1. Introduction
Melanoma is a relatively aggressive form of skin malignancy that accounts for only about 1% of skin cancers but causes most deaths. There are currently more than 132,000 cases of melanoma skin cancer worldwide each year. The accuracy of diagnosis by patients and dermatologists by using visual inspection is only about 60%. In addition, the shortage of dermatologists per capita prompted the need for computer-aided methods in detecting skin cancer. The American Cancer Society’s 2022 homegrown statistics estimate that there will be approximately 99,780 new melanoma cases (about 57,180 cases in men and 42,600 cases in women) and an estimated 7650 deaths from melanoma (about 5080 men and 2570 women). In addition to this, there are other types of cancer. For example, colon cancer, lung cancer, stomach cancer, and so on are still the leading causes of human suffering and death.
With the development of computer vision technology and artificial intelligence, image analyses have been widely used in various scene-parsing tasks. Medical image analyses play vital roles in computer-aided diagnosis and detection [
1,
2,
3]. The amount of medical image data acquired is growing faster than the available human expert interpretation. Therefore, automated segmentation techniques are desired in helping physicians achieve accurate and timely imaging-based diagnoses [
4,
5]. However, due to insufficient original training samples of medical images or the lack of a clear demarcation line between some subtle lesion areas and normal tissues and organs (as shown in
Figure 1). They are making the task of skin lesion segmentation more difficult.
In recent years, with in-depth research on deep learning theory, convolutional neural network-based [
6,
7,
8,
9] deep learning methods for image recognition and classification have shown excellent performance [
10,
11,
12], including the recently popular BP neural network algorithm for image processing [
13]. Moreover, with respect to multi-level dilated residual network [
14] for processing skin lesions and MRIs, Long et al. [
7] proposed an FCN architecture based on CNNs to solve the semantic level image segmentation problem by performing end-to-end pixel-level classification of the input raw images. Most medical images are large, so the feature vector obtained by training using raw images is large. It also has high requirements for computer performances, leading to substantial computational costs. Fischer et al. [
15] proposed U-Net, which consists of mutually symmetric systolic and dilated paths. Among them, the systolic path is used to obtain context information and the dilated path is used for precise localizations. In the dilation path, feature vectors are fused with corresponding low-level features to add multi-scale information. Finally, the overlap tile strategy alleviates the computational resource issue. High IoU values of 0.9203 and 0.7756 were obtained on the PhC-U373 and DIC-HELa datasets, respectively. Later, Zhou et al. [
16] proposed a new architecture U-Net++ that enables flexible feature fusion by redesigning multiple dense skip connections, reducing the semantic gap between feature representations and encoder sub-networks. Moreover, the multi-scale feature aggregation of U-Net++ can synthesize the segmentation results step by step, thus improving the accuracy and accelerating the convergence speed of the network.
2. Related Work
As the complexity of computer vision tasks and task demands increase, deeper [
17] convolutional neural networks are required for feature extraction. As a result, the gradient disappearance problem sometimes occurs during feature propagation. Huang et al. [
18] proposed Dense Net, which not only alleviates the gradient vanishing problem but also enhances the feature’s propagation and can dramatically reduce the number of parameters. Subsequently, Zhang et al. [
19] proposed Res-Net, which utilizes skip connections (Identity mapping) to alleviate the vanishing gradient problem while increasing the network’s depth. The authors of [
20] proposed PSP-Net using a pyramid pooling module to aggregate global contextual information from different regions to increase the target receptive field. Later, Ibtehaz et al. [
21] proposed MultiResUNet to introduce contextual multi-scale information into the U-Net architecture via different residual modules, adding local detail information.
However, FCNs and CNN models face the same issue: a lack of long-term global correlation modeling capabilities. The main reason is that CNN extracts local information simply and cannot measure global relevance efficiently. A transformer [
22] is an essential model in natural language processing, and was used initially to improve NMT (neural machine translation) models using attention mechanisms. The transformer network has a cleaner structure and is quicker in training and inferencing. The transformer focuses on extracting global information but weakens local information, so it also has some disadvantages in medical image segmentation tasks. How to properly highlight foreground information, weaken background information, and how to better jointly model local information and global correlation dependence become focuses of the study. The authors of [
23] combined the transformer structure with the U-Net model, using the transformer’s powerful encoding ability and U-Net’s local localization ability to complete the segmentation of multiple abdominal organs and the heart. Extensive experiments demonstrate that TransU-Net outperforms the original U-Net architecture in various image classification tasks.
Based on existing approaches, in this paper, we propose a novel CNN for medical image segmentation. The training results on three different datasets outperformed the current state-of-the-art models in three main areas of work:
Standard convolution is replaced by dilated convolution; original image information of varying resolution sizes is introduced into the encoder at all levels;
Feature fusion at each level uses hybrid attention for detail enhancement of feature vectors in both channel and spatial dimensions;
Slicing experiments are conducted to verify the contribution of HRA, dilation convolution, and cross-validation pieces relative to the MHAU-Net model.
Image pre-processing Since the lesion areas in the original dermoscopic images vary in shape, size, and pixel intensity, some lesion areas are hidden under human hair or shadows, which will inevitably affect segmentation results, thereby reducing the generalization ability of the model. Therefore, to minimize the impact of these factors on the model segmentation performance, we introduce an image preprocessing method.
We used a morphological manipulation approach to remove artifacts from the original dermoscopic images. First, the input RGB image is converted into a grayscale image. The morphological operation with black hat transform is used [
24], followed by artifact removal using a thresholding operation (as shown in
Figure 2 see legend information for details). We continuously adjusted the experimental parameters and selected a cross-shaped two-dimensional array of size 25 × 25 as the structural element, which has the middle row and column consisting of 1 and the remaining elements composed of 0. All images are resized to a shape of 256 × 256 using bilinear interpolation to achieve faster convolution operations and to solve the excessive memory consumption problem.
K-Fold Cross-validation: The medical image datasets that we acquired are limited, and the question of how to train models with high generalization performances on limited resources poses a new challenge to researchers. In this paper, we use a cross-validation strategy, which is also known as loop estimation. Cross-validation tests estimate the general validity of an algorithm on an independent data set, ensuring a balance between bias and variance. In the K-Fold cross-validation test, dataset D is randomly divided into k equal or nearly equal-sized mutually exclusive subsets D1, D2, …, Dk [
25], which is then run k times, each time using one of the k blocks as the validation set and the rest as the training set. To evaluate the segmentation accuracy of the baseline U-Net and the proposed MSHMU-Net architecture, we perform a 5-fold cross-validation test on each of the different datasets.
3. Method
3.1. Attention Mechanism
In computer vision, attention mechanisms have been widely used in different task scenarios [
26,
27]. As an adaptive spatial region selection mechanism, spatial attention has been used in image classification [
26] and image captioning [
27], etc.
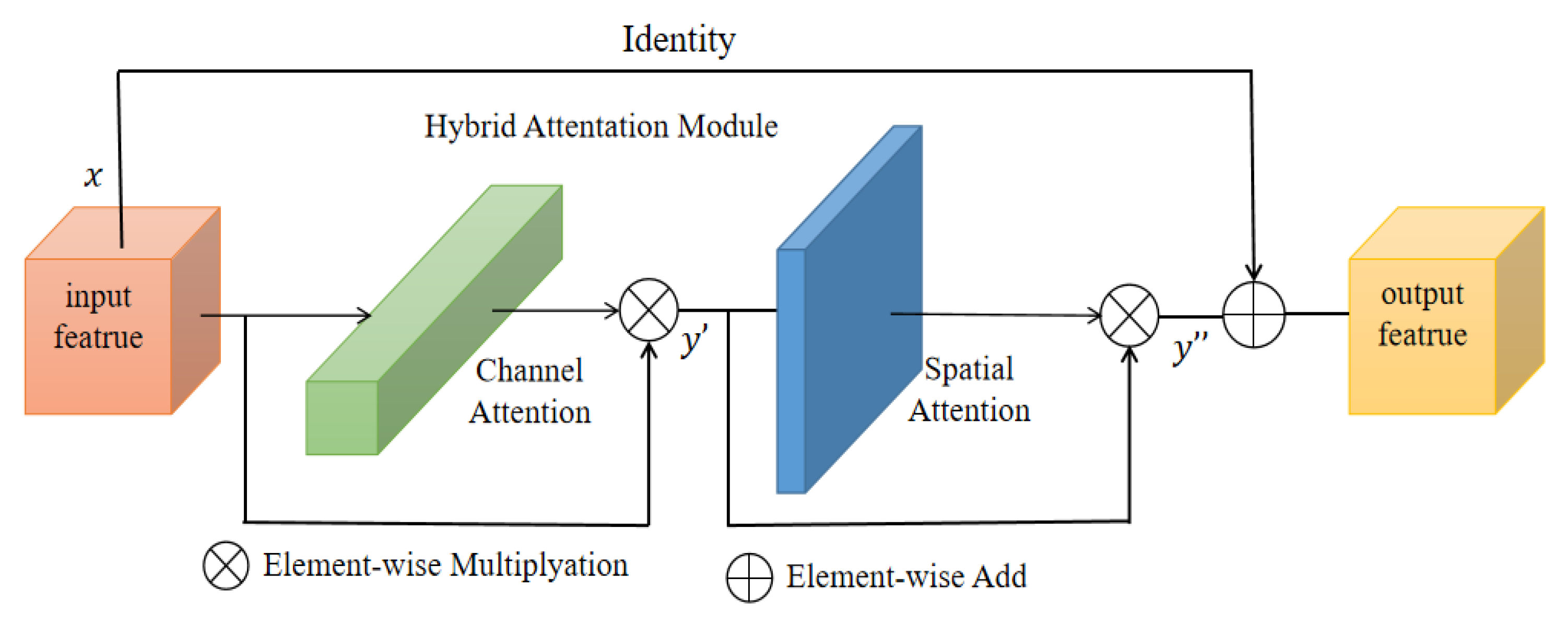
To obtain a better segmentation output, we introduce a hybrid residual attention (HRA) module combined with identity mapping (as shown in
Figure 3). First, the channel attention [
28] module enhances the specific semantic responsiveness of channels by establishing associations between channels, thereby focusing on more meaningful parts. The second is the spatial attention module [
29], which uses the association between any two point features to enhance the representation of their respective features mutually. Finally, the output results are added and fused to obtain the final features for pixel classification. The approach of these attention mechanisms is to achieve feature reinforcement by generating a context vector to assign the weights of the input sequence.
They take the input of feature vector
as an example. First, the channel attention mapping is used. Using both Max-Pool and Average-Pool algorithms, the transformation results are then obtained after several MLP layers and finally applied to two channels. The attention results of the channels are obtained using the sigmoid function (details of the operation are shown in
Table 1). The output result is multiplied element-by-element with the original input feature vector (as shown in Equation (1)) to obtain the one-dimensional feature,
. Next, the spatial attention mapping is used, and the two-dimensional spatial attention map
is then obtained using the same method (as shown in Equation (2)).
and
represent the channel and spatial attention mapping operators, respectively.
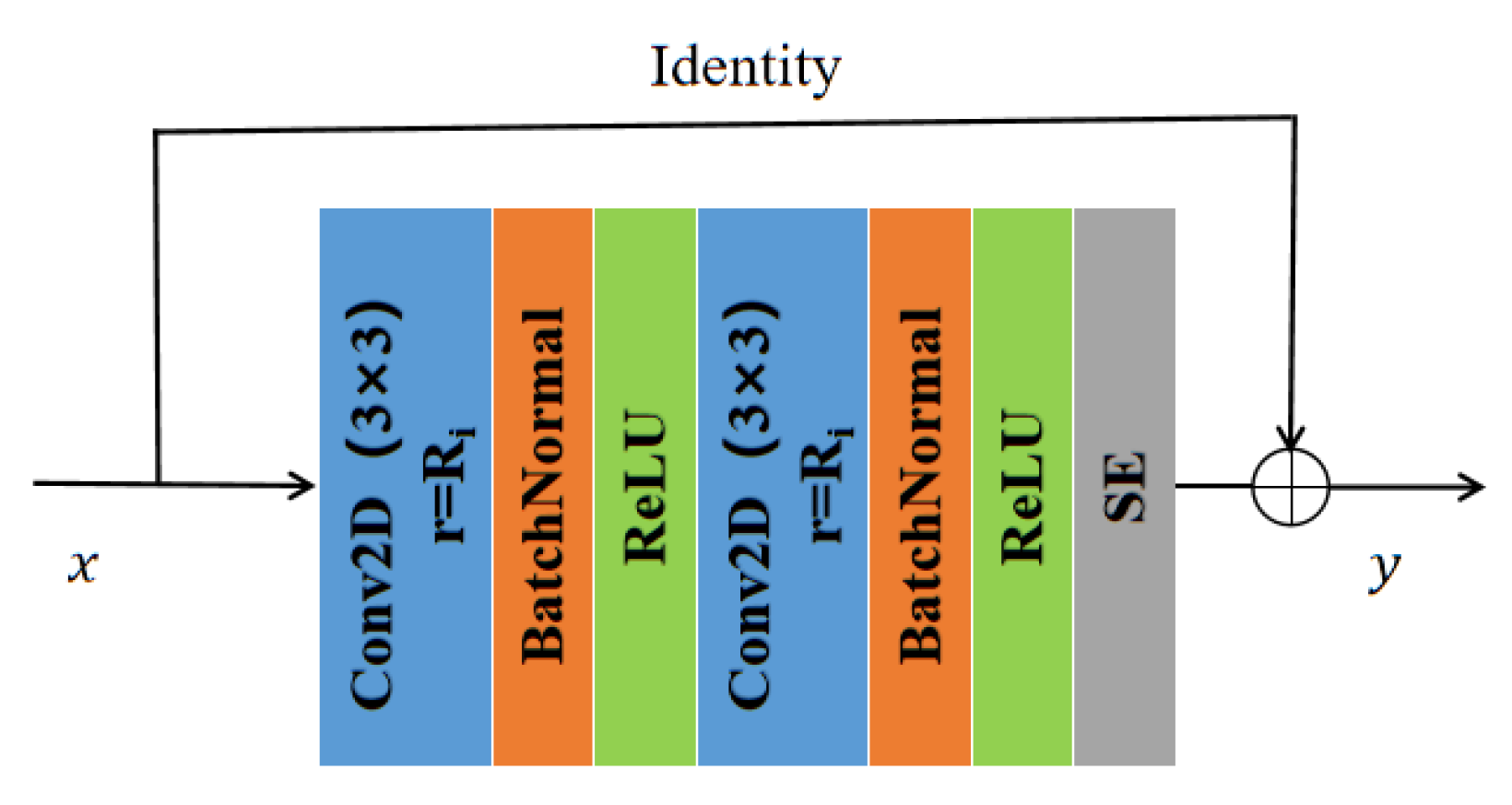
3.2. Residual Atrous Convolution
In general, the method for increasing the receptive field and reducing the amount of computation in deep neural networks is down-sampling. However, down-sampling sacrifices part of the spatial resolution and loses some information, which limits the effect of semantic segmentation. In contrast, atrous convolutions [
30] enable effectively increasing target receptive field without increasing model parameters and without changing the size of the feature map. In addition, we introduce residual connectivity [
10]. Residual connection not only reduces the complexity of model training to minimize overfitting but also prevents the gradient from vanishing. The RA convolutional network is proposed by combining the above two methods. In the RA module, we replace the standard convolution in the original CNN with dilated convolutions. On the one hand, the receptive field increases, and significant targets can be detected and segmented. On the other, the increased resolution compared with down-sampling can accurately locate the target. Combining residual connections can improve the mobility of information and prevent serious information loss. Significantly, RA can be integrated into other convolutional neural networks, which is a crucial reference for improving the propagation of feature vectors.
For the input feature vector, two 3 × 3 dilated convolutions (as shown in
Figure 4) followed by normalization were used to prevent the occurrence of gradient explosions. Then, a rectified linear unit was used for activations to alleviate the overfitting problem. Finally, an identity mapping and squeeze excitation unit [
28] was introduced to add and fuse the output with the original feature vector.
3.3. MHAU-Net Architecture
Coding Phase: The encoding stage uses RA blocks with different dilation rates for feature information extraction (as shown in
Table 1). The RA block uses a 3 × 3 convolution with stride 2 instead of pooling during down-sampling. To avoid overfitting and underutilizing resources, after each layer of convolution operation, the feature maps of each layer are normalized using a batch normalization layer and then activated using the leaky ReLU activation function (as shown in
Figure 4). Then, the feature vectors are input into HRA, and the dependencies between channels are established. Using the dependency relationship between feature channels, the feature representation of specific semantics can be improved to generate channel attention maps. The spatial attention module encodes a vast range of contextual information into local features, thus enhancing their expressive power. The spatial relations among the elements generate a spatial attention graph. HRA has powerful feature representation capabilities that can be integrated into other CNN architectures. However, frequently using channels and spatial attention mechanisms increases spatial and time complexities. The high resolution low-level features and the smaller field of perception of individual pixels enable the use of more fine-grained feature information to capture more small targets. The validation shows that increasing too many attention mechanisms does not bring about significant improvements but instead increases the training burden. Therefore, we choose to use attention mapping after three more low-level features of RA, R
3A, and R
4A.
Meanwhile, we perform four 3 × 3 pooling convolution operations with different steps on the original image. Images with varying resolution sizes are input to each encoder level in a multi-scale manner and are encoded with multi-scale contextual information (as shown in
Figure 5). Given an original image with a size of 256 × 256, after four sampling operations, images of sizes 128 × 128, 64 × 64, 32 × 32, and 16 × 16 are obtained, respectively, and then added to the feature vector of the corresponding coding level.
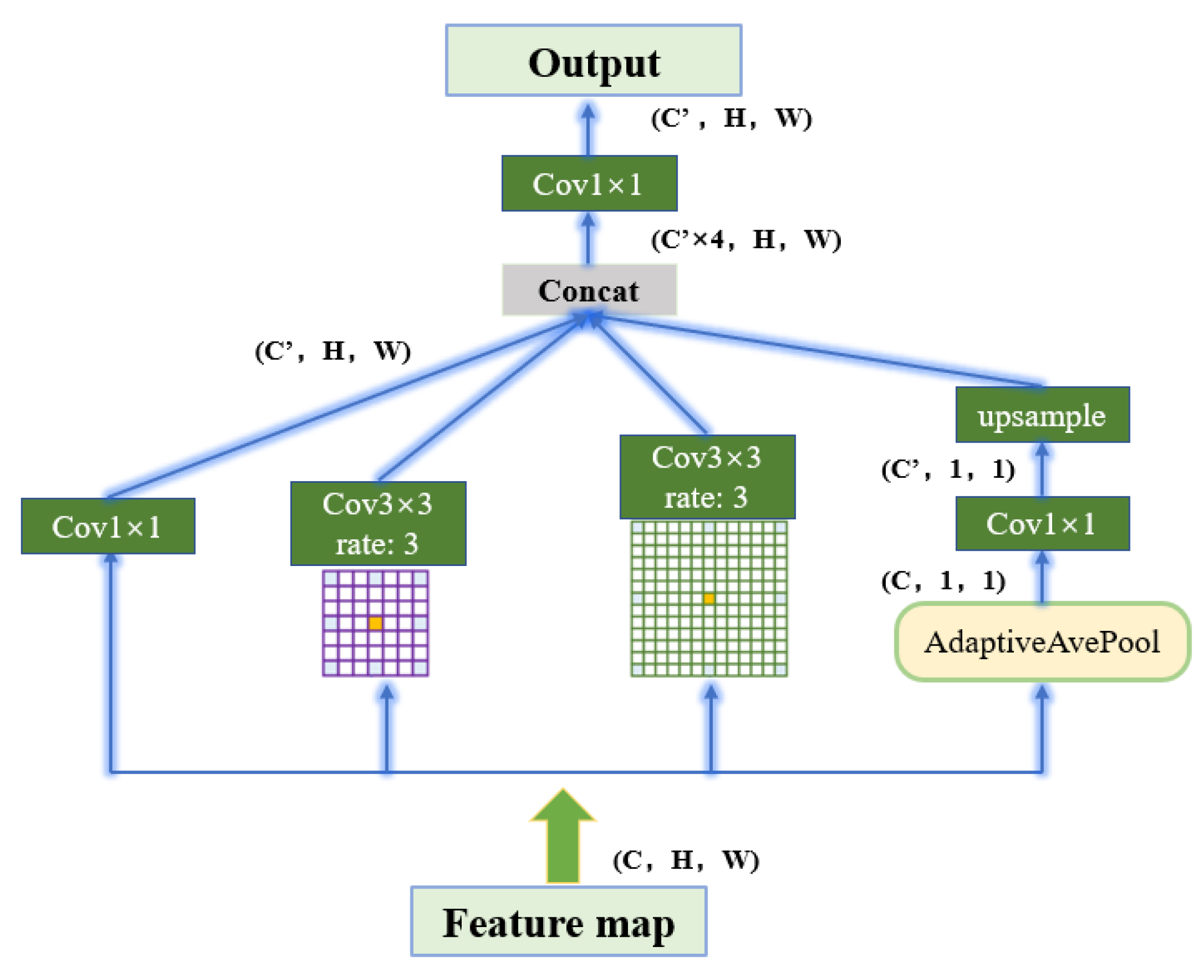
Transition Phase: ASPP is composed of a 1 × 1 convolution (shown on the far left in
Figure 6), a pooling pyramid (two 3 × 3 convolution blocks in the middle), and an adaptive pooling layer (far right). The dilation factor of each layer of the pooled pyramid can be customized, and different scales of perceptual fields can be obtained by extra padding and dilation. The advantage of using AdaptiveAvgPool2d layers is that there is no need to assign a convolution kernel and step size, as only the final output size needs to be specified. The purpose is to compress the feature maps of each channel to 1 × 1, respectively, to extract the features of each channel and thus obtain the global features.
Decoding Phase: The decoding path adopts the exact opposite operation of the encoding path. First, the output features of ASPP are up-sampled using bilinear interpolation with a step size of 2. The output features corresponding to the encoder level are extracted and spliced with up-sampling features. Second, feature reduction is performed using convolutions of size 3 × 3, followed by batch normalization and then followed by activation operations. Finally, a 1 × 1 convolution and a sigmoid activation function are applied to output mask features.
4. Experiments and Results
4.1. Datasets
To evaluate MHAU-Net, we conducted experiments on three public medical image datasets. In this paper, data augmentation techniques, including vertical flip and transpose (as shown in
Figure 7), were used in advance for all datasets participating in the experiments (details of the data set are shown in
Table 2). However, we do not establish the validation dataset. Since we use a cross-validation strategy, some data are randomly divided as the validation set in each training round. Cross-validation enables an increase in the randomness of the validation dataset and the training parameters are adjusted in time, thus effectively improving the generalization performance of the model.
4.2. Evaluation Metrics
In this paper, we use the standard metrics commonly used for semantic segmentation to demonstrate that MHAU-Net has a more accurate segmentation output than other popular models. The Dice Similarity Coefficient (DSC) (as shown in Equation (3)) is used to evaluate the similarity between the segmentation output and the actual labels, and the value ranges from [0, 1]; the larger the value, the higher the similarity between the two sets. The Intersection over Union (IoU) is the ratio of the intersection of the true and predicted values of a prediction category to the union (as shown in Equation (4)). The sensitivity (SEN), defined as Equation (5), indicates the proportion of correctly segmented lesion pixels, and high sensitivity (close to 1.0) shows a good segmentation effect. Specificity (SPE) (as shown in Equation (6)) indicates the proportion of non-lesioned skin pixels that are not correctly segmented. The high specificity suggests the ability of the method to segment non-lesioned pixels:
where the relationship between
DSC and
IoU can be expressed as Equation (7). The
IoU of each prediction category is found and the output mIoU is obtained by taking the average value.
TP is the True Positive.
FP is the False Positive.
FN is the False Negative.
TN is the True Negative.
4.3. Experimental Configuration
All experimental programs are implemented in the PyTorch 1.11.0 framework and run on a single-core NVIDIA GeForce RTX 3090 with a 24 GB dedicated GPU. A stochastic gradient descent optimization strategy is used, with an initial learning rate of 10−3 and a learning rate reduction of 1/5 for every 15 epochs. The batch size and the number of epochs are set to 16 and 150, respectively.
4.4. Results
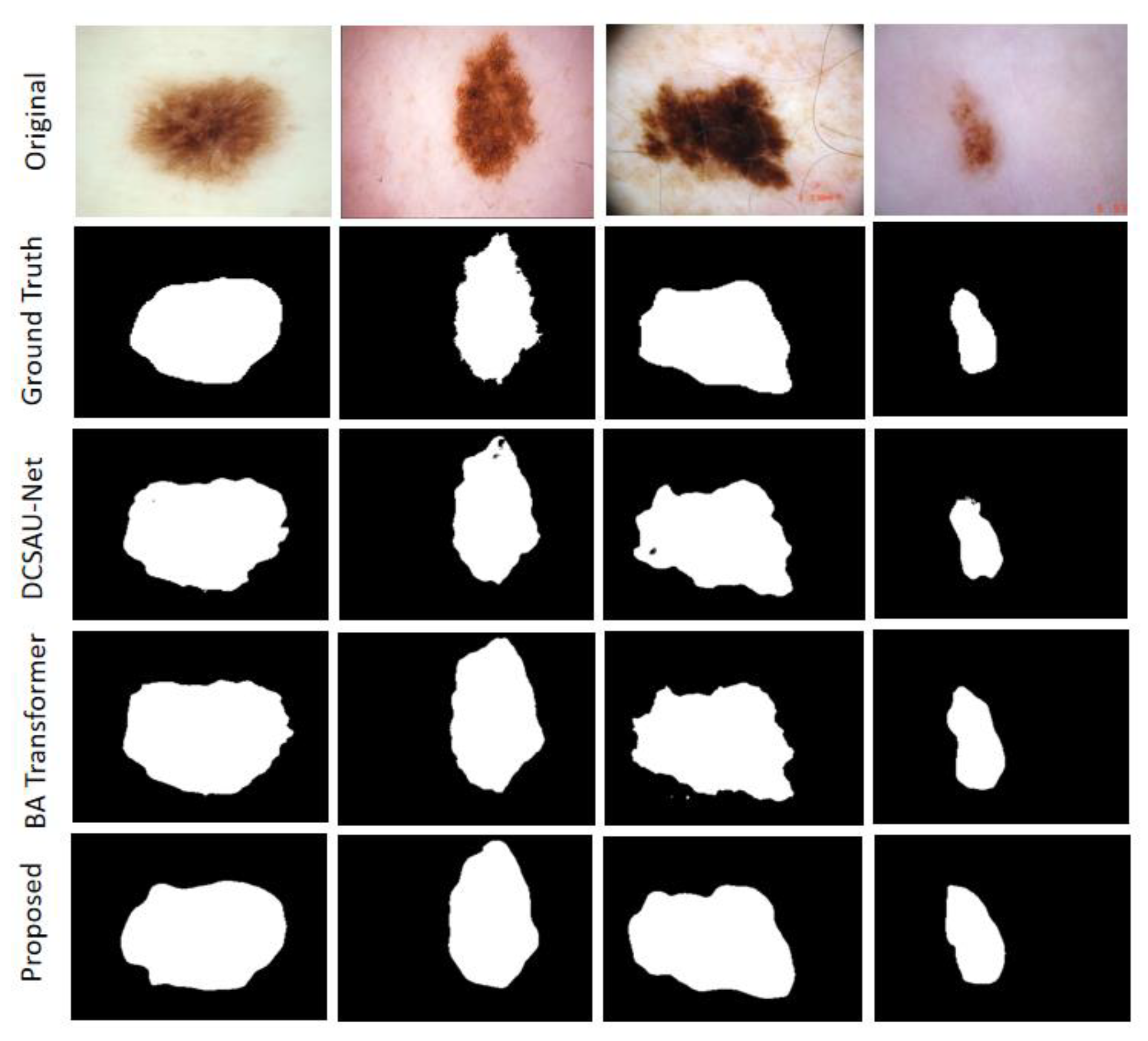
This section presents the segmentation results of the MAHU-Net method exhibited in different datasets. Quantitatively, in the ISIC 2018 Task1 challenge dataset, our proposes has better segmentation performances compared to the original U-Net and the latest DCSAU-Net [
31] architectures, with 7.67% and 4.91% improvements in mIoU, respectively, and DSC compared to DCSAU-Net with a 3.41% improvement compared to DCSAU-Net. It was 93.9%, 92.7%, 94.69%, and 87.92% in overall sensitivity, specificity, DSC, and mIoU, respectively; DSC, mIoU, and SPE were superior to DCSAU-Net by 3.41%, 4.91%, and 3.11%, respectively (as shown in
Table 3). The case of the final segmentation of our proposed method on the ISIC 2018 validation set is provided in
Figure 8.
On ISIC-2017 Task1, we merged 150 images data from the validation set into the training set and then used data augmentation techniques. Finally, the results are derived using the cross-validation strategy. The overall sensitivity and specificity outperformed the original U-Net by 2.47% and 3.01%, respectively (as shown in
Table 4). The case of the final segmentation of our proposed method on the ISIC 2017 task validation set is provided in
Figure 9.
On the Kvasir-SEG dataset, we compared it with the currently popular Double U-Net, Pra-Net, and U-Net. The results show that our method is highly competitive. DSC is 1.34%, 1.92%, and 9.92% higher than Double U-Net, Pra-Net, and U-Net, respectively; it only has a 0.12% difference from the TransFuse-S architecture. However, we show the large advantage with a high mIoU of 0.9025. SEN is 1.65% and 6.72% higher than U-Net and Double U-Net, respectively (as shown in
Table 5). An experimental comparison between U-Net and our method is given in
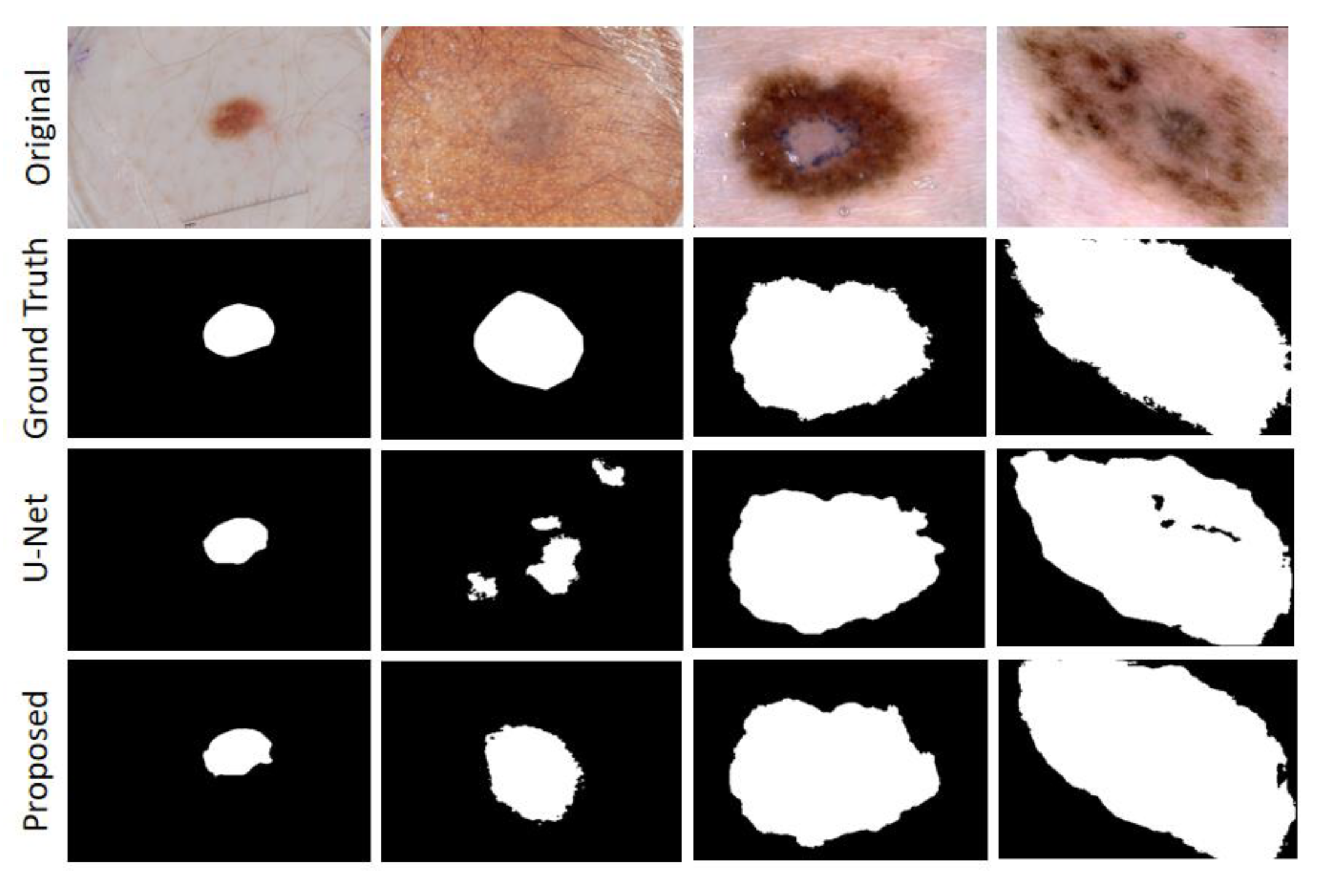
Figure 10, and our proposal shows a more robust output in tiny tissue regions.
The comparative experimental data in
Table 3,
Table 4 and
Table 5 are from the original article cited, and the code is publicly available on GitHub.
4.5. Slice Experimental
In this paper, we use a slice experimental approach to evaluate the contribution of HRA and other vital components to semantic segmentation. The experiments use the original U-Net as the segmentation baseline to verify the gains from repeated HRA, dilated residual convolution, and cross-validation experiments. The same hyperparameter settings are used for all experiments. ISIC 2018 Task1 is used as an example to illustrate the effect of these network components. Starting with the baseline model U-Net, experiments were progressively performed with HRA #1, HRA #2, and HRA #3. For mIoU, applying HRA #1 improves segmentation performances by 1.38%, using both HRA #1 and HRA #2 improves it by 3.71%, and using HRA #1, HRA #2, and HRA #3 together improves it by 4.92% (as shown in
Table 6). The segmentation performance is raised by 5.84% by adding dilation convolution. Lastly, the model generalization performance is boosted using cross-validation methods.
The end experimental results indicate that MHAU-Net demonstrates good performance compared to the original U-Net and the recently popular Double U-Net, DSCAU-Net, and MFS-Net in different metrics. From the training results in
Table 6, it can be seen that SPE has a decreasing trend. This error may be related to the increase in false positives (i.e., classifying some non-lesioned pixels as lesioned pixels). However, in terms of experimental results, the training result of MHAU-Net is about 9% higher than that of U-Net. It provides a reference for the improvement of subsequent network models.
5. Discussion and Future Work
The MHAU-Net architecture proposed in this paper achieved satisfactory results from ISIC-2018 Task1, ISIC-2017, and Kvasir-SEG dataset. From the information shown in
Figure 8,
Figure 9 and
Figure 10, it could be concluded that the segmentation maps generated by the MHAU-Net outperformed the other architectures in capturing the boundary information, demonstrating that the segmentation masks generated in the MHAU-Net showed more precise information in the target area than the existing models. The full convolutional network has more room for improvements in capturing skin lesion locations and edge details.
In this paper, we combine the binary cross-entropy loss function and the dice loss function to train the proposed model. With the same loss function, the proposed model achieves higher dice coefficient values than the other models. Based on the empirical evaluation, the dice coefficient loss function is chosen to achieve better segmentation results. In addition, the effects of batch size, optimizer, and loss function selection on the results are observed.
We speculate that the performance of the model can be further improved by increasing the size of the dataset, applying more enhancement techniques, and applying some post-processing steps. The application of MHAU-Net should not be limited to biomedical image segmentation; it can also be extended to natural image segmentation and other pixel-level classification tasks that require further detailed validation. The proposed method could be feasible for future medical imaging analyses and clinical exam routines.
Future improvements will include the following:
We attempt to develop an enhanced version of MHAU-Net for video analyses in the medical field.
Future studies will integrate the hybrid residual attention module proposed in this paper in other skin lesion segmentation models to verify its enhancement of the model’s results.
6. Conclusions
Inspired by U-Net and the attention mechanism, we propose MHAU-Net to address the need for the automated detection of lesion areas in dermoscopy and its related medical fields. MHAU-Net consists of four parts: multi-scale resolution input, hybrid residual attention (HRA), dilated convolution, and atrous spatial pyramid pooling. HRA fully utilizes the benefits of the attention mechanism to achieve feature enhancements and conducts slicing experiments to verify the contribution of network components to the overall network architecture. Use a 5-fold cross-validation strategy during training to improve the generalization performance of the model. We validated MHAU-Net on three datasets with better performance than the BA-Transformer and U-Net. To achieve the goal of model generalizability, the proposed architecture in this paper should be further investigated for improvements to obtain better segmentation results.
Author Contributions
Methodology, Y.L.; software, Y.L. and Z.A.; validation, J.H., D.W. and H.M.; formal analysis, C.L.; data curation, D.W.; writing—original draft preparation, Y.L.; writing—review and editing, C.X.; visualization, H.M.; supervision, C.X.; project administration, C.X.; funding acquisition, C.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (No. 2019YFC0117800).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, S.K.; Greenspan, H.; Davatzikos, C.; Duncan, J.S.; Van Ginneken, B.; Madabhushi, A.; Prince, J.L.; Rueckert, D.; Summers, R.M. A Review of Deep Learning in Medical Imaging: Imaging Traits, Technology Trends, Case Studies With Progress Highlights, and Future Promises. Proc. IEEE 2021, 109, 820–838. [Google Scholar] [CrossRef]
- Bai, W.; Suzuki, H.; Huang, J.; Francis, C.; Wang, S.; Tarroni, G.; Guitton, F.; Aung, N.; Fung, K.; Petersen, S.E.; et al. A population-based phenome-wide association study of cardiac and aortic structure and function. Nat. Med. 2020, 26, 1654. [Google Scholar] [CrossRef]
- Mei, X.; Lee, H.-C.; Diao, K.-Y.; Huang, M.; Lin, B.; Liu, C.; Xie, Z.; Ma, Y.; Robson, P.M.; Chung, M.; et al. Artificial intelligence-enabled rapid diagnosis of patients with COVID-19. Nat. Med. 2020, 26, 1224. [Google Scholar] [CrossRef] [PubMed]
- Khened, M.; Kollerathu, V.A.; Krishnamurthi, G. Fully convolutional multi-scale residual DenseNets for cardiac segmentation and automated cardiac diagnosis using ensemble of classifiers. Med. Image Anal. 2019, 51, 21–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Chen, X.; Liu, Y.; Lu, Z.; You, J.; Yang, M.; Yao, S.; Zhao, G.; Xu, Y.; Chen, T.; et al. Clinically applicable deep learning framework for organs at risk delineation in CT images. Nat. Mach. Intell. 2019, 1, 480–491. [Google Scholar] [CrossRef]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster R-CNN: Towards Real-Time Object Detection with Region Proposal Networks. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 1137–1149. [Google Scholar] [CrossRef] [Green Version]
- Shelhamer, E.; Long, J.; Darrell, T. Fully Convolutional Networks for Semantic Segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 640–651. [Google Scholar] [CrossRef]
- Khan, M.A.; Akram, T.; Zhang, Y.-D.; Sharif, M. Attributes based skin lesion detection and recognition: A mask RCNN and transfer learning-based deep learning framework. Pattern Recognit. Lett. 2021, 143, 58–66. [Google Scholar] [CrossRef]
- Dosovitskiy, A.; Beyer, L.; Kolesnikov, A.; Weissenborn, D.; Zhai, X.; Unterthiner, T.; Dehghani, M.; Minderer, M.; Heigold, G.; Gelly, S.; et al. An Image is Worth 16x16 Words: Transformers for Image Recognition at Scale. arXiv 2020, arXiv:2010.11929. [Google Scholar]
- Hesamian, M.H.; Jia, W.; He, X.; Kennedy, P. Deep Learning Techniques for Medical Image Segmentation: Achievements and Challenges. J. Digit. Imaging 2019, 32, 582–596. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, H.; Qi, X.; Dou, Q.; Fu, C.W.; Heng, P.A. H-DenseUNet: Hybrid Densely Connected UNet for Liver and Tumor Segmentation from CT Volumes. IEEE Trans. Med. Imaging 2018, 37, 2663–2674. [Google Scholar] [CrossRef] [PubMed]
- Dolz, J.; Gopinath, K.; Yuan, J.; Lombaert, H.; Desrosiers, C.; Ben Ayed, I. HyperDense-Net: A Hyper-Densely Connected CNN for Multi-Modal Image Segmentation. IEEE Trans. Med. Imaging 2019, 38, 1116–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, Y.; Ma, X.X. Image noise recognition algorithm based on BP neural network. In Proceedings of the 32nd Chinese Control and Decision Conference (CCDC), Hefei, China, 22–24 August 2020. [Google Scholar]
- Gudhe, N.R.; Behravan, H.; Sudah, M.; Okuma, H.; Vanninen, R.; Kosma, V.M.; Mannermaa, A. Multi-level dilated residual network for biomedical image segmentation. Sci. Rep. 2021, 11, 14105. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the 18th International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI), Munich, Germany, 5–9 October 2015. [Google Scholar]
- Zhou, Z.; Siddiquee, M.M.R.; Tajbakhsh, N.; Liang, J. UNet plus plus: Redesigning Skip Connections to Exploit Multiscale Features in Image Segmentation. IEEE Trans. Med. Imaging 2020, 39, 1856–1867. [Google Scholar] [CrossRef] [Green Version]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Huang, G.; Liu, Z.; Van Der Maaten, L.; Weinberger, K.Q. Densely Connected Convolutional Networks. In Proceedings of the 30th IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- Zhang, Z.; Liu, Q.; Wang, Y. Road Extraction by Deep Residual U-Net. IEEE Geosci. Remote Sens. Lett. 2018, 15, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Shi, J.; Qi, X.; Wang, X.; Jia, J. Pyramid Scene Parsing Network. In Proceedings of the 30th IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- Ibtehaz, N.; Rahman, M.S. MultiResUNet: Rethinking the U-Net architecture for multimodal biomedical image segmentation. Neural Netw. 2020, 121, 74–87. [Google Scholar] [CrossRef]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention Is All You Need. In Proceedings of the 31st Annual Conference on Neural Information Processing Systems (NIPS), Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Chen, J.; Lu, Y.; Yu, Q.; Luo, X.; Adeli, E.; Wang, Y.; Lu, L.; Yuille, A.L.; Zhou, Y. TransUNet: Transformers Make Strong Encoders for Medical Image Segmentation. arXiv 2021, arXiv:2102.04306. [Google Scholar]
- Wang, G.; Wang, Y.; Li, H.; Chen, X.; Lu, H.; Ma, Y.; Peng, C.; Wang, Y.; Tang, L. Morphological background detection and illumination normalization of text image with poor lighting. PLoS ONE 2017, 9, e110991. [Google Scholar] [CrossRef] [Green Version]
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proceedings of the International Joint Conference on Artificial Intelligence, Montreal, QC, Canada, 20–25 August 1995. [Google Scholar]
- Fu, J.; Liu, J.; Tian, H.; Li, Y.; Bao, Y.; Fang, Z.; Lu, H. Dual Attention Network for Scene Segmentation. arXiv 2018, arXiv:1809.02983. [Google Scholar]
- Lu, J.; Xiong, C.; Parikh, D.; Socher, R. Knowing When to Look: Adaptive Attention via A Visual Sentinel for Image Captioning. arXiv 2016, arXiv:1612.01887. [Google Scholar]
- Hu, J.; Shen, L.; Sun, G. Squeeze-and-Excitation Networks. arXiv 2017, arXiv:1709.01507. [Google Scholar]
- Woo, S.; Park, J.; Lee, J.Y.; Kweon, I.S. CBAM: Convolutional Block Attention Module. In Proceedings of the European Conference on Computer Vision, Munich, Germany, 8–14 September 2018. [Google Scholar]
- Yu, F.; Koltun, V. Multi-Scale Context Aggregation by Dilated Convolutions. arXiv 2015, arXiv:1511.07122. [Google Scholar]
- Xu, Q.; Duan, W.; He, N. DCSAU-Net: A Deeper and More Compact Split-Attention U-Net for Medical Image Segmentation. arXiv 2022, arXiv:2202.00972. [Google Scholar]
- Valanarasu, J.M.J.; Patel, V.M. UNeXt: MLP-based Rapid Medical Image Segmentation Network. arXiv 2022, arXiv:2203.04967. [Google Scholar]
- Wang, J.; Wei, L.; Wang, L.; Zhou, Q.; Zhu, L.; Qin, J. Boundary-Aware Transformers for Skin Lesion Segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI), Electr Network, Singapore, 18–22 September 2021. [Google Scholar]
- Basak, H.; Kundu, R.; Sarkar, R. MFSNet: A multi focus segmentation network for skin lesion segmentation. Pattern Recognit. 2022, 128, 108673. [Google Scholar] [CrossRef]
- Fan, D.-P.; Ji, G.-P.; Zhou, T.; Chen, G.; Fu, H.; Shen, J.; Shao, L. PraNet: Parallel Reverse Attention Network for Polyp Segmentation. arXiv 2020, arXiv:2006.11392. [Google Scholar]
- Jha, D.; Riegler, M.A.; Johansen, D.; Halvorsen, P.; Johansen, H.D. DoubleU-Net: A Deep Convolutional Neural Network for Medical Image Segmentation. In Proceedings of the 33rd IEEE International Symposium on Computer-Based Medical Systems (CBMS), Electr Network, Rochester, MN, USA, 28–30 July 2020. [Google Scholar]
- Zhang, Y.; Liu, H.; Hu, Q. TransFuse: Fusing Transformers and CNNs for Medical Image Segmentation. arXiv 2021, arXiv:2102.08005. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).