Gas-Sensitive Characteristics of Graphene Composite Tungsten Disulfide to Ammonia
Abstract
:1. Introduction
2. Materials and Methods
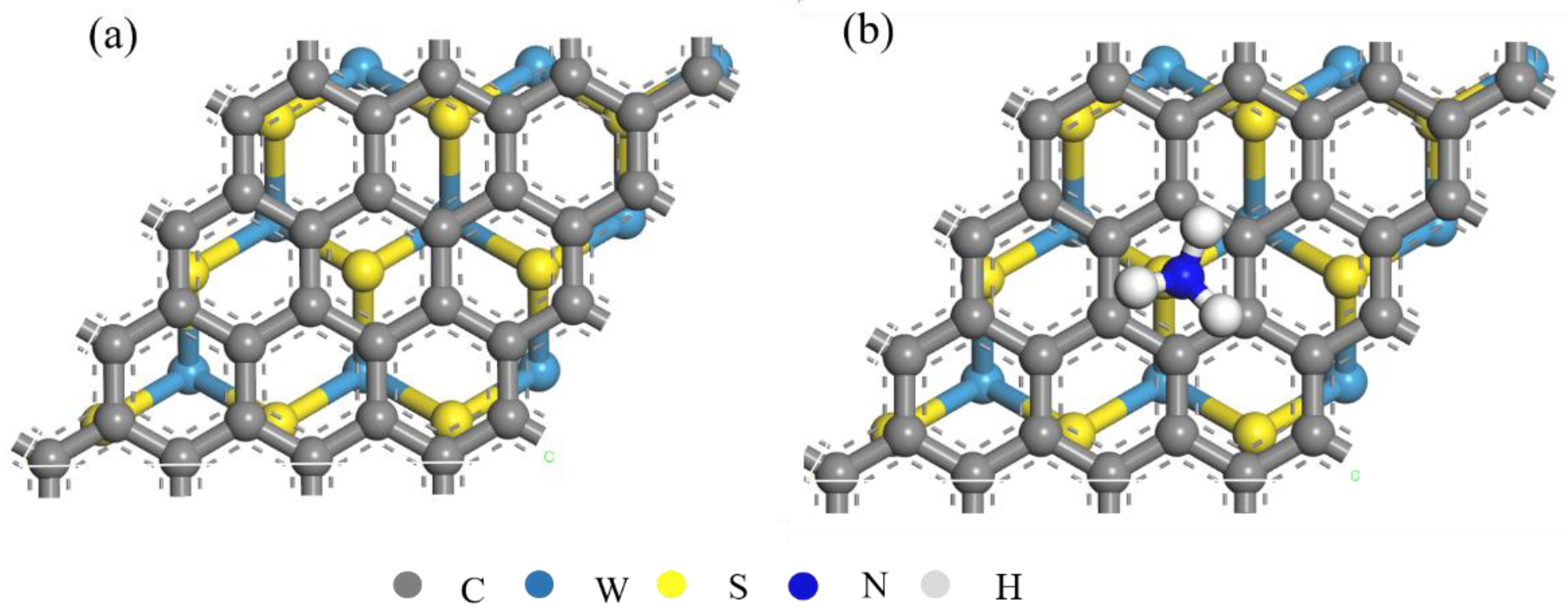
2.1. Simulation Model and Calculation Method
2.2. Experimental Steps
3. Results and Discussion
3.1. Discussion of Band Structure
3.2. Gas Sensitivity Test Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hong, S.; Wu, M.; Hong, Y. FET-type gas sensors: A review. Sens. Actuators B Chem. 2020, 330, 129240. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Ping, J.F.; Fan, Z.X.; Sindoro, M. Recent advances in sensing applications of two-dimensional transition metal dichalcogenide nanosheets and their composites. Adv. Funct. Mater. 2017, 27, 1605817. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Karaduman, I.; Er, E.; Çelikkan, H. Room-temperature ammonia gas sensor based on reduced graphene oxide nanocomposites decorated by Ag, Au and Pt nanoparticles. J. Alloys Compd. 2017, 722, 569–578. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Han, T.Y.; Fei, T. The investigation of microstructure effect on NO2 sensors based on SnO2 nanoparticles/reduced graphene oxide hybrids. ACS Appl. Mater. Interfaces 2018, 10, 41773–41783. [Google Scholar] [CrossRef]
- Yang, L.; Jin, Y.Q.; Wang, X.J. Antifouling field-effect transistor sensing interface based on covalent organic frameworks. Adv. Electron. Mater. 2020, 6, 1901169. [Google Scholar] [CrossRef]
- Islam, M.A.; Li, H.; Moon, S. Vertically aligned 2D MoS2 layers with strain-engineered serpentine patterns for high performance stretchable gas sensors: Experimental and theoretical demonstration. ACS Appl. Mater. Interfaces 2020, 12, 53174–53183. [Google Scholar] [CrossRef]
- Wu, E.X.; Xie, Y.; Yuan, B. Ultrasensitive and fully reversible NO2 gas sensing based on p-type MoTe2 under ultraviolet illumination. ACS Sens. 2018, 3, 1719–1726. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, Y.H.; Park, S.Y. Two-dimensional transition metal disulfides for chemoresistive gas sensing: Perspective and challenges. Chemosensors 2017, 5, 15. [Google Scholar] [CrossRef]
- Li, J.H.; Yan, H.T.; Wei, W. Enhanced lithium storage performance of liquid-phase exfoliated graphene supported WS2 heterojunctions. ChemElectroChem 2018, 5, 3222–3228. [Google Scholar] [CrossRef]
- Senkovskiy, B.V.; Nenashev, A.V.; Alavi, S.K. Tunneling current modulation in atomically precise graphene nanoribbon heterojunctions. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Liang, X.Y.; Ding, N.; Ng, S.P. Adsorption of gas molecules on Ga-doped graphene and effect of applied electric field: A DFT study. Appl. Surf. Sci. 2017, 411, 11–17. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, C.; Wu, G. DFT study on the electronic structure and optical properties of N, Al, and N-Al doped graphene. Appl. Surf. Sci. 2018, 459, 354–362. [Google Scholar] [CrossRef]
- Gui, Y.G.; Hao, Z.S.; Li, X. Gas sensing of graphene and graphene oxide nanoplatelets to ClO2 and its decomposed species. Superlattices Microstruct. 2019, 135, 106248. [Google Scholar] [CrossRef]
- Gutierrez, H.R.; Perea, N.; Elias, A. Extraordinary room-temperature photoluminescence in triangular WS2 monolayers. Nano Lett. 2013, 13, 3447–3454. [Google Scholar] [CrossRef] [Green Version]
- Georgiou, T.; Yang, H.; Jalil, R. Electrical and optical characterization of atomically thin WS2. Dalton Trans. 2014, 43, 10388–10391. [Google Scholar] [CrossRef]
- Yang, M.Y.; Wang, L.; Hou, T. Controlling of the electronic properties of WS2 and graphene oxide heterostructures from first-principles calculations. J. Mater. Chem. C 2017, 5, 201–207. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Q.; Wang, J. Performance of intrinsic and modified graphene for the adsorption of H2S and CH4: A DFT study. Nanomaterials 2020, 10, 299. [Google Scholar] [CrossRef]
- Gao, W.; Xiao, P.H.; Henkelman, G. Interfacial adhesion between graphene and silicon dioxide by density functional theory with van der Waals corrections. J. Phys. D Appl. Phys. 2014, 47, 255301. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, C.; Chen, D.C. The adsorption performance of harmful gas on Cu doped WS2: A first-principal study. Mater. Today Commun. 2021, 28, 102488. [Google Scholar] [CrossRef]
- Koo, W.T.; Cha, J.H.; Jung, J.W. Few-layered WS2 nanoplates confined in Co, N-doped hollow carbon nanocages: Abundant WS2 edges for highly sensitive gas sensors. Adv. Funct. Mater. 2018, 28, 1802575. [Google Scholar] [CrossRef]
- Jarvinen, T.; Lorite, G.S.; Perantie, J. WS2 and MoS2 thin film gas sensors with high response to NH3 in air at low temperature. Nanotechnology 2019, 30, 405501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvi, A.; Ferrari, A.; Sbuelz, L. Recognizing physisorption and chemisorption in carbon nanotubes gas sensors by double exponential fitting of the response. Sensors 2016, 16, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.F.; Xie, G.Z.; Su, Y.J. A new model and its application for the dynamic response of RGO resistive gas sensor. Sensors 2019, 19, 889. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, Z.; Wang, J. WS2 nanoflakes based selective ammonia sensors at room temperature. Sens. Actuators B Chem. 2017, 240, 273–277. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Tian, F. Edge-enriched WS2 nanosheets on carbon nanofibers boosts NO2 detection at room temperature. J. Hazard. Mater. 2021, 411, 125120. [Google Scholar] [CrossRef]
- Chen, X.; Hu, J.; Yin, M. UV-light-assisted NO2 gas sensor based on WS2/PbS heterostructures with full recoverability and reliable anti-humidity ability. Sens. Actuators B Chem. 2021, 339, 2. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Lim, S. Chemical sensing of 2D graphene/MoS2 heterostructure device. ACS Appl. Mater. Interfaces 2015, 7, 30. [Google Scholar] [CrossRef]
- Navazani, S.; Shokuhfar, A.; Hassanisadi, M. Fabrication and characterization of a sensitive, room temperature methane sensor based on SnO2@reduced graphene oxide-polyaniline ternary nanohybrid. Mater. Sci. Semicond. Process. 2018, 88, 139–147. [Google Scholar] [CrossRef]





| Material | Target Gas | Concentration (ppm) | Response (%) | Response Time (s) | Operating Temperature (°C) | |
|---|---|---|---|---|---|---|
| WS2 NFs | NH3 | 5 | 252.4 | 921 | 25 | [27] |
| WS2/CNFs | NO2 | 50 | 279.1 | 54 | 25 | [28] |
| WS2/PbS | NO2 | 5 | 592.8 | 612 | 25 | [29] |
| MoS2/GO | NH3 | 100 | 3.6 | 2751 | 150 | [30] |
| SnO2/rGO | CH4 | 10000 | 91.3 | 426 | 25 | [31] |
| WS2/GO | NH3 | 100 | 2.4 | 280 | 30 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Li, Z.; Fu, Y.; Wang, Q. Gas-Sensitive Characteristics of Graphene Composite Tungsten Disulfide to Ammonia. Sensors 2022, 22, 8672. https://doi.org/10.3390/s22228672
Zhao F, Li Z, Fu Y, Wang Q. Gas-Sensitive Characteristics of Graphene Composite Tungsten Disulfide to Ammonia. Sensors. 2022; 22(22):8672. https://doi.org/10.3390/s22228672
Chicago/Turabian StyleZhao, Fei, Zhongxue Li, Yongzhong Fu, and Quan Wang. 2022. "Gas-Sensitive Characteristics of Graphene Composite Tungsten Disulfide to Ammonia" Sensors 22, no. 22: 8672. https://doi.org/10.3390/s22228672
APA StyleZhao, F., Li, Z., Fu, Y., & Wang, Q. (2022). Gas-Sensitive Characteristics of Graphene Composite Tungsten Disulfide to Ammonia. Sensors, 22(22), 8672. https://doi.org/10.3390/s22228672






