A Modified Spectroscopic Approach for the Real-Time Detection of Pollen and Fungal Spores at a Semi-Urban Site Using the WIBS-4+, Part I
Abstract
:1. Introduction
2. Materials and Methods
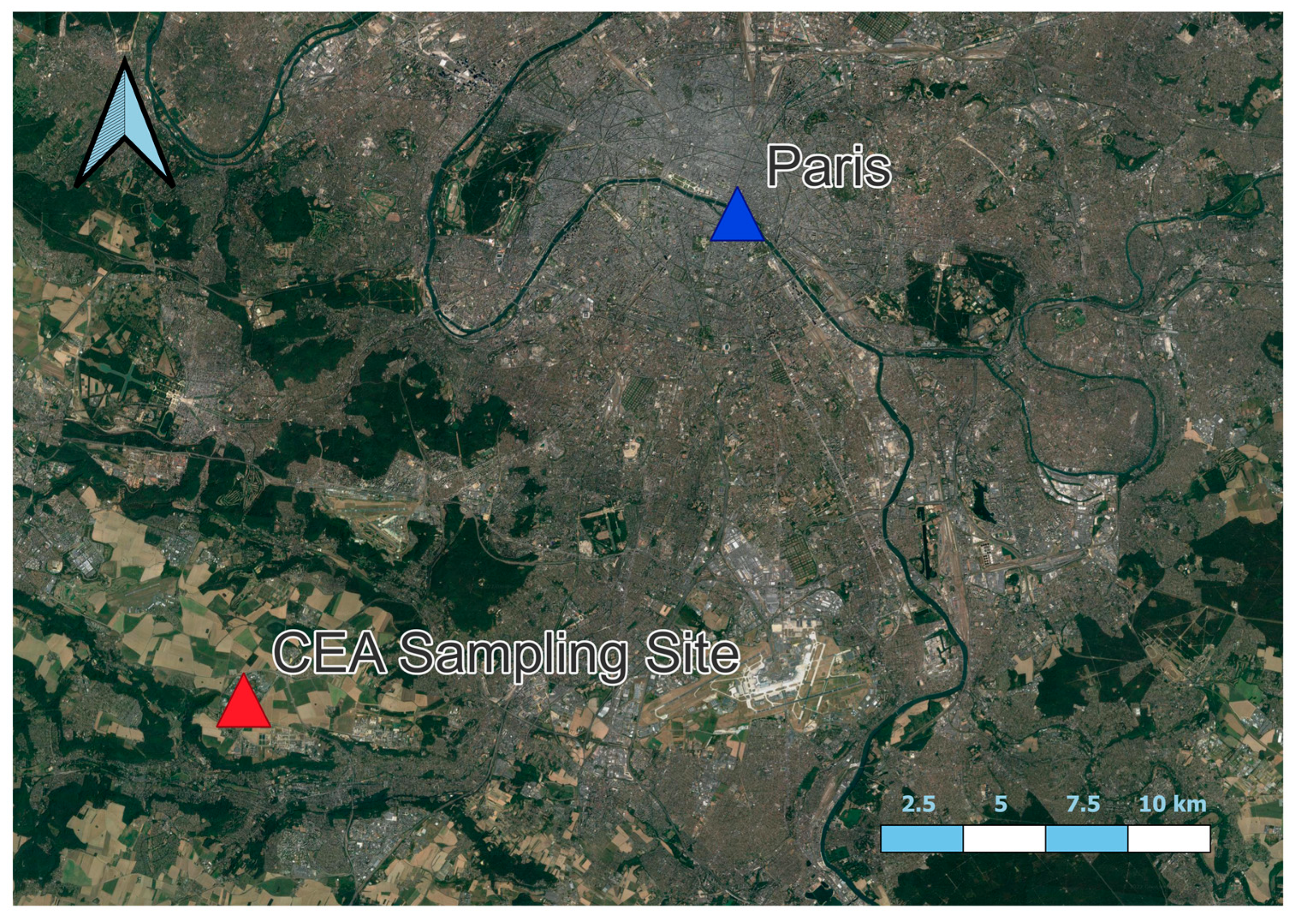
2.1. Site
2.2. Hirst Instrumentation
2.3. WIBS Instrumentation and Data Acquisition
2.4. K-Means
3. Results and Discussion
3.1. An Overview of Pollen and Fungal Spore Concentrations Determined by the Hirst Instrument during the Monitoring Campaign
3.2. An Overview of Fluorescent Particles Detected by WIBS-4+ during the Monitoring Campaign
3.3. Hirst vs. WIBS-4+
3.3.1. Comparison of Fungal Spore Concentration with Fluorescent Particles (WIBS)
3.3.2. Comparison of Pollen Concentration with Fluorescent Particles (WIBS)
3.4. K-Means Clustering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Gorman, C.M.; Fuller, H.T. Prevalence of Culturable Airborne Spores of Selected Allergenic and Pathogenic Fungi in Outdoor Air. Atmos. Environ. 2008, 42, 4355–4368. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Sadyś, M.; Skjøth, C.A.; Healy, D.A.; Kennedy, R.; Sodeau, J.R. Atmospheric Concentrations of Alternaria, Cladosporium, Ganoderma and Didymella Spores Monitored in Cork (Ireland) and Worcester (England) during the Summer of 2010. Aerobiologia 2014, 30, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Skjøth, C.A.; Sommer, J.; Stach, A.; Smith, M.; Brandt, J. The Long-Range Transport of Birch (Betula) Pollen from Poland and Germany Causes Significant Pre-Season Concentrations in Denmark. Clin. Exp. Allergy 2007, 37, 1204–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galán, C.; Alcázar, P.; Cariñanos, P.; Garcia, H.; Domínguez-Vilches, E. Meteorological Factors Affecting Daily Urticaceae Pollen Counts in Southwest Spain. Int. J. Biometeorol. 2000, 43, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Matthias-Maser, S.; Gruber, S.; Jaenicke, R. The Size Distribution of Primary Biological Aerosol Particles in Rain-Water. Nucleation Atmos. Aerosols 1996 1996, 39, 526–529. [Google Scholar] [CrossRef]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Jensen-Jarolim, E.; Einhorn, L.; Herrmann, I.; Thalhammer, J.G.; Panakova, L. Pollen Allergies in Humans and Their Dogs, Cats and Horses: Differences and Similarities. Clin. Transl. Allergy 2015, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- D’Amato, G.; Annesi-Maesano, I.; Cecchi, L.; D’Amato, M. Latest News on Relationship between Thunderstorms and Respiratory Allergy, Severe Asthma, and Deaths for Asthma. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Séguin, V.; Lemauviel-Lavenant, S.; Garon, D.; Bouchart, V.; Gallard, Y.; Blanchet, B.; Diquelou, S.; Personeni, E.; Gauduchon, P.; Ourry, A. Effect of Agricultural and Environmental Factors on the Hay Characteristics Involved in Equine Respiratory Disease. Agric. Ecosyst. Environ. 2010, 135, 206–215. [Google Scholar] [CrossRef]
- Peraica, M.; Radić, B.; Lucić, A.; Pavlović, M. Toxic Effects of Mycotoxins in Humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar]
- Després, V.R.; Alex Huffman, J.; Burrows, S.M.; Hoose, C.; Safatov, A.S.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.O.; Pöschl, U.; et al. Primary Biological Aerosol Particles in the Atmosphere: A Review. Tellus Ser. B Chem. Phys. Meteorol. 2012, 64, 15598. [Google Scholar] [CrossRef]
- Diehl, K.; Matthias-Maser, S.; Jaenicke, R.; Mitra, S.K. The Ice Nucleating Ability of Pollen: Part II. Laboratory Studies in Immersion and Contact Freezing Modes. Atmos. Res. 2002, 61, 125–133. [Google Scholar] [CrossRef]
- Diehl, K.; Quick, C.; Matthias-Maser, S.; Mitra, S.K.; Jaenicke, R. The Ice Nucleating Ability of Pollen Part I: Laboratory Studies in Deposition and Condensation Freezing Modes. Atmos. Res. 2001, 58, 75–87. [Google Scholar] [CrossRef]
- Pummer, B.G.; Bauer, H.; Bernardi, J.; Bleicher, S.; Grothe, H. Suspendable Macromolecules Are Responsible for Ice Nucleation Activity of Birch and Conifer Pollen. Atmos. Chem. Phys. 2012, 12, 2541–2550. [Google Scholar] [CrossRef] [Green Version]
- Bauer, H.; Kasper-Giebl, A.; Löflund, M.; Giebl, H.; Hitzenberger, R.; Zibuschka, F.; Puxbaum, H. The Contribution of Bacteria and Fungal Spores to the Organic Carbon Content of Cloud Water, Precipitation and Aerosols. Atmos. Res. 2002, 64, 109–119. [Google Scholar] [CrossRef]
- Haga, D.I.; Burrows, S.M.; Iannone, R.; Wheeler, M.J.; Mason, R.H.; Chen, J.; Polishchuk, E.A.; Pöschl, U.; Bertram, A.K. Ice Nucleation by Fungal Spores from the Classes Agaricomycetes, Ustilaginomycetes, and Eurotiomycetes, and the Effect on the Atmospheric Transport of These Spores. Atmos. Chem. Phys. 2014, 14, 8611–8630. [Google Scholar] [CrossRef] [Green Version]
- Haga, D.I.; Iannone, R.; Wheeler, M.J.; Mason, R.; Polishchuk, E.A.; Fetch, T.; Van Der Kamp, B.J.; McKendry, I.G.; Bertram, A.K. Ice Nucleation Properties of Rust and Bunt Fungal Spores and Their Transport to High Altitudes, Where They Can Cause Heterogeneous Freezing. J. Geophys. Res. Atmos. 2013, 118, 7260–7272. [Google Scholar] [CrossRef]
- Schnell, R.C.; Vali, G. Atmospheric Ice Nuclei from Decomposing Vegetation. Nature 1972, 236, 163–165. [Google Scholar] [CrossRef]
- Conen, F.; Eckhardt, S.; Gundersen, H.; Stohl, A.; Yttri, K.E. Rainfall Drives Atmospheric Ice Nucleating Particles in the Maritime Climate of Southern Norway. Atmos. Chem. Phys. Discuss. 2017, 1–13. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Healy, D.A.; Hellebust, S.; Buters, J.T.M.; Sodeau, J.R. Using the WIBS-4 (Waveband Integrated Bioaerosol Sensor) Technique for the on-Line Detection of Pollen Grains. Aerosol Sci. Technol. 2014, 48, 341–349. [Google Scholar] [CrossRef]
- Healy, D.A.; Huffman, J.A.; O’Connor, D.J.; Pöhlker, C.; Pöschl, U.; Sodeau, J.R. Ambient Measurements of Biological Aerosol Particles near Killarney, Ireland: A Comparison between Real-Time Fluorescence and Microscopy Techniques. Atmos. Chem. Phys. 2014, 14, 8055–8069. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, S.; Tormo-Molina, R.; Lemonis, N.; Clot, B.; O’Connor, D.J.; Sodeau, J.R. Comparison of Fungal Spores Concentrations Measured with Wideband Integrated Bioaerosol Sensor and Hirst Methodology. Atmos. Environ. 2018, 175, 1–14. [Google Scholar] [CrossRef]
- Ziemba, L.D.; Beyersdorf, A.J.; Chen, G.; Corr, C.A.; Crumeyrolle, S.N.; Diskin, G.; Hudgins, C.; Martin, R.; Mikoviny, T.; Moore, R.; et al. Airborne Observations of Bioaerosol over the Southeast United States Using a Wideband Integrated Bioaerosol Sensor. J. Geophys. Res. Atmos. 2016, 121, 8506–8524. [Google Scholar] [CrossRef] [Green Version]
- Negron, A.; Deleon-Rodriguez, N.; Waters, S.M.; Ziemba, L.D.; Anderson, B.; Bergin, M.; Konstantinidis, K.T.; Nenes, A. Using Flow Cytometry and Light-Induced Fluorescence to Characterize the Variability and Characteristics of Bioaerosols in Springtime in Metro Atlanta, Georgia. Atmos. Chem. Phys. 2020, 20, 1817–1838. [Google Scholar] [CrossRef] [Green Version]
- Cheng, B.; Yue, S.; Hu, W.; Ren, L.; Deng, J.; Wu, L.; Fu, P. Summertime Fluorescent Bioaerosol Particles in the Coastal Megacity Tianjin, North China. Sci. Total Environ. 2020, 723, 137966. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Ren, L.; Song, T.; Li, L.; Xie, Q.; Li, W.; Kang, M.; Zhao, W.; Wei, L.; Ren, H.; et al. Abundance and Diurnal Trends of Fluorescent Bioaerosols in the Troposphere over Mt. Tai, China, in Spring. J. Geophys. Res. Atmos. 2019, 124, 4158–4173. [Google Scholar] [CrossRef]
- Perring, A.E.; Schwarz, J.P.; Baumgardner, D.; Hernandez, M.T.; Spracklen, D.V.; Heald, C.L.; Gao, R.S.; Kok, G.; McMeeking, G.R.; McQuaid, J.B.; et al. Airborne Observations of Regional Variation in Fluorescent Aerosol across the United States. J. Geophys. Res. Atmos. 2015, 120, 1153–1170. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Healy, D.A.; Sodeau, J.R. The On-Line Detection of Biological Particle Emissions from Selected Agricultural Materials Using the WIBS-4 (Waveband Integrated Bioaerosol Sensor) Technique. Atmos. Environ. 2013, 80, 415–425. [Google Scholar] [CrossRef]
- Healy, D.A.; O’Connor, D.J.; Sodeau, J.R. Measurement of the Particle Counting Efficiency of the “Waveband Integrated Bioaerosol Sensor” Model Number 4 (WIBS-4). J. Aerosol Sci. 2012, 47, 94–99. [Google Scholar] [CrossRef]
- Healy, D.A.; O’Connor, D.J.; Burke, A.M.; Sodeau, J.R. A Laboratory Assessment of the Waveband Integrated Bioaerosol Sensor (WIBS-4) Using Individual Samples of Pollen and Fungal Spore Material. Atmos. Environ. 2012, 60, 534–543. [Google Scholar] [CrossRef]
- Toprak, E.; Schnaiter, M. Fluorescent Biological Aerosol Particles Measured with the Waveband Integrated Bioaerosol Sensor WIBS-4: Laboratory Tests Combined with a One Year Field Study. Atmos. Chem. Phys. 2013, 13, 225–243. [Google Scholar] [CrossRef]
- Robinson, E.S.; Gao, R.S.; Schwarz, J.P.; Fahey, D.W.; Perring, A.E. Fluorescence Calibration Method for Single-Particle Aerosol Fluorescence Instruments. Atmos. Meas. Tech. 2017, 10, 1755–1768. [Google Scholar] [CrossRef] [Green Version]
- Lieberherr, G.; Auderset, K.; Calpini, B.; Clot, B.; Crouzy, B.; Gysel-Beer, M.; Konzelmann, T.; Manzano, J.; Mihajlovic, A.; Moallemi, A.; et al. Assessment of Real-Time Bioaerosol Particle Counters Using Reference Chamber Experiments. Atmos. Meas. Tech. Discuss. 2021, 14, 7693–7706. [Google Scholar] [CrossRef]
- Whitehead, J.D.; Gallagher, M.W.; Dorsey, J.R.; Robinson, N.; Gabey, A.M.; Coe, H.; McFiggans, G.; Flynn, M.J.; Ryder, J.; Nemitz, E.; et al. Aerosol Fluxes and Dynamics within and above a Tropical Rainforest in South-East Asia. Atmos. Chem. Phys. 2010, 10, 9369–9382. [Google Scholar] [CrossRef] [Green Version]
- Gabey, A.M.; Gallagher, M.W.; Whitehead, J.; Dorsey, J.R.; Kaye, P.H.; Stanley, W.R. Measurements and Comparison of Primary Biological Aerosol above and below a Tropical Forest Canopy Using a Dual Channel Fluorescence Spectrometer. Atmos. Chem. Phys. 2010, 10, 4453–4466. [Google Scholar] [CrossRef] [Green Version]
- Gabey, A.M.; Stanley, W.R.; Gallagher, M.W.; Kaye, P.H. The Fluorescence Properties of Aerosol Larger than 0.8 μ in Urban and Tropical Rainforest Locations. Atmos. Chem. Phys. 2011, 11, 5491–5504. [Google Scholar] [CrossRef] [Green Version]
- Tummon, F.; Adamov, S.; Clot, B.; Crouzy, B.; Gysel-Beer, M.; Kawashima, S.; Lieberherr, G.; Manzano, J.; Markey, E.; Moallemi, A.; et al. A First Evaluation of Multiple Automatic Pollen Monitors Run in Parallel. Aerobiologia (Bologna) 2021. [Google Scholar] [CrossRef]
- Feeney, P.; Rodríguez, S.F.; Molina, R.; McGillicuddy, E.; Hellebust, S.; Quirke, M.; Daly, S.; O’Connor, D.; Sodeau, J. A Comparison of On-Line and off-Line Bioaerosol Measurements at a Biowaste Site. Waste Manag. 2018, 76, 323–338. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Daly, S.M.; Sodeau, J.R. On-Line Monitoring of Airborne Bioaerosols Released from a Composting/Green Waste Site. Waste Manag. 2015, 42, 23–30. [Google Scholar] [CrossRef]
- Daly, S.M.; O’Connor, D.J.; Healy, D.A.; Hellebust, S.; Arndt, J.; McGillicuddy, E.; Feeney, P.; Quirke, M.; Wenger, J.; Sodeau, J. Investigation of Coastal Sea-Fog Formation Using the WIBS (Wideband Integrated Bioaerosol Sensor) Technique. Atmos. Chem. Phys. 2019, 19, 5737–5751. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wan, M.P.; Schiavon, S.; Tham, K.W.; Zuraimi, S.; Xiong, J.; Fang, M.; Gall, E. Size-Resolved Dynamics of Indoor and Outdoor Fluorescent Biological Aerosol Particles in a Bedroom: A One-Month Case Study in Singapore. Indoor Air 2020, 30, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Fennelly, M.; Gallagher, C.; Harding, M.; Hellebust, S.; Wenger, J.; O’Sullivan, N.; O’Connor, D.; Prentice, M. Real-Time Monitoring of Aerosol Generating Dental Procedures. J. Dent. 2022, 120, 104092. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, V.; Huston, A.; Lin, H.-B.; Eversole, J.; Falkenstein, P.; Schultz, A. Field Test Results and Ambient Aerosol Measurements Using Dual Wavelength Fluorescence Excitation and Elastic Scatter for Bioaerosols. Chem. Biol. Sens. VIII 2007, 6554, 65540R. [Google Scholar] [CrossRef]
- Sivaprakasam, V.; Huston, A.L.; Scotto, C.; Eversole, J.D. Multiple UV Wavelength Excitation and Fluorescence of Bioaerosols. Opt. Express 2004, 12, 4457. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Iacopino, D.; Healy, D.A.; O’Sullivan, D.; Sodeau, J.R. The Intrinsic Fluorescence Spectra of Selected Pollen and Fungal Spores. Atmos. Environ. 2011, 45, 6451–6458. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Lovera, P.; Iacopino, D.; O’Riordan, A.; Healy, D.A.; Sodeau, J.R. Using Spectral Analysis and Fluorescence Lifetimes to Discriminate between Grass and Tree Pollen for Aerobiological Applications. Anal. Methods 2014, 6, 1633–1639. [Google Scholar] [CrossRef]
- Pöhlker, C.; Huffman, J.A.; Pöschl, U. Autofluorescence of Atmospheric Bioaerosols: Spectral Fingerprints and Taxonomic Trends of Pollen. Atmos. Meas. Tech. 2013, 6, 3369–3392. [Google Scholar] [CrossRef] [Green Version]
- Sodeau, J.; O’connor, D.; Feeney, P.; Quirke, M.; Daly, S.; Fennelly, M.; Buckley, P.; Hellebust, S.; McGillicuddy, E.; Wenger, J. Online Bioaerosol Sensing (OLBAS); The Environmental Protection Agency: Wexford, Ireland, 2019; ISBN 9781840958157.
- Könemann, T.; Savage, N.; Klimach, T.; Walter, D.; Fröhlich-Nowoisky, J.; Su, H.; Pöschl, U.; Alex Huffman, J.; Pöhlker, C. Spectral Intensity Bioaerosol Sensor (SIBS): An Instrument for Spectrally Resolved Fluorescence Detection of Single Particles in Real Time. Atmos. Meas. Tech. 2019, 12, 1337–1363. [Google Scholar] [CrossRef] [Green Version]
- Hirst, J.M. An Automatic Volumetric Spore Trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Kaye, P.H.; Hirst, E.; Foot, V.E.; Clark, J.M.; Baxter, K.L. A Low-Cost Multichannel Aerosol Fluorescence Sensor for Networked Deployment. Opt. Based Biol. Chem. Sens. Def. 2004, 5617, 388. [Google Scholar] [CrossRef] [Green Version]
- Kaye, P.H.; Stanley, W.R.; Hirst, E.; Foot, E.V.; Baxter, K.L.; Barrington, S.J. Single Particle Multichannel Bio-Aerosol Fluorescence Sensor. Opt. Express 2005, 13, 3583. [Google Scholar] [CrossRef] [PubMed]
- Savage, N.J.; Krentz, C.E.; Könemann, T.; Han, T.T.; Mainelis, G.; Pöhlker, C.; Alex Huffman, J. Systematic Characterization and Fluorescence Threshold Strategies for the Wideband Integrated Bioaerosol Sensor (WIBS) Using Size-Resolved Biological and Interfering Particles. Atmos. Meas. Tech. 2017, 10, 4279–4302. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Hennig, C. fpc: Flexible Procedures for Clustering. Available online: https://cran.r-project.org/web/packages/fpc/fpc.pdf (accessed on 11 October 2022).
- Sarda-Estève, R.; Baisnée, D.; Guinot, B.; Sodeau, J.; O’Connor, D.; Belmonte, J.; Besancenot, J.-P.; Petit, J.-E.; Thibaudon, M.; Oliver, G.; et al. Variability and Geographical Origin of Five Years Airborne Fungal Spore Concentrations Measured at Saclay, France from 2014 to 2018. Remote Sens. 2019, 11, 1671. [Google Scholar] [CrossRef] [Green Version]
- Estève, R.S.; Baisnée, D.; Guinot, B.; Petit, J.-E.; Sodeau, J.; O’Connor, D.; Besancenot, J.-P.; Thibaudon, M.; Gros, V. Temporal Variability and Geographical Origins of Airborne Pollen Grains Concentrations from 2015 to 2018 at Saclay, France. Remote Sens. 2018, 10, 1932. [Google Scholar] [CrossRef] [Green Version]
- Sarda-Estève, R.; Baisnée, D.; Guinot, B.; Mainelis, G.; Sodeau, J.; O’connor, D.; Besancenot, J.P.; Thibaudon, M.; Monteiro, S.; Petit, J.E.; et al. Atmospheric Biodetection Part i: Study of Airborne Bacterial Concentrations from January 2018 to May 2020 at Saclay, France. Int. J. Environ. Res. Public Health 2020, 17, 6292. [Google Scholar] [CrossRef] [PubMed]
- Rapiejko, P.; Stankiewicz, W.; Szczygielski, K.; Jurkiewicz, D. Threshold Pollen Count Necessary to Evoke Allergic Symptoms. Otolaryngol. Pol. 2007, 61, 591–594. [Google Scholar] [CrossRef] [Green Version]
- Negrini, A.C.; Voltolini, S.; Troise, C.; Arobba, D. Comparison between Urticaceae (Parietaria) Pollen Count and Hay Fever Symptoms: Assessment of a «threshold-Value». Aerobiologia (Bologna) 1992, 8, 325–329. [Google Scholar] [CrossRef]
- Piotrowska-Weryszko, K.; Weryszko-Chmielewska, E. Plant Pollen Content in the Air of Lublin (Central-Eastern Poland) and Risk of Pollen Allergy. Ann. Agric. Environ. Med. 2014, 21, 693–696. [Google Scholar] [CrossRef] [Green Version]
- Vélez-Pereira, A.M.; De Linares, C.; Canela, M.-A.; Belmonte, J. Logistic Regression Models for Predicting Daily Airborne Alternaria and Cladosporium Concentration Levels in Catalonia (NE Spain). Int. J. Biometeorol. 2019, 63, 1541–1553. [Google Scholar] [CrossRef]
- Martinez-Bracero, M.; Markey, E.; Clancy, J.H.; McGillicuddy, E.J.; Sewell, G.; O’connor, D.J. Airborne Fungal Spore Review, New Advances and Automatisation. Atmosphere 2022, 13, 308. [Google Scholar] [CrossRef]
- Vélez-Pereira, A.M.; De Linares, C.; Canela, M.; Belmonte, J. Spatial Distribution of Fungi from the Analysis of Aerobiological Data with a Gamma Function. Aerobiologia (Bologna) 2021, 37, 461–477. [Google Scholar] [CrossRef]
- Hernandez, M.; Perring, A.E.; McCabe, K.; Kok, G.; Granger, G.; Baumgardner, D. Chamber Catalogues of Optical and Fluorescent Signatures Distinguish Bioaerosol Classes. Atmos. Meas. Tech. 2016, 9, 3283–3292. [Google Scholar] [CrossRef] [Green Version]
- Savage, N.J.; Huffman, J.A. Evaluation of a Hierarchical Agglomerative Clustering Method Applied to WIBS Laboratory Data for Improved Discrimination of Biological Particles by Comparing Data Preparation Techniques. Atmos. Meas. Tech. 2018, 11, 4929–4942. [Google Scholar] [CrossRef] [Green Version]
- Twohy, C.H.; McMeeking, G.R.; DeMott, P.J.; McCluskey, C.S.; Hill, T.C.J.; Burrows, S.M.; Kulkarni, G.R.; Tanarhte, M.; Kafle, D.N.; Toohey, D.W. Abundance of Fluorescent Biological Aerosol Particles at Temperatures Conducive to the Formation of Mixed-Phase and Cirrus Clouds. Atmos. Chem. Phys. 2016, 16, 8205–8225. [Google Scholar] [CrossRef] [Green Version]
- Ila Gosselin, M.; Rathnayake, C.M.; Crawford, I.; Pöhlker, C.; Fröhlich-Nowoisky, J.; Schmer, B.; Després, V.R.; Engling, G.; Gallagher, M.; Stone, E.; et al. Fluorescent Bioaerosol Particle, Molecular Tracer, and Fungal Spore Concentrations during Dry and Rainy Periods in a Semi-Arid Forest. Atmos. Chem. Phys. 2016, 16, 15165–15184. [Google Scholar] [CrossRef] [Green Version]
- Pöhlker, C.; Huffman, J.A.; Pöschl, U. Autofluorescence of Atmospheric Bioaerosols - Fluorescent Biomolecules and Potential Interferences. Atmos. Meas. Tech. 2012, 5, 37–71. [Google Scholar] [CrossRef] [Green Version]
- Fennelly, M.J.; Sewell, G.; Prentice, M.B.; O’Connor, D.J.; Sodeau, J.R. Review: The Use of Real-Time Fluorescence Instrumentation to Monitor Ambient Primary Biological Aerosol Particles (PBAP). Atmosphere 2017, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, D.J.; Healy, D.A.; Sodeau, J.R. A 1-Month Online Monitoring Campaign of Ambient Fungal Spore Concentrations in the Harbour Region of Cork, Ireland. Aerobiologia (Bologna) 2015, 31, 295–314. [Google Scholar] [CrossRef]
- Pan, Y.L.; Kalume, A.; Wang, C.; Santarpia, J. Atmospheric Aging Processes of Bioaerosols under Laboratory-Controlled Conditions: A Review. J. Aerosol Sci. 2021, 155, 105767. [Google Scholar] [CrossRef]
- Yue, S.; Ren, H.; Fan, S.; Sun, Y.; Wang, Z.; Fu, P. Springtime Precipitation Effects on the Abundance of Fluorescent Biological Aerosol Particles and HULIS in Beijing. Sci. Rep. 2016, 6, 29618. [Google Scholar] [CrossRef] [Green Version]
- Moran-Zuloaga, D.; Ditas, F.; Walter, D.; Saturno, J.; Brito, J.; Carbone, S.; Chi, X.; Hrabě De Angelis, I.; Baars, H.; H M Godoi, R.; et al. Long-Term Study on Coarse Mode Aerosols in the Amazon Rain Forest with the Frequent Intrusion of Saharan Dust Plumes. Atmos. Chem. Phys. 2018, 18, 10055–10088. [Google Scholar] [CrossRef]
- Von Der Weiden, S.L.; Drewnick, F.; Borrmann, S. Particle Loss Calculator - A New Software Tool for the Assessment of the Performance of Aerosol Inlet Systems. Atmos. Meas. Tech. 2009, 2, 479–494. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.M.; Thien, F.; Hew, M. Thunderstorm Asthma: Controlling (Deadly) Grass Pollen Allergy. BMJ 2018, 360, 4–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buters, J.; Clot, B.; Galán, C.; Gehrig, R.; Gilge, S.; Hentges, F.; O’Connor, D.; Sikoparija, B.; Skjoth, C.; Tummon, F.; et al. Automatic Detection of Airborne Pollen: An Overview. Aerobiologia (Bologna) 2022. [Google Scholar] [CrossRef]
- Adamov, S.; Lemonis, N.; Clot, B.; Crouzy, B.; Gehrig, R.; Graber, M.J.; Sallin, C.; Tummon, F. On the Measurement Uncertainty of Hirst-Type Volumetric Pollen and Spore Samplers. Aerobiologia (Bologna) 2021, 5. [Google Scholar] [CrossRef]
- West, J.S.; Kimber, R.B.E. Innovations in Air Sampling to Detect Plant Pathogens. Ann. Appl. Biol. 2015, 166, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Miki, K.; Kawashima, S.; Clot, B.; Nakamura, K. Comparative Efficiency of Airborne Pollen Concentration Evaluation in Two Pollen Sampler Designs Related to Impaction and Changes in Internal Wind Speed. Atmos. Environ. 2019, 203, 18–27. [Google Scholar] [CrossRef]
- Heffer, M.J.; Ratz, J.D.; Miller, J.D.; Day, J.H. Comparison of the Rotorod to Other Air Samplers for the Determination of Ambrosia Artemisiifolia Pollen Concentrations Conducted in the Environmental Exposure Unit. Aerobiologia (Bologna) 2005, 21, 233–239. [Google Scholar] [CrossRef]
- Melnikova, E.V.; Roshchina, V.V.; Karnaukhov, V.N. Microspectrofluorimetry of Intact Pollen. Biofizika 1997, 42, 233. [Google Scholar]
- Crawford, I.; Ruske, S.; Topping, D.O.; Gallagher, M.W. Evaluation of Hierarchical Agglomerative Cluster Analysis Methods for Discrimination of Primary Biological Aerosol. Atmos. Meas. Tech. 2015, 8, 4979–4991. [Google Scholar] [CrossRef] [Green Version]
- Robinson, N.H.; Allan, J.D.; Huffman, J.A.; Kaye, P.H.; Foot, V.E.; Gallagher, M. Cluster Analysis of WIBS Single-Particle Bioaerosol Data. Atmos. Meas. Tech. 2013, 6, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Ruske, S.; Topping, D.O.; Foot, V.E.; Morse, A.P.; Gallagher, M.W. Machine Learning for Improved Data Analysis of Biological Aerosol Using the WIBS. Atmos. Meas. Tech. 2018, 11, 6203–6230. [Google Scholar] [CrossRef]
- Swanson, B.E.; Huffman, J.A. Development and Characterization of an Inexpensive Single-Particle Fluorescence Spectrometer for Bioaerosol Monitoring. Opt. Express 2018, 26, 3646. [Google Scholar] [CrossRef] [PubMed]








| Channel | Excitation (nm) | Emission (nm) |
|---|---|---|
| A | 280 | 310–400 |
| B | 280 | 420–650 |
| C | 370 | 420–650 |
| AB | 280 | 310–400 |
| 420–650 | ||
| AC | 280 | 310–400 |
| 370 | 420–650 | |
| BC | 280 | 420–650 |
| 370 | ||
| ABC | 280 | 310–400 |
| 420–650 | ||
| 370 | 420–650 | |
| D | 280 | 600–750 |
| E | 370 | 600–750 |
| DE | 280 | 600–750 |
| 370 |
| Particle Class | Percentage Contribution |
|---|---|
| AB | 49% |
| B | 30% |
| ABC | 10% |
| A | 7% |
| BC | 3% |
| C | <0.5% |
| AC | <0.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markey, E.; Hourihane Clancy, J.; Martínez-Bracero, M.; Neeson, F.; Sarda-Estève, R.; Baisnée, D.; McGillicuddy, E.J.; Sewell, G.; O’Connor, D.J. A Modified Spectroscopic Approach for the Real-Time Detection of Pollen and Fungal Spores at a Semi-Urban Site Using the WIBS-4+, Part I. Sensors 2022, 22, 8747. https://doi.org/10.3390/s22228747
Markey E, Hourihane Clancy J, Martínez-Bracero M, Neeson F, Sarda-Estève R, Baisnée D, McGillicuddy EJ, Sewell G, O’Connor DJ. A Modified Spectroscopic Approach for the Real-Time Detection of Pollen and Fungal Spores at a Semi-Urban Site Using the WIBS-4+, Part I. Sensors. 2022; 22(22):8747. https://doi.org/10.3390/s22228747
Chicago/Turabian StyleMarkey, Emma, Jerry Hourihane Clancy, Moisés Martínez-Bracero, Finnian Neeson, Roland Sarda-Estève, Dominique Baisnée, Eoin J. McGillicuddy, Gavin Sewell, and David J. O’Connor. 2022. "A Modified Spectroscopic Approach for the Real-Time Detection of Pollen and Fungal Spores at a Semi-Urban Site Using the WIBS-4+, Part I" Sensors 22, no. 22: 8747. https://doi.org/10.3390/s22228747
APA StyleMarkey, E., Hourihane Clancy, J., Martínez-Bracero, M., Neeson, F., Sarda-Estève, R., Baisnée, D., McGillicuddy, E. J., Sewell, G., & O’Connor, D. J. (2022). A Modified Spectroscopic Approach for the Real-Time Detection of Pollen and Fungal Spores at a Semi-Urban Site Using the WIBS-4+, Part I. Sensors, 22(22), 8747. https://doi.org/10.3390/s22228747








