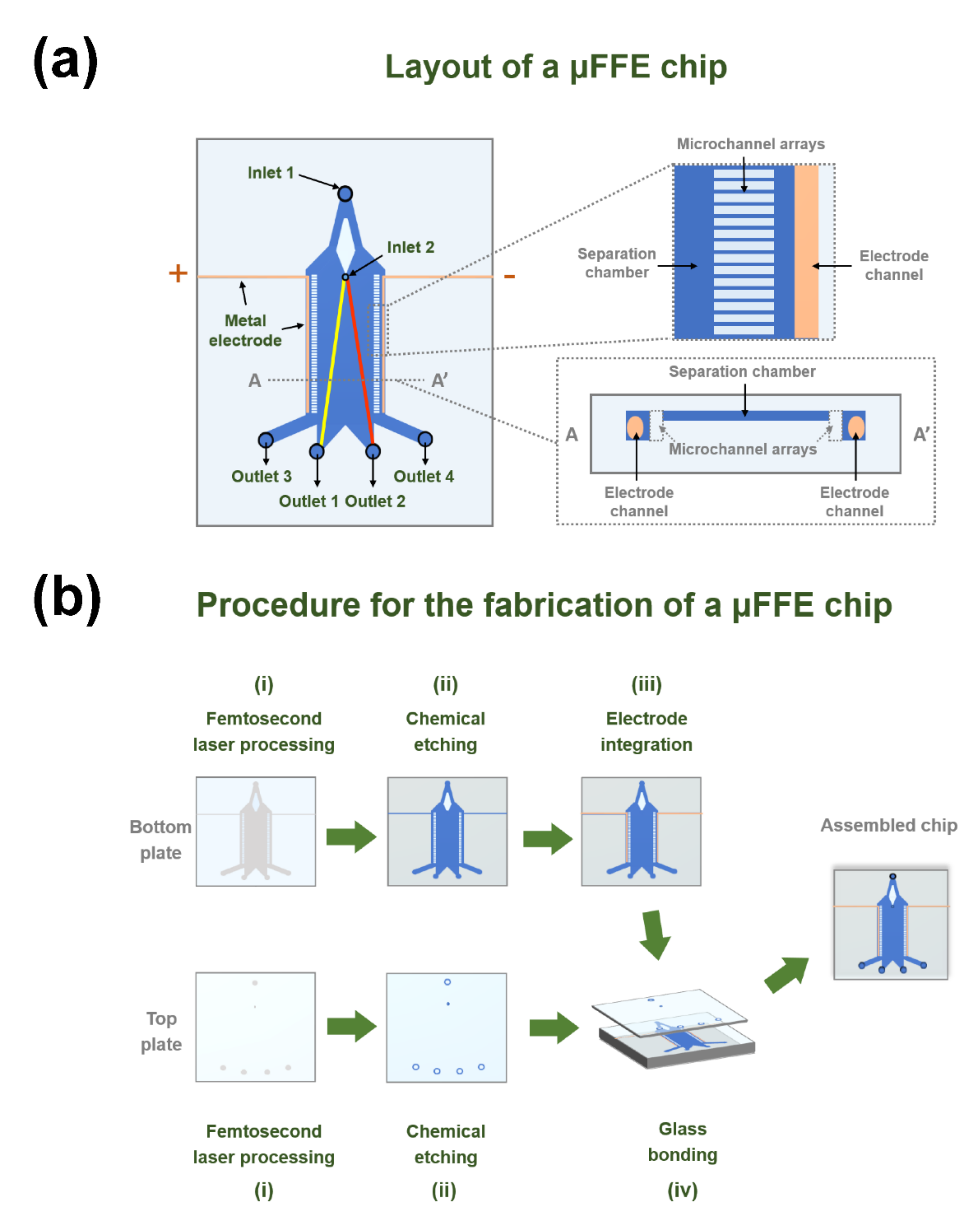
2.1. Chip Design
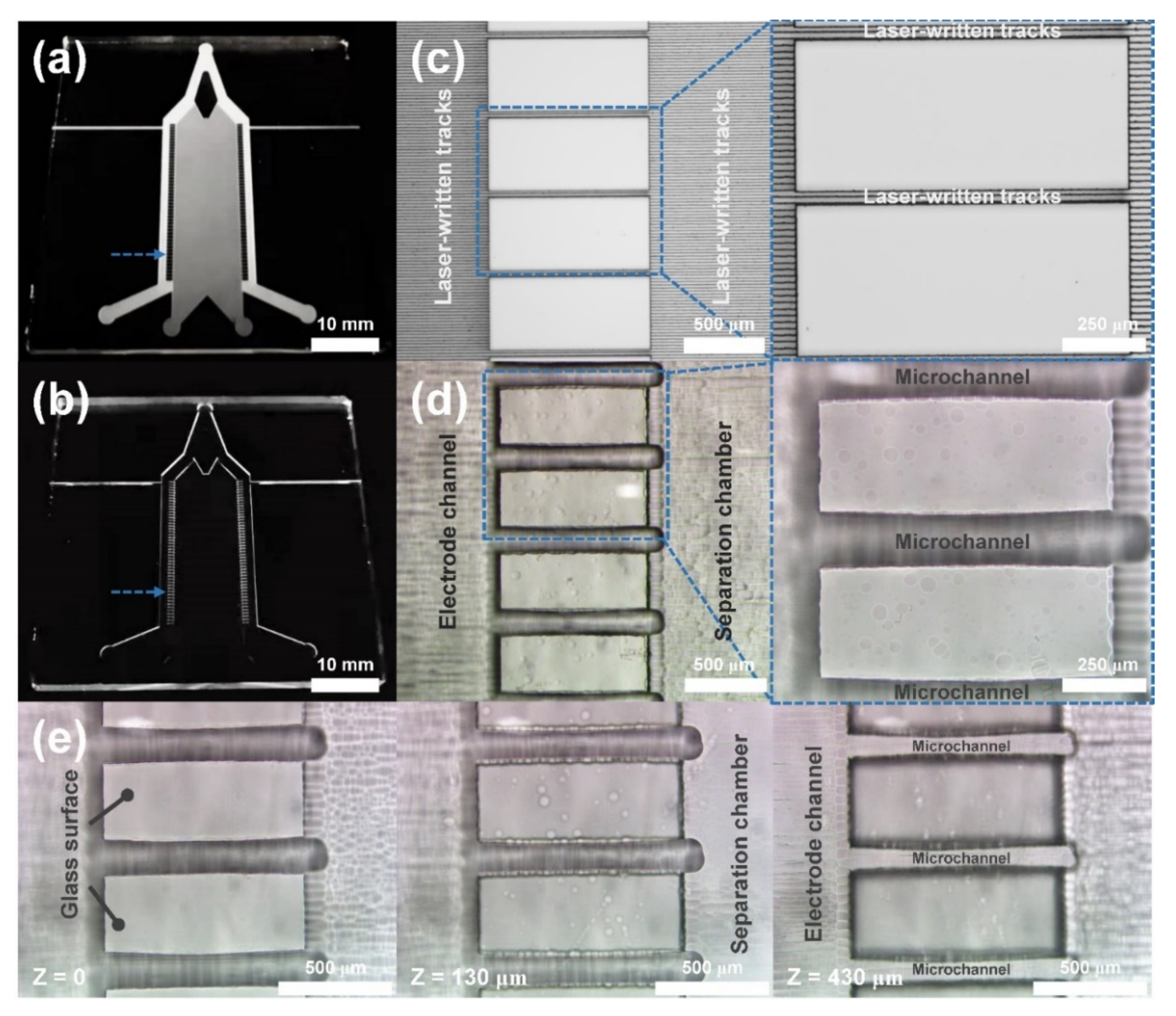
2.2. Fabrication of a μFFE Chip
2.3. On-Chip Separation
2.4. Characterization
3. Results
3.1. Femtosecond Laser-Assisted Chemical Etching of Fused Silica Glass
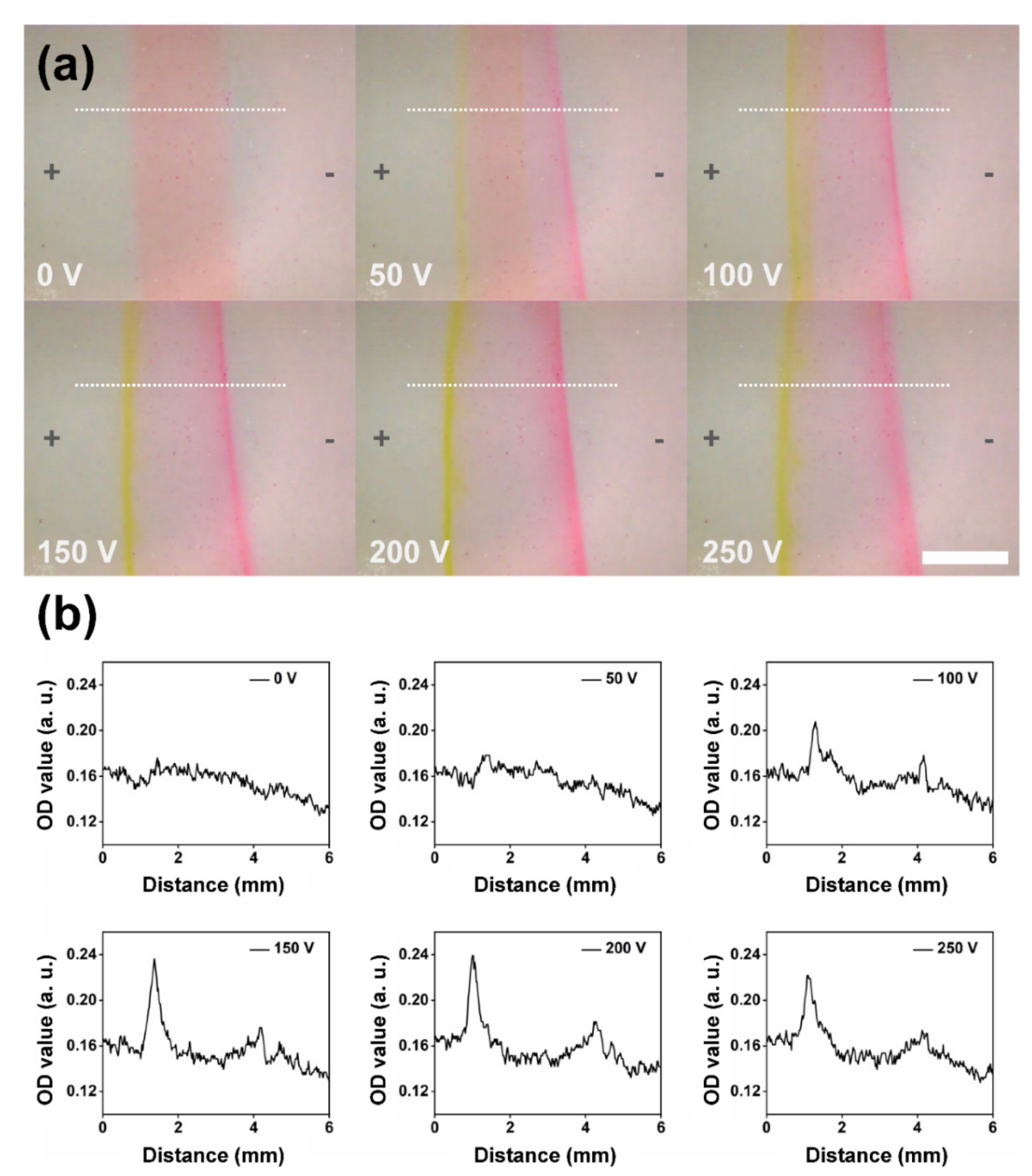
3.2. On-Chip Separation under Different Applied Voltages
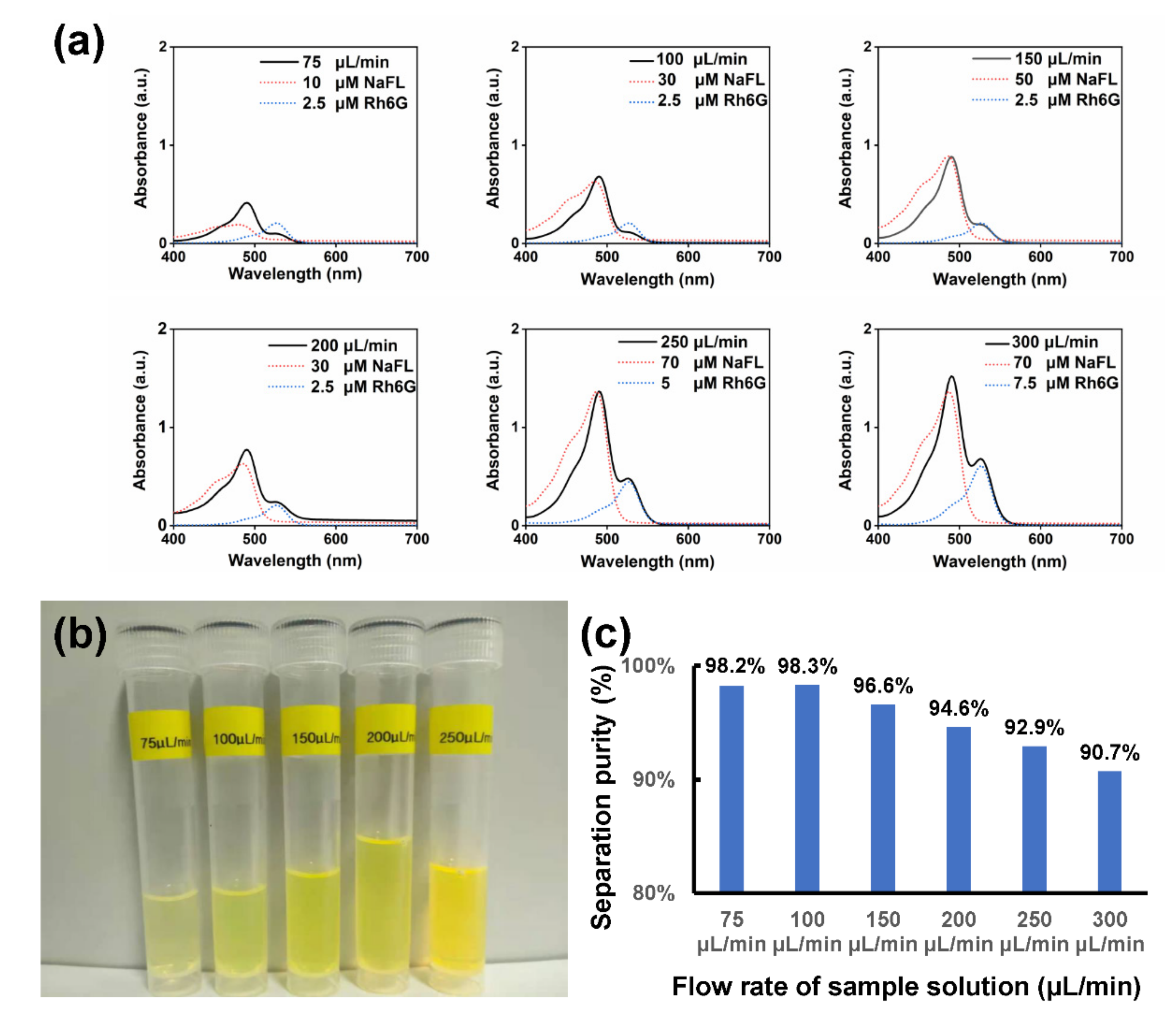
3.3. On-Chip Separation under Different Flow Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kohlheyer, D.; Eijkel, J.C.T.; van den Berg, A.; Schasfoort, R.B.M. Miniaturizing free-flow electrophoresis—A critical review. Electrophoresis 2008, 29, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, R.T.; Bowser, M.T. Micro free-flow electrophoresis: Theory and applications. Anal. Bioanal. Chem. 2009, 394, 187–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostino, F.J.; Krylov, S.N. Advances in steady-state continuous-flow purification by small-scale free-flow electrophoresis. Trends Anal. Chem. 2015, 72, 68–79. [Google Scholar] [CrossRef]
- Novo, P.; Janasek, D. Current advances and challenges in microfluidic free-flow electrophoresis—A critical review. Anal. Chim. Acta 2017, 991, 9–29. [Google Scholar] [CrossRef]
- Johnson, A.C.; Bowser, M.T. Micro free flow electrophoresis. Lab Chip 2018, 18, 27–40. [Google Scholar] [CrossRef]
- Stastna, M. Continuous flow electrophoretic separation—Recent developments and applications to biological sample analysis. Electrophoresis 2020, 41, 36–55. [Google Scholar] [CrossRef]
- Köhler, S.; Benz, C.; Becker, H.; Beckert, E.; Beushausend, V.; Belder, D. Micro free-flow electrophoresis with injection molded chips. RSC Adv. 2012, 2, 520–525. [Google Scholar] [CrossRef]
- Song, Y.A.; Wu, L.; Tannenbaum, S.R.; Wishnok, J.S.; Han, J. Tunable membranes for free-flow zone electrophoresis in PDMS microchip using guided self-assembly of silica microbeads. Anal. Chem. 2013, 85, 11695–11699. [Google Scholar] [CrossRef] [Green Version]
- Anciaux, S.K.; Geiger, M.; Bowser, M.T. 3D printed micro free-flow electrophoresis device. Anal. Chem. 2016, 88, 7675–7682. [Google Scholar] [CrossRef]
- Köhler, S.; Nagl, S.; Fritzsche, S.; Belder, D. Label-free real-time imaging in microchip free-flow electrophoresis applying high speed deep UV fluorescence scanning. Lab Chip 2012, 12, 458–463. [Google Scholar] [CrossRef]
- Kohlheyer, D.; Besselink, G.A.J.; Schlautmann, S.; Schasfoort, R.B.M. Free-flow zone electrophoresis and isoelectric focusing using a microfabricated glass device with ion permeable membranes. Lab Chip 2006, 6, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Podszun, S.; Vulto, P.; Heinz, H.; Hakenberg, S.; Hermann, C.; Hankemeier, T.; Urban, G.A. Enrichment of viable bacteria in a micro-volume by free-flow electrophoresis. Lab Chip 2012, 12, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Kohlheyer, D.; Eijkel, J.C.T.; Schlautmann, S.; van den Berg, A.; Schasfoort, R.B.M. Bubble-free operation of a microfluidic free-flow electrophoresis chip with integrated Pt electrodes. Anal. Chem. 2008, 80, 4111–4118. [Google Scholar] [CrossRef]
- Fonslow, B.R.; Barocas, V.H.; Bowser, M.T. Using channel depth to isolate and control flow in a micro free-flow electrophoresis device. Anal. Chem. 2006, 78, 5369–5374. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Mavrogiannis, N.; Ibo, M.; Crivellari, F.; Gagnon, Z.R. Microfluidic free-flow zone electrophoresis and isotachophoresis using carbon black nano-composite PDMS sidewall membranes. Electrophoresis 2017, 38, 327–334. [Google Scholar] [CrossRef]
- Novo, P.; Dell’Aica, M.; Jender, M.; Höving, S.; Zahedi, R.P.; Janasek, D. Integration of polycarbonate membranes in microfluidic free-flow electrophoresis. Analyst 2017, 142, 4228–4239. [Google Scholar] [CrossRef]
- Cheng, L.J.; Chang, H.C. Switchable pH actuators and 3D integrated salt bridges as new strategies for reconfigurable microfluidic free-flow electrophoretic separation. Lab Chip 2014, 14, 979–987. [Google Scholar] [CrossRef]
- Frost, N.W.; Bowser, M.T. Using buffer additives to improve analyte stream stability in micro free flow electrophoresis. Lab Chip 2010, 10, 1231–1236. [Google Scholar] [CrossRef]
- Jezierski, S.; Tehsmer, V.; Nagl, S.; Belder, D. Integrating continuous microflow reactions with subsequent micropreparative separations on a single microfluidic chip. Chem. Commun. 2013, 49, 11644–11646. [Google Scholar] [CrossRef]
- Janasek, D.; Schilling, M.; Franzke, J.; Manz, A. Isotachophoresis in free-flow using a miniaturized device. Anal. Chem. 2006, 78, 3815–3819. [Google Scholar] [CrossRef]
- Zhang, C.X.; Manz, A. High-speed free-flow electrophoresis on chip. Anal. Chem. 2003, 75, 5759–5766. [Google Scholar] [CrossRef] [PubMed]
- Bellouard, Y.; Said, A.; Dugan, M.; Bado, P. Fabrication of high-aspect-ratio, microfluidic channels and tunnels using femtosecond laser pulses and chemical etching. Opt. Express 2004, 12, 2120–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyama, S.; Matsuo, S.; Hashimoto, S.; Morihira, Y. Examination of etching agent and etching mechanism on femtosecond laser microfabrication of channels inside vitreous silica substrates. J. Phys. Chem. C 2009, 113, 11560–11566. [Google Scholar] [CrossRef]
- Sugioka, K.; Xu, J.; Wu, D.; Hanada, Y.; Wang, Z.; Cheng, Y.; Midorikawa, K. Femtosecond laser 3D micromachining: A powerful tool for the fabrication of microfluidic, optofluidic, and electrofluidic devices based on glass. Lab Chip 2014, 14, 3447–3458. [Google Scholar] [CrossRef]
- Cheng, Y. Internal laser writing of high-aspect-ratio microfluidic structures in silicate glasses for lab-on-a-chip applications. Micromachines 2017, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Xu, J.; Song, Y.; Li, X.; Wang, P.; Chu, W.; Wang, Z.; Cheng, Y. Freeform microfluidic networks encapsulated in laser printed 3D macro-scale glass objects. Adv. Mater. Technol. 2020, 5, 1900989. [Google Scholar] [CrossRef]
- Fonslow, B.R.; Bowser, M.T. Optimizing band width and resolution in micro-free flow electrophoresis. Anal. Chem. 2006, 78, 8236–8244. [Google Scholar] [CrossRef]
- Song, J.; Liu, T.; Shen, X.; Wu, G.; Xia, Q. Application of free-flow electrophoresis to the purification of trichosanthin from a crude product of acetone fractional precipitation. Electrophoresis 1998, 19, 1097–1103. [Google Scholar] [CrossRef]
- Kuhn, R.; Wagner, H. Free flow electrophoresis as a method for the purification of enzymes from E. coli cell extract. Electrophoresis 1989, 10, 165–172. [Google Scholar] [CrossRef]
- Saar, K.L.; Müller, T.; Charmet, J.; Challa, P.K.; Knowles, T.P.J. Enhancing the resolution of micro free flow electrophoresis through spatially controlled sample injection. Anal. Chem. 2018, 90, 8998–9005. [Google Scholar] [CrossRef] [Green Version]


| Manufacturing Method | Flow Rate of the Sample Stream | Electric-Field Intensity | Height of Separation Chamber | Height of Electrode Channel |
|---|---|---|---|---|
| Soft lithography of PDMS [8] | 2 μL/min | ~137 V/cm | 13 μm | 13 μm |
| 3D printing of ABS plastics [9] | 1 μL/min | ~100 V/cm | 80 μm | 345 μm |
| Lithographic processing of borosilicate glass followed by glass/glass bonding [11] | 0.18 μL/min | ~250 V/cm | 15 μm | 15 μm |
| Lithographic processing of Pyrex glass followed by glass/glass bonding [12] | 3 μL/min | ~61 V/cm | 116 μm | 116 μm |
| Lithographic processing of Borofloat33 glass followed by glass/glass bonding [13] | 0.1 μL/min | ~150 V/cm | 10 μm | 10 μm |
| Lithographic processing of Borofloat glass followed by glass/glass bonding [14] | 0.21 μL/min | ~264 V/cm | 20 μm | 78 μm |
| PDMS/glass bonding [30] | 0.33 μL/min | ~140 V/cm | 50 μm | 50 μm |
| This work | 300 μL/min | ~192 V/cm | 130 μm | 430 μm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Xu, J.; Li, X.; Lin, Z.; Song, Y.; Li, X.; Wang, Z.; Cheng, Y. High-Throughput Continuous-Flow Separation in a Micro Free-Flow Electrophoresis Glass Chip Based on Laser Microfabrication. Sensors 2022, 22, 1124. https://doi.org/10.3390/s22031124
Zhang A, Xu J, Li X, Lin Z, Song Y, Li X, Wang Z, Cheng Y. High-Throughput Continuous-Flow Separation in a Micro Free-Flow Electrophoresis Glass Chip Based on Laser Microfabrication. Sensors. 2022; 22(3):1124. https://doi.org/10.3390/s22031124
Chicago/Turabian StyleZhang, Aodong, Jian Xu, Xiaolong Li, Zijie Lin, Yunpeng Song, Xin Li, Zhenhua Wang, and Ya Cheng. 2022. "High-Throughput Continuous-Flow Separation in a Micro Free-Flow Electrophoresis Glass Chip Based on Laser Microfabrication" Sensors 22, no. 3: 1124. https://doi.org/10.3390/s22031124
APA StyleZhang, A., Xu, J., Li, X., Lin, Z., Song, Y., Li, X., Wang, Z., & Cheng, Y. (2022). High-Throughput Continuous-Flow Separation in a Micro Free-Flow Electrophoresis Glass Chip Based on Laser Microfabrication. Sensors, 22(3), 1124. https://doi.org/10.3390/s22031124







