Homogeneity Measurements of Li-Ion Battery Cathodes Using Laser-Induced Breakdown Spectroscopy
Abstract
1. Introduction
2. Materials
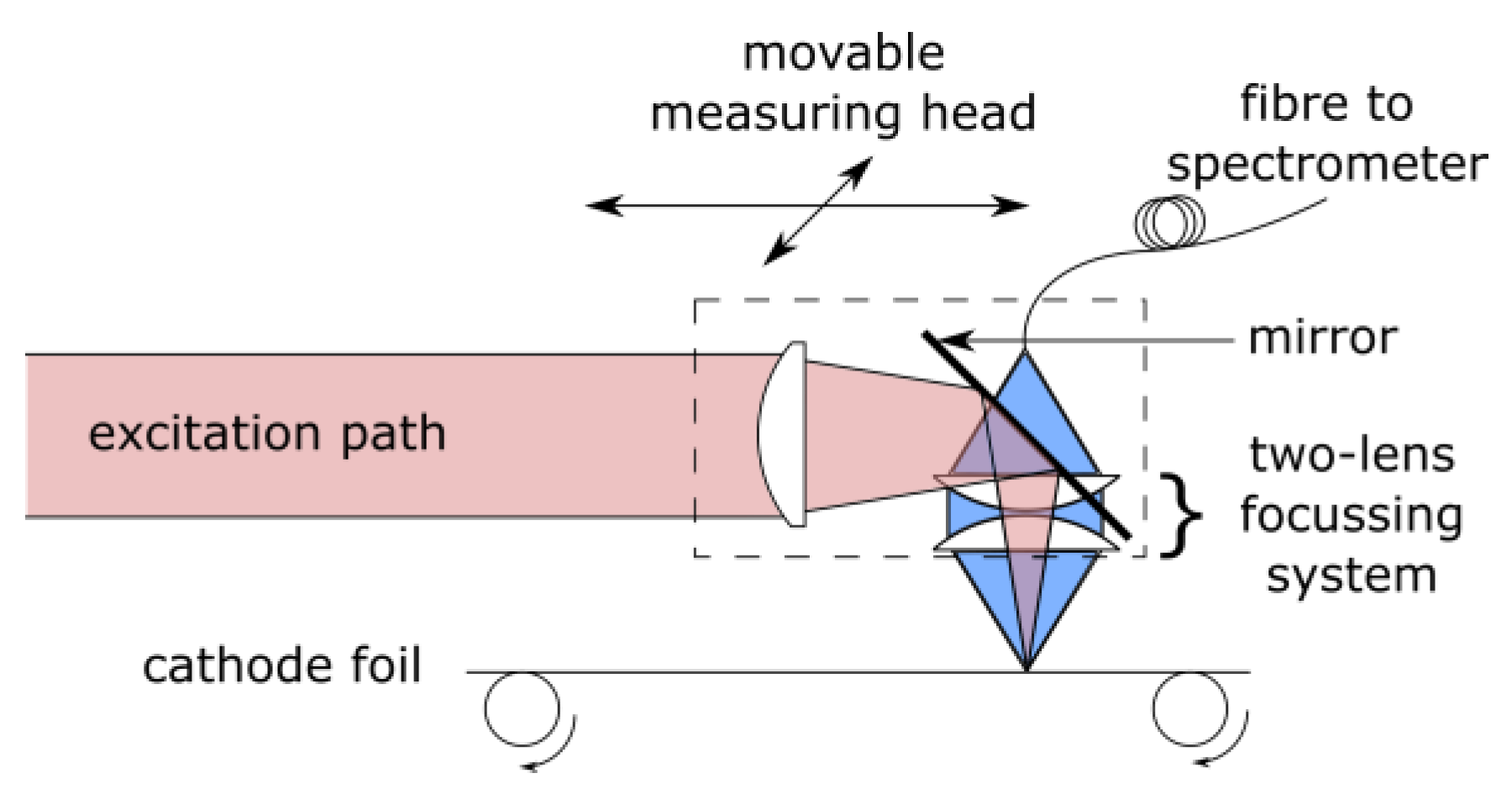
3. Methods
4. Results
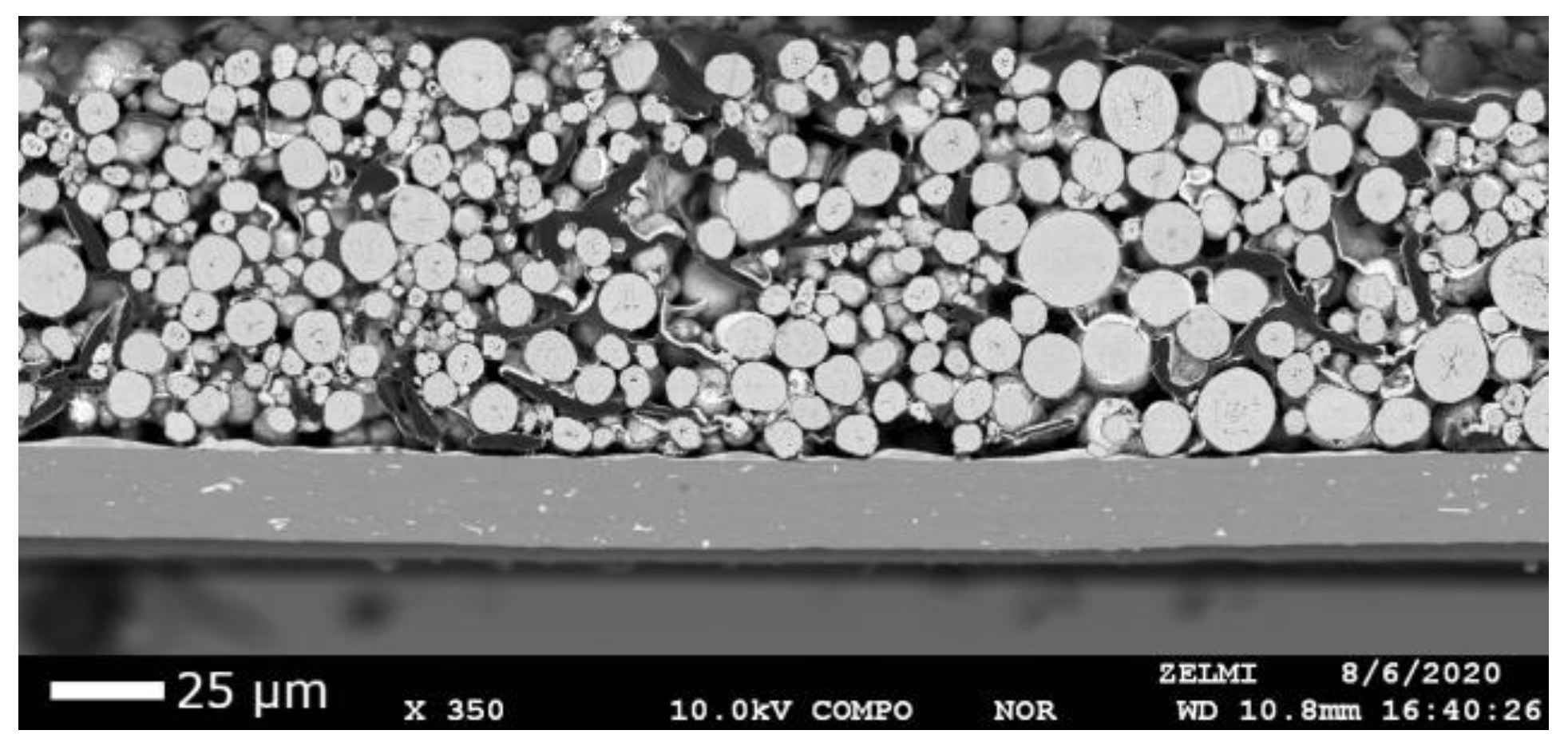
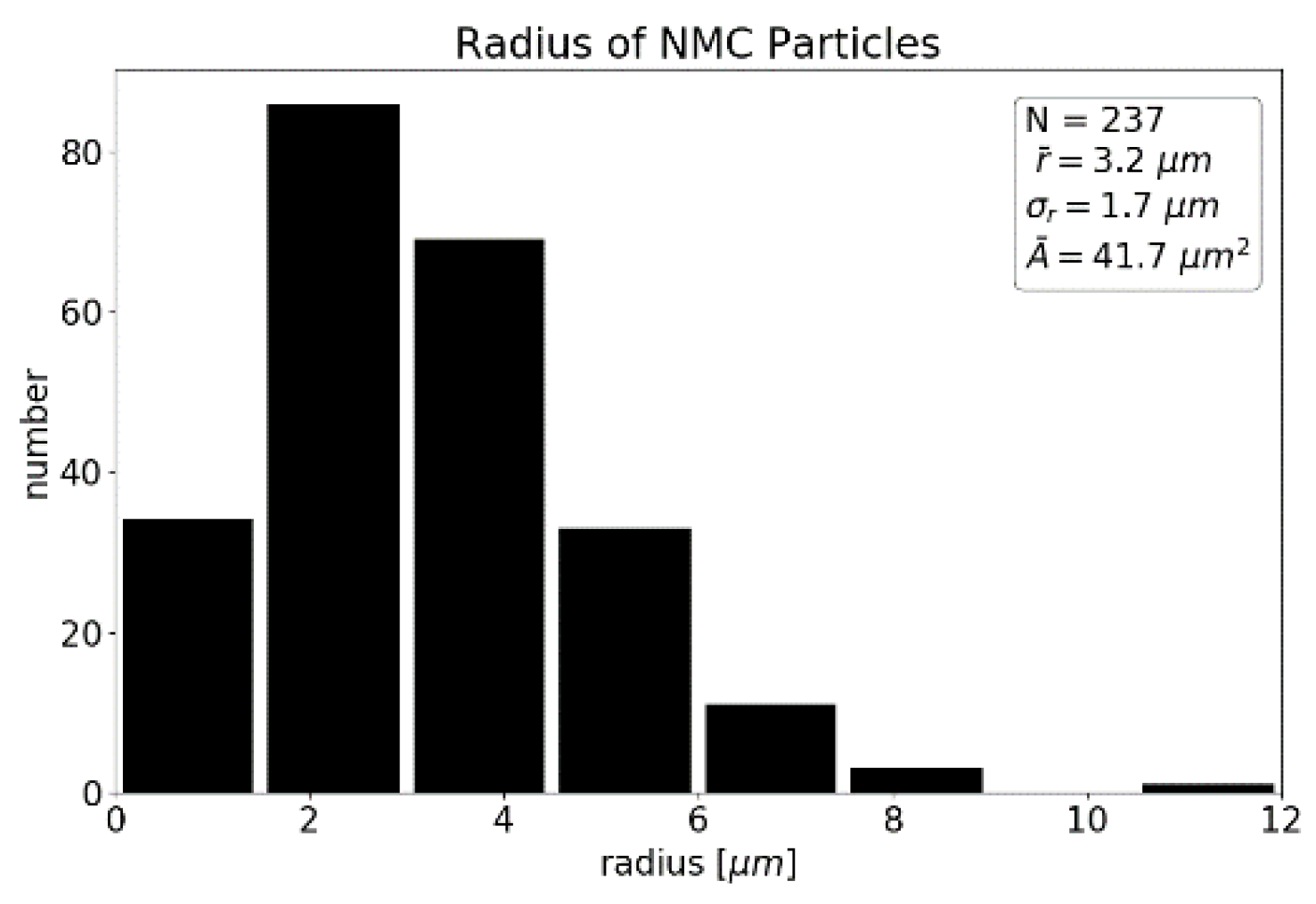
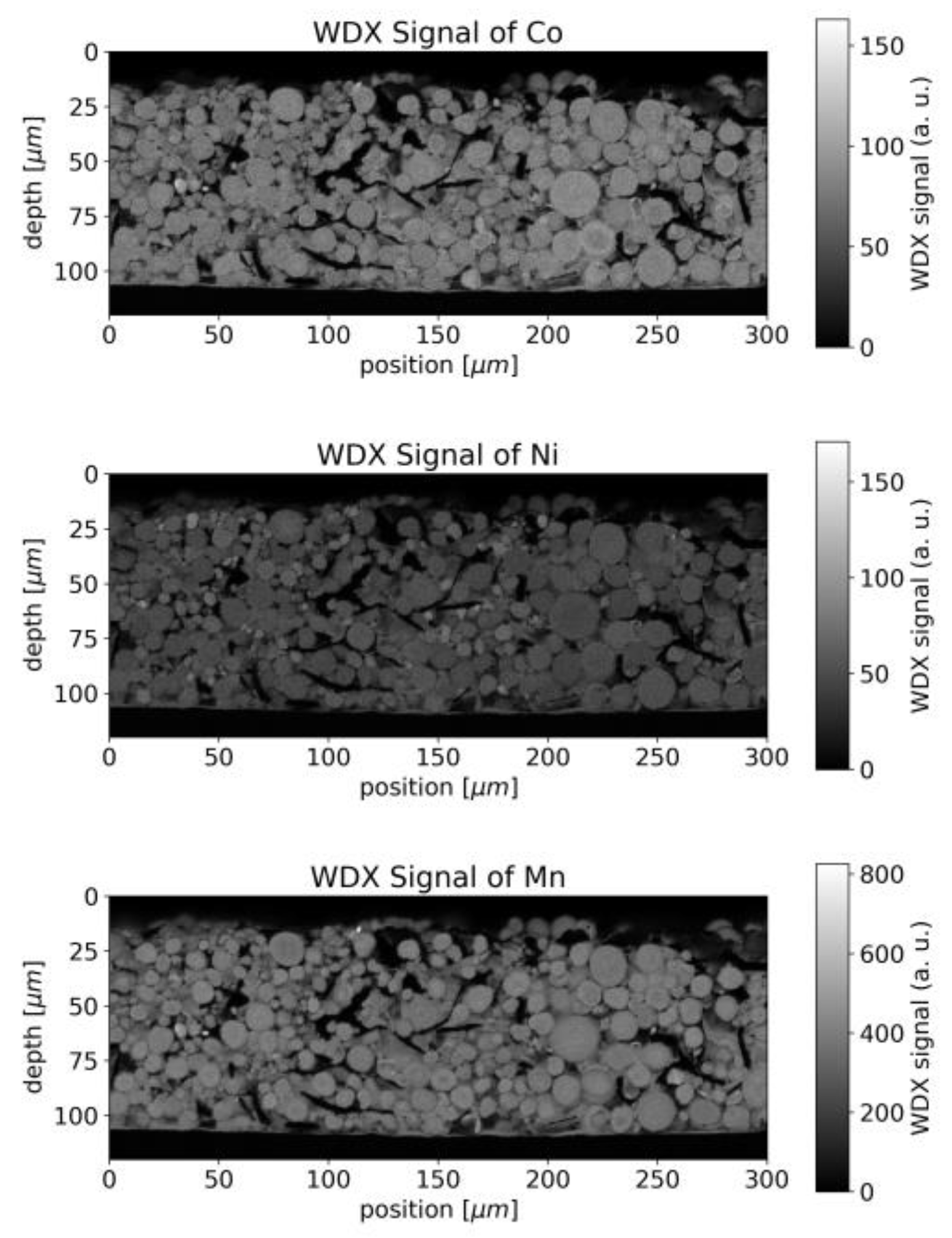
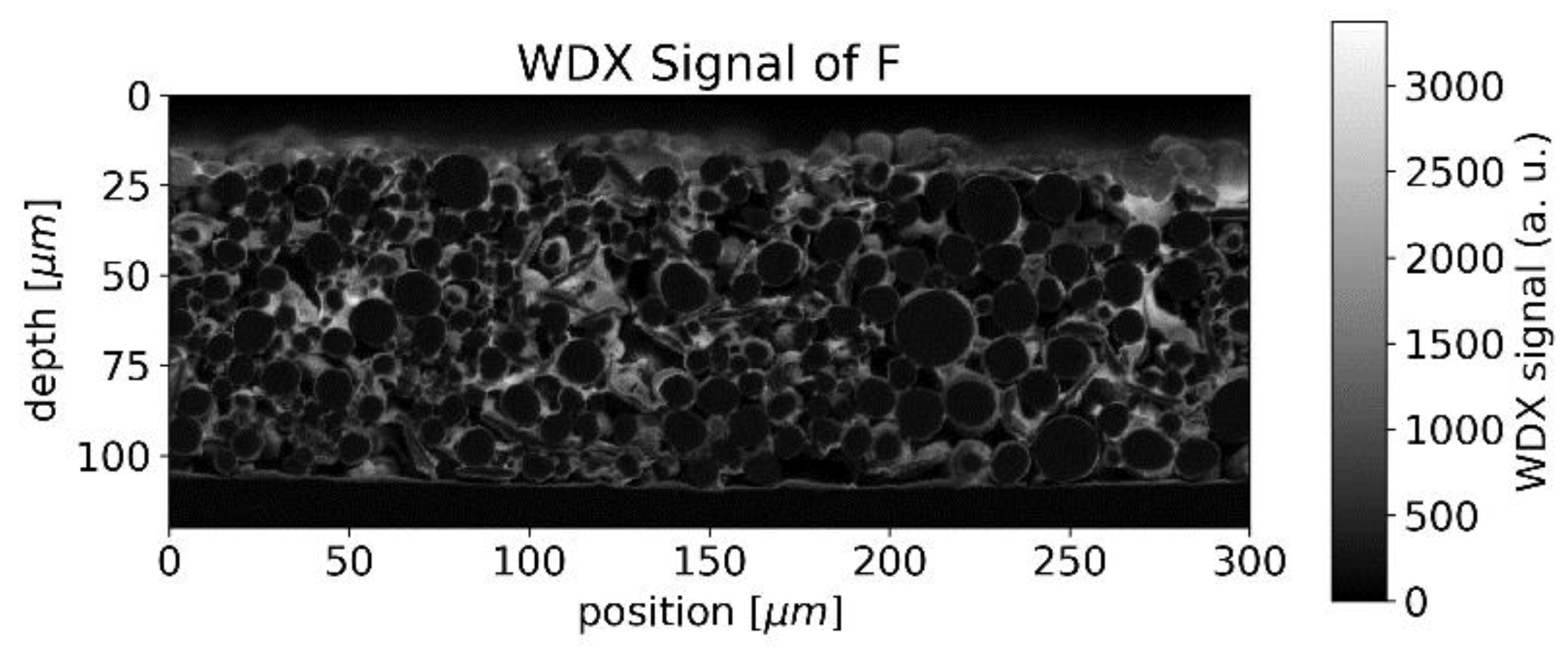
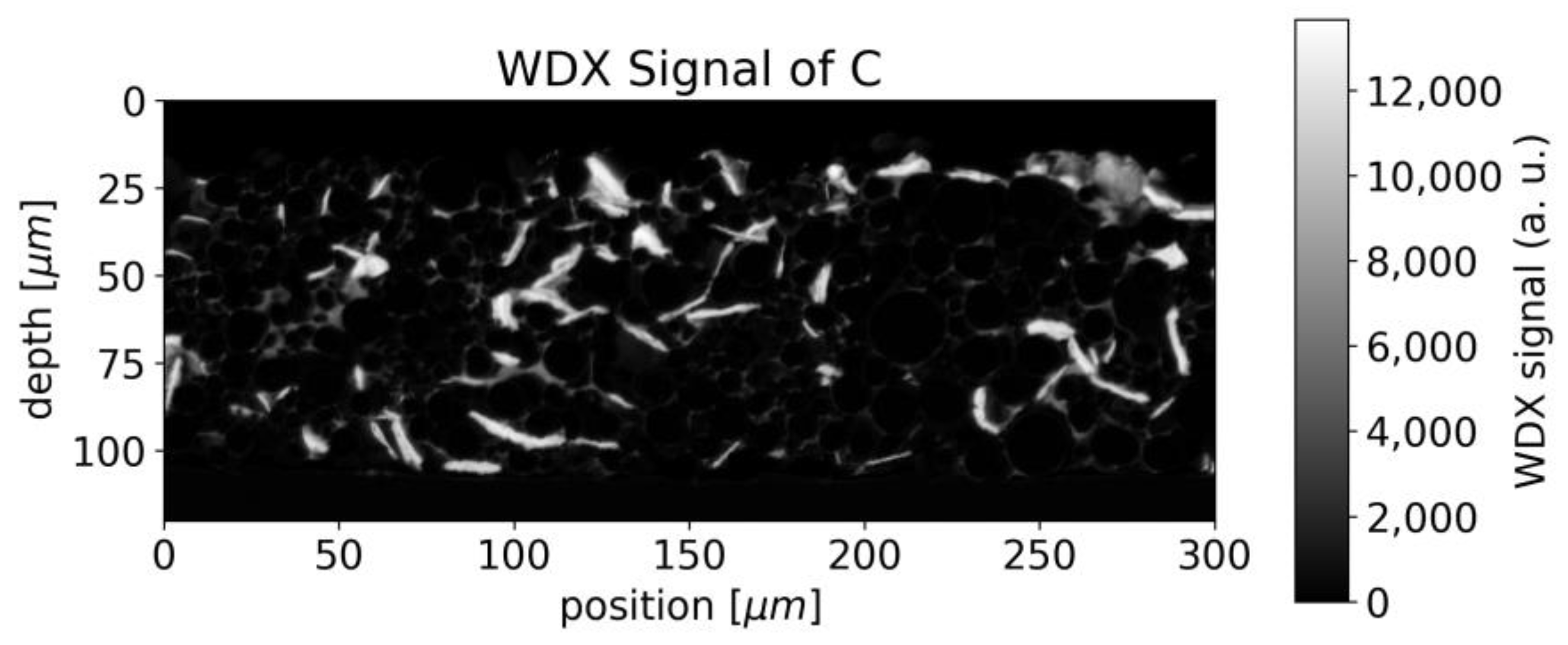
4.1. Cathode Characterization with EMPA
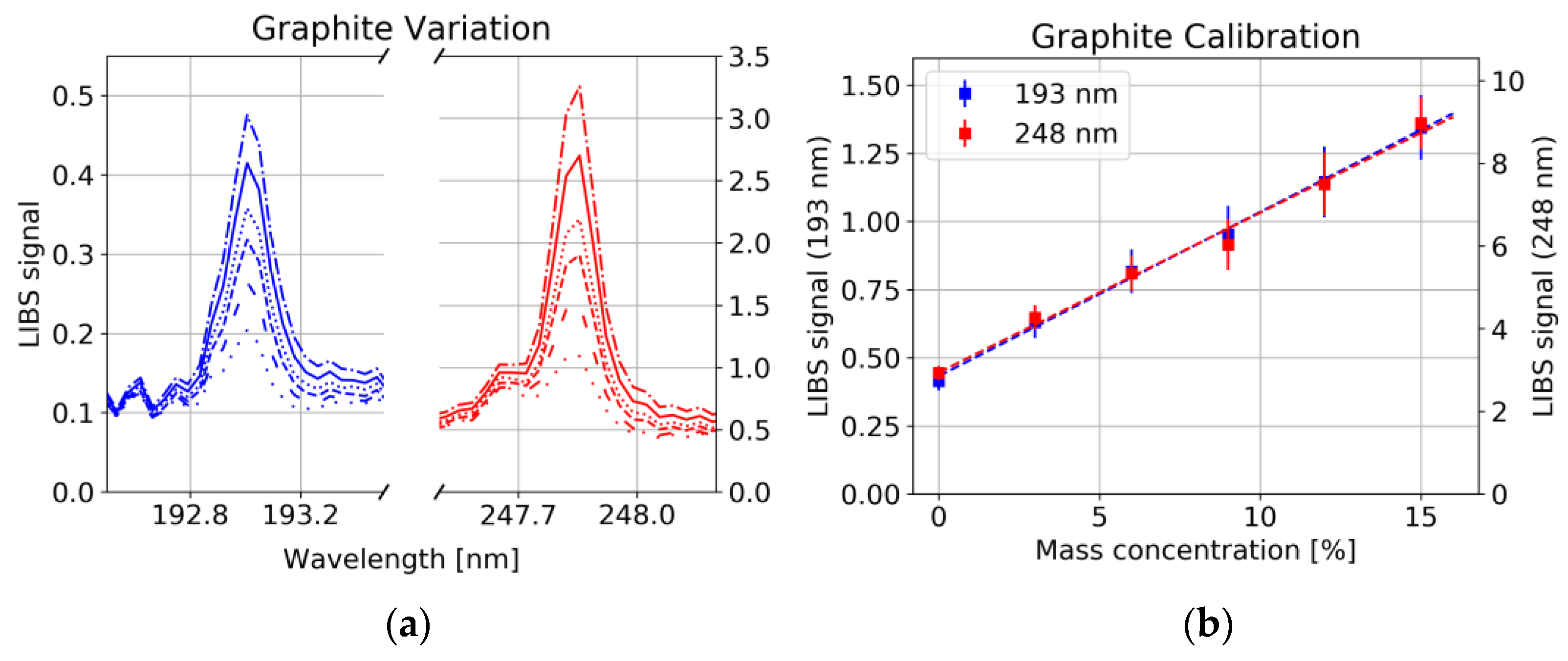
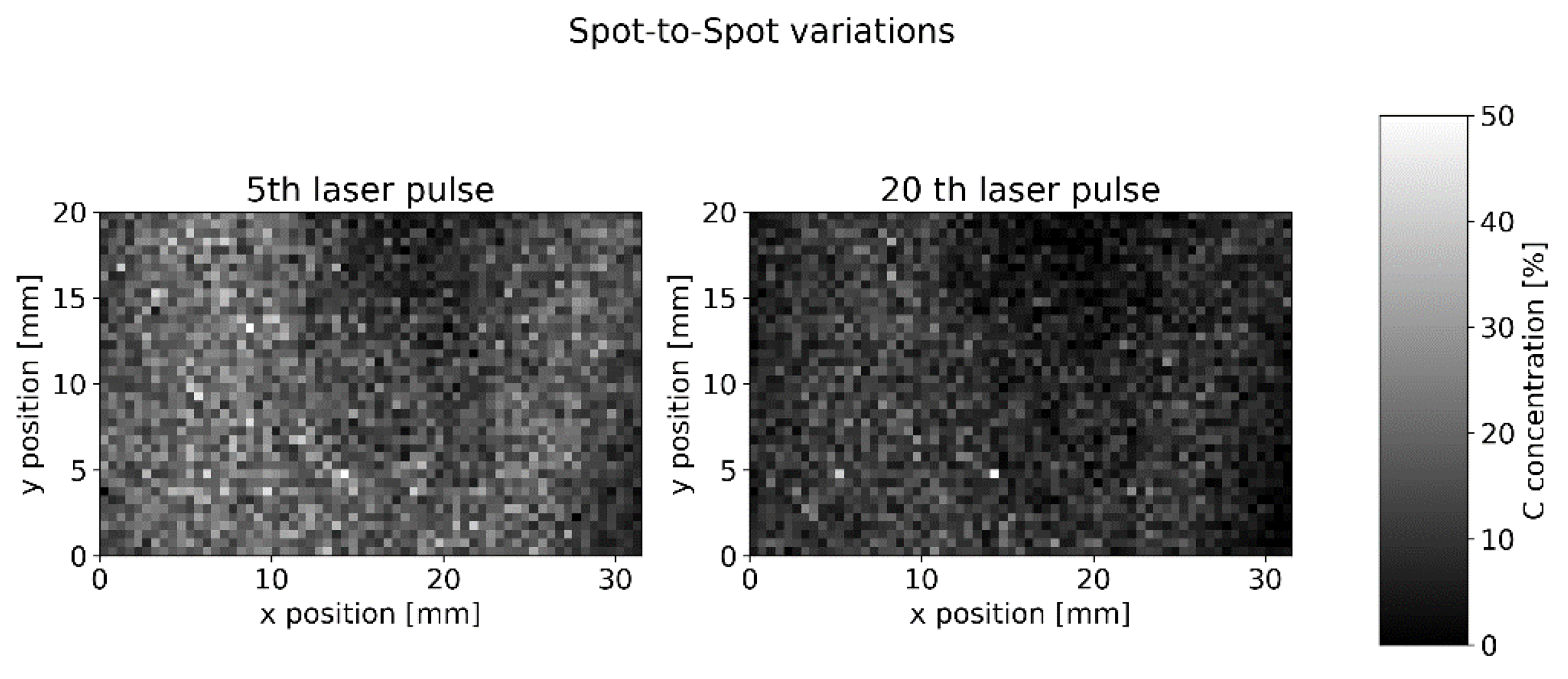
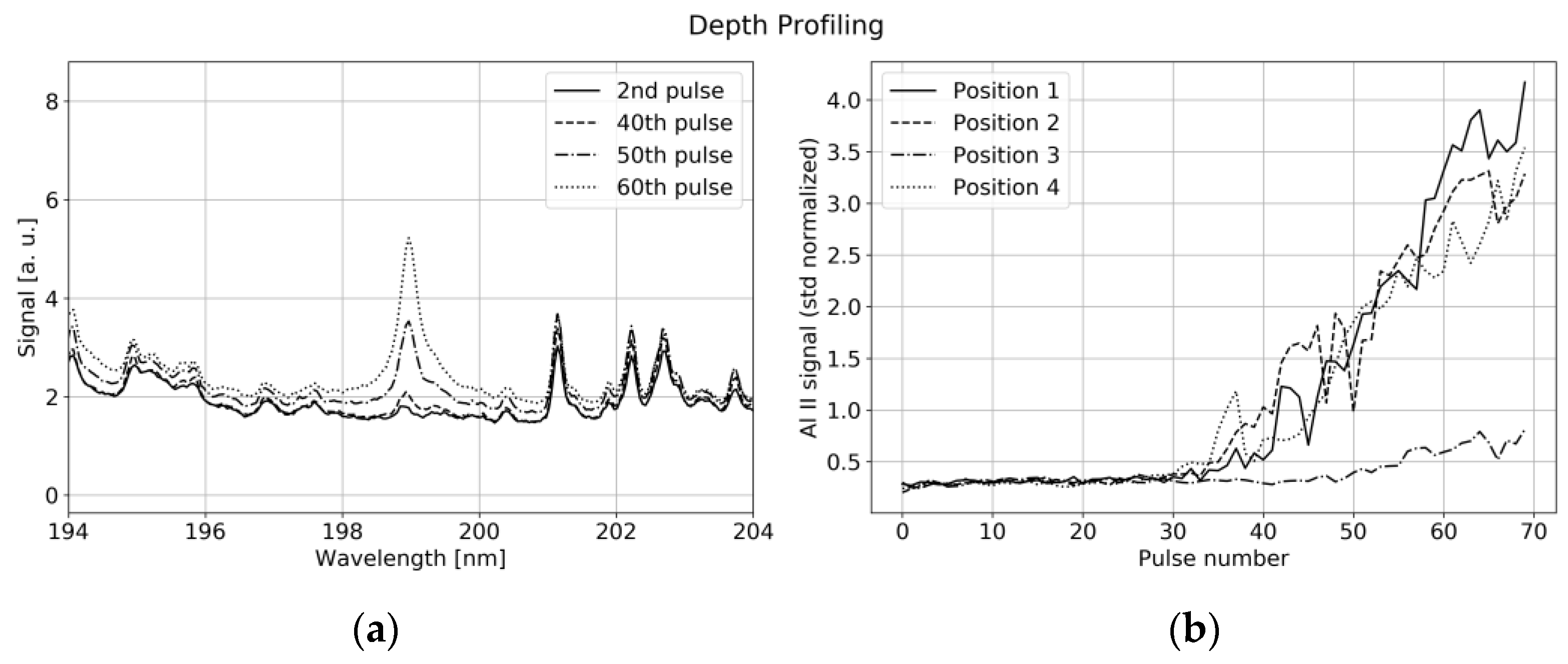
4.2. LIBS Measurement
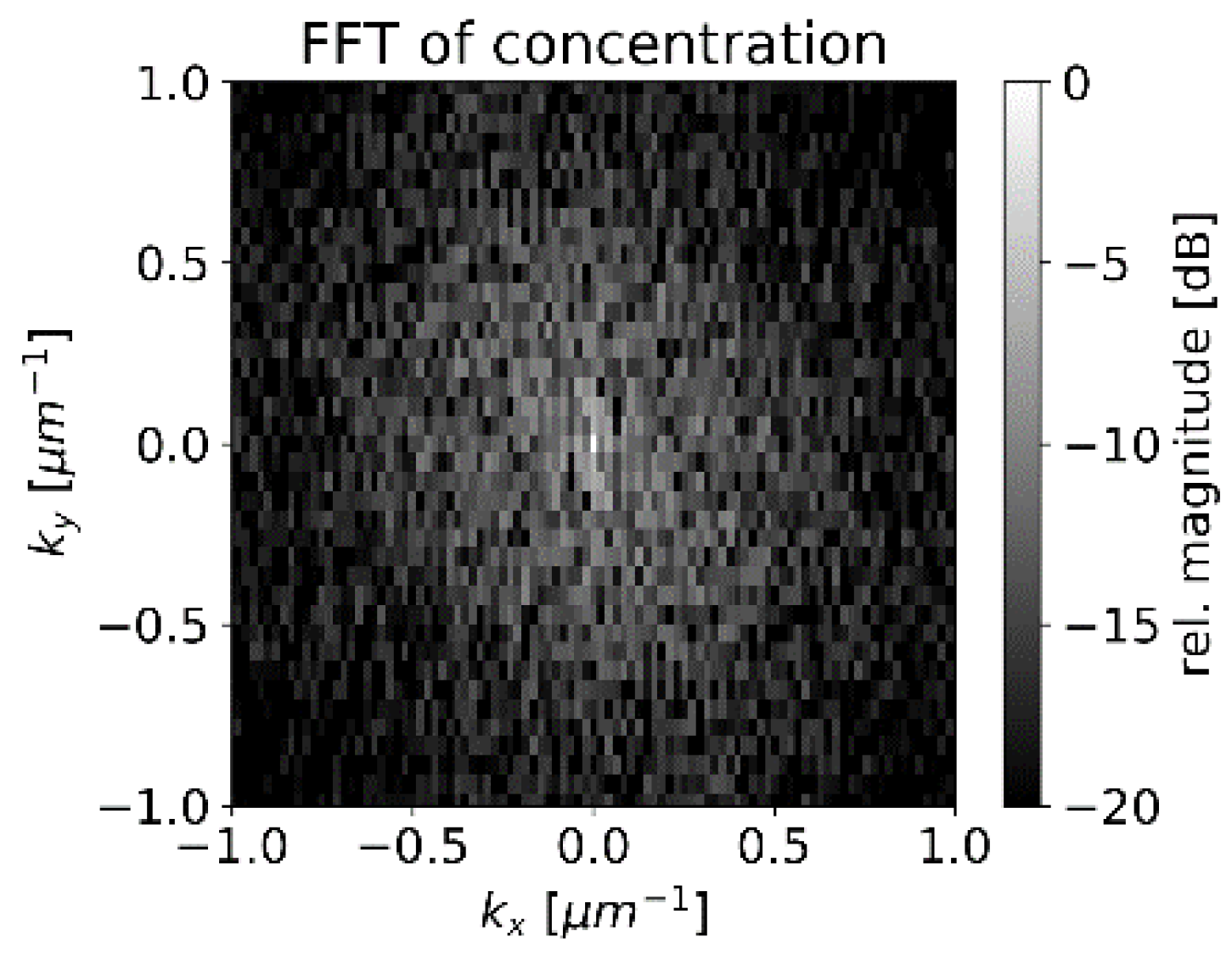
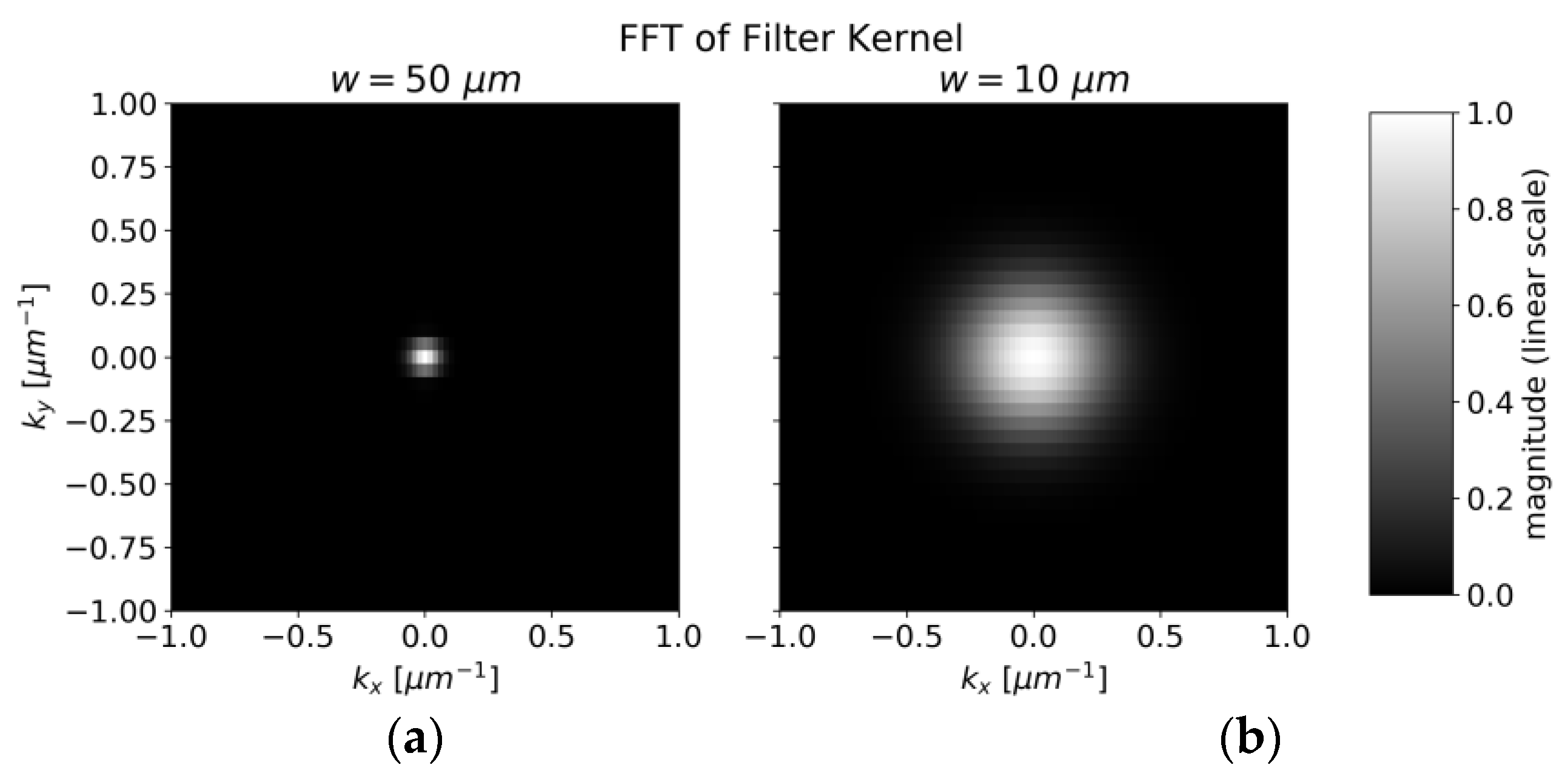
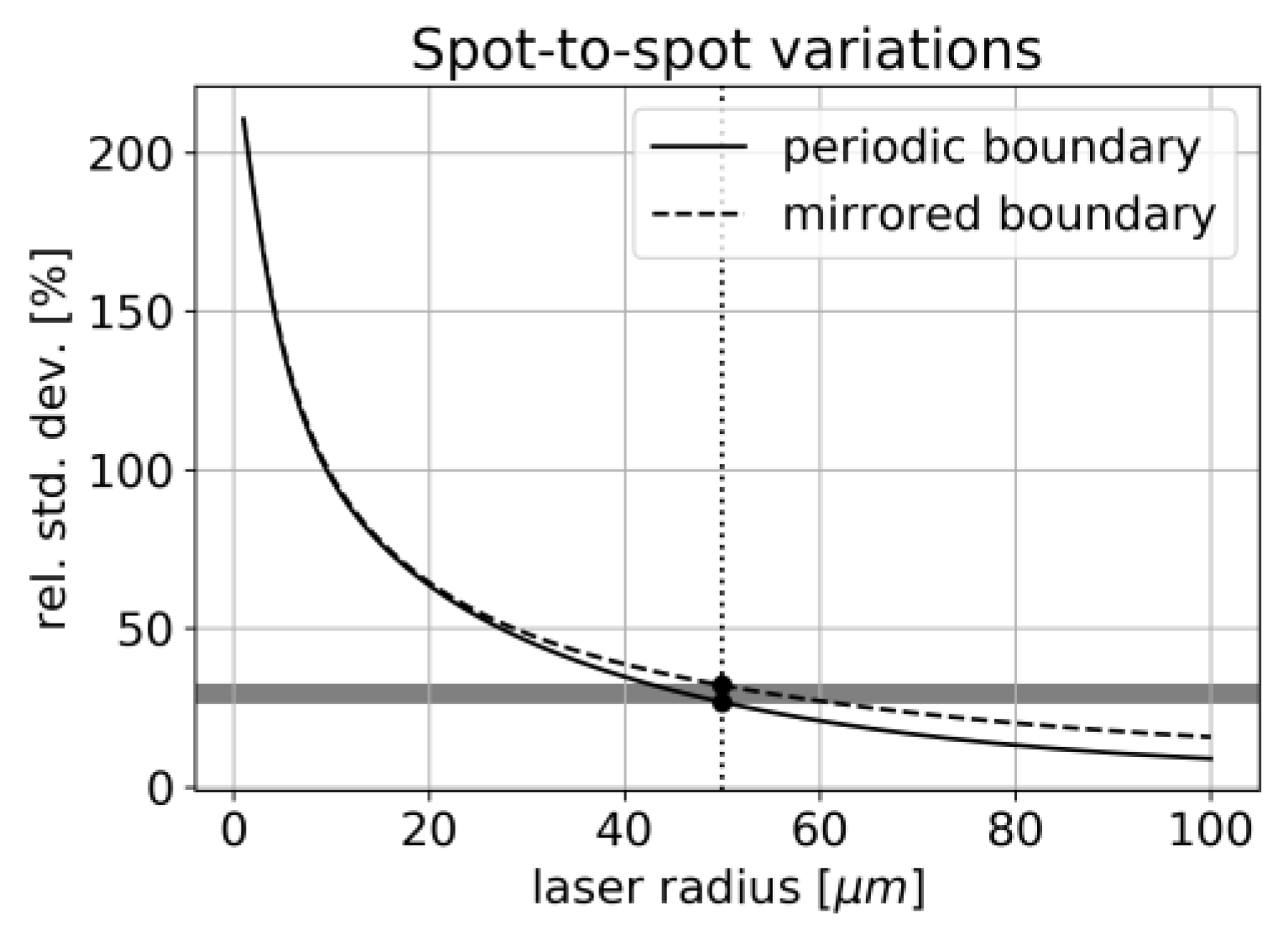
4.3. Concentration Measurement
4.4. Cathode Characterization with EMPA
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Cyclable Lithium and Capacity Loss in Li-Ion Cells. J. Power Sources 2005, 152, A818. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize battery degradation modes via a diagnostic and prognostic model. J. Power Sources 2012, 219, 204–216. [Google Scholar]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W.; et al. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Ross, P.N. Catalysis and Interfacial Chemistry in Lithium Batteries: A Surface Science Approach. Catal. Lett. 2014, 144, 1370–1376. [Google Scholar] [CrossRef]
- Nowak, S.; Winter, M. Elemental analysis of lithium ion batteries. J. Anal. At. Spectrom. 2017, 32, 1833–1847. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Evertz, M.; Streipert, B.; Wagner, R.; Klöpsch, R.; Vortmann, B.; Hahn, H.; Nowak, S.; Amereller, M.; Gentschev, A.-C.; et al. The truth about the 1st cycle Coulombic efficiency of LiNi1/3Co1/3Mn1/3O2 (NCM) cathodes. Phys. Chem. Chem. Phys. 2016, 18, 3956–3965. [Google Scholar] [CrossRef]
- Kida, Y.; Kinoshita, A.; Yanagida, K.; Funahashi, A.; Nohma, T.; Yonezu, I. Study on capacity fade factors of lithium secondary batteries using LiNi0.7Co0.3O2 and graphite–coke hybrid carbon. Electrochim. Acta 2002, 47, 4157–4162. [Google Scholar] [CrossRef]
- Zorba, V.; Syzdek, J.; Mao, X.; Russo, R.E.; Kostecki, R. Ultrafast laser induced breakdown spectroscopy of electrode/electrolyte interfaces. Appl. Phys. Lett. 2012, 100, 234101. [Google Scholar] [CrossRef]
- Imashuku, S.; Taguchi, H.; Kawamata, T.; Fujieda, S.; Kashiwakura, S.; Suzuki, S.; Wagatsuma, K. Quantitative lithium mapping of lithium-ion battery cathode using laser-induced breakdown spectroscopy. J. Power Sources 2018, 399, 186–191. [Google Scholar] [CrossRef]
- Smyrek, P.; Bergfeldt, T.; Seifert, H.J.; Pfleging, W. Laser-induced breakdown spectroscopy for the quantitative measurement of lithium concentration profiles in structured and unstructured electrodes. J. Mater. Chem. A 2019, 7, 5656–5665. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database (Version 5.9); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2021.
- Johnson, R.C.V. The structure and origin of the Swan band spectrum of carbon. Phil. Trans. R. Soc. Lond. A 1927, 226, 157–230. [Google Scholar]
- Swan, W. XXIX—On the Prismatic Spectra of the Flames of Compounds of Carbon and Hydrogen. Trans. R. Soc. Edinb. 1857, 21, 411–429. [Google Scholar] [CrossRef]
- Fernández-Menéndez, L.J.; Méndez-López, C.; Alvarez-Llamas, C.; González-Gago, C.; Pisonero, J.; Bordel, N. Spatio-temporal distribution of atomic and molecular excited species in Laser-Induced Breakdown Spectroscopy: Potential implications on the determination of halogens. Spectrochim. Acta Part B At. Spectrosc. 2020, 168, 105848. [Google Scholar] [CrossRef]
- Drake, G.W.F. Atomic, Molecular, & Optical Physics Handbook; Drake, G.W.F., Ed.; AIP Press: Melville, NY, USA, 1996. [Google Scholar]
- Gornushkin, I.B.; Stevenson, C.L.; Smith, B.W.; Omenetto, N.; Winefordner, J.D. Modeling an inhomogeneous optically thick laser induced plasma: A simplified theoretical approach. Spectrochim. Acta Part B At. Spectrosc. 2016, 56, 1769–1785. [Google Scholar] [CrossRef]
- Cowan, R.D.; Dieke, G.H. Self-Absorption of Spectrum Lines. Rev. Mod. Phys. 1948, 20, 418–455. [Google Scholar] [CrossRef]
- Ackerman, M.; Biaume, F. Structure of the Schumann-Runge bands from the 0-0 to the 13-0 band. J. Mol. Spectrosc. 1970, 35, 73–82. [Google Scholar] [CrossRef]
- Chan, W.T.; Russo, R.E. Study of laser-material interactions using inductively coupled plasma-atomic emission spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1991, 46, 1471–1486. [Google Scholar] [CrossRef]
- El Haddad, J.; Canioni, L.; Bousquet, B. Good practices in LIBS analysis: Review and advices. Spectrochim. Acta Part B At. Spectrosc. 2014, 101, 171–182. [Google Scholar] [CrossRef]
- Takahashi, T.; Thornton, B. Quantitative methods for compensation of matrix effects and self-absorption in Laser Induced Breakdown Spectroscopy signals of solids. Spectrochim. Acta Part B At. Spectrosc. 2017, 138, 31–42. [Google Scholar]
- Aragón, C.; Aguilera, J.A.; Peñalba, F. Improvements in Quantitative Analysis of Steel Composition by Laser-Induced Breakdown Spectroscopy at Atmospheric Pressure Using an Infrared Nd:YAG Laser. Appl. Spectrosc. 1999, 53, 1259–1267. [Google Scholar] [CrossRef]
- Dong, M.; Mao, X.; Gonzalez, J.J.; Lu, J.; Russo, R.E. Time-resolved LIBS of atomic and molecular carbon from coal in air, argon and helium. J. Anal. At. Spectrom. 2012, 27, 2066. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kappeler, M.; Basler, C.; Brandenburg, A.; Carl, D.; Wöllenstein, J. Homogeneity Measurements of Li-Ion Battery Cathodes Using Laser-Induced Breakdown Spectroscopy. Sensors 2022, 22, 8816. https://doi.org/10.3390/s22228816
Kappeler M, Basler C, Brandenburg A, Carl D, Wöllenstein J. Homogeneity Measurements of Li-Ion Battery Cathodes Using Laser-Induced Breakdown Spectroscopy. Sensors. 2022; 22(22):8816. https://doi.org/10.3390/s22228816
Chicago/Turabian StyleKappeler, Moritz, Carl Basler, Albrecht Brandenburg, Daniel Carl, and Jürgen Wöllenstein. 2022. "Homogeneity Measurements of Li-Ion Battery Cathodes Using Laser-Induced Breakdown Spectroscopy" Sensors 22, no. 22: 8816. https://doi.org/10.3390/s22228816
APA StyleKappeler, M., Basler, C., Brandenburg, A., Carl, D., & Wöllenstein, J. (2022). Homogeneity Measurements of Li-Ion Battery Cathodes Using Laser-Induced Breakdown Spectroscopy. Sensors, 22(22), 8816. https://doi.org/10.3390/s22228816





