Screening for Obstructive Sleep Apnea Risk by Using Machine Learning Approaches and Anthropometric Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Population
2.3. Body Composition
2.4. Sleep Parameter
2.5. Statistical Analysis
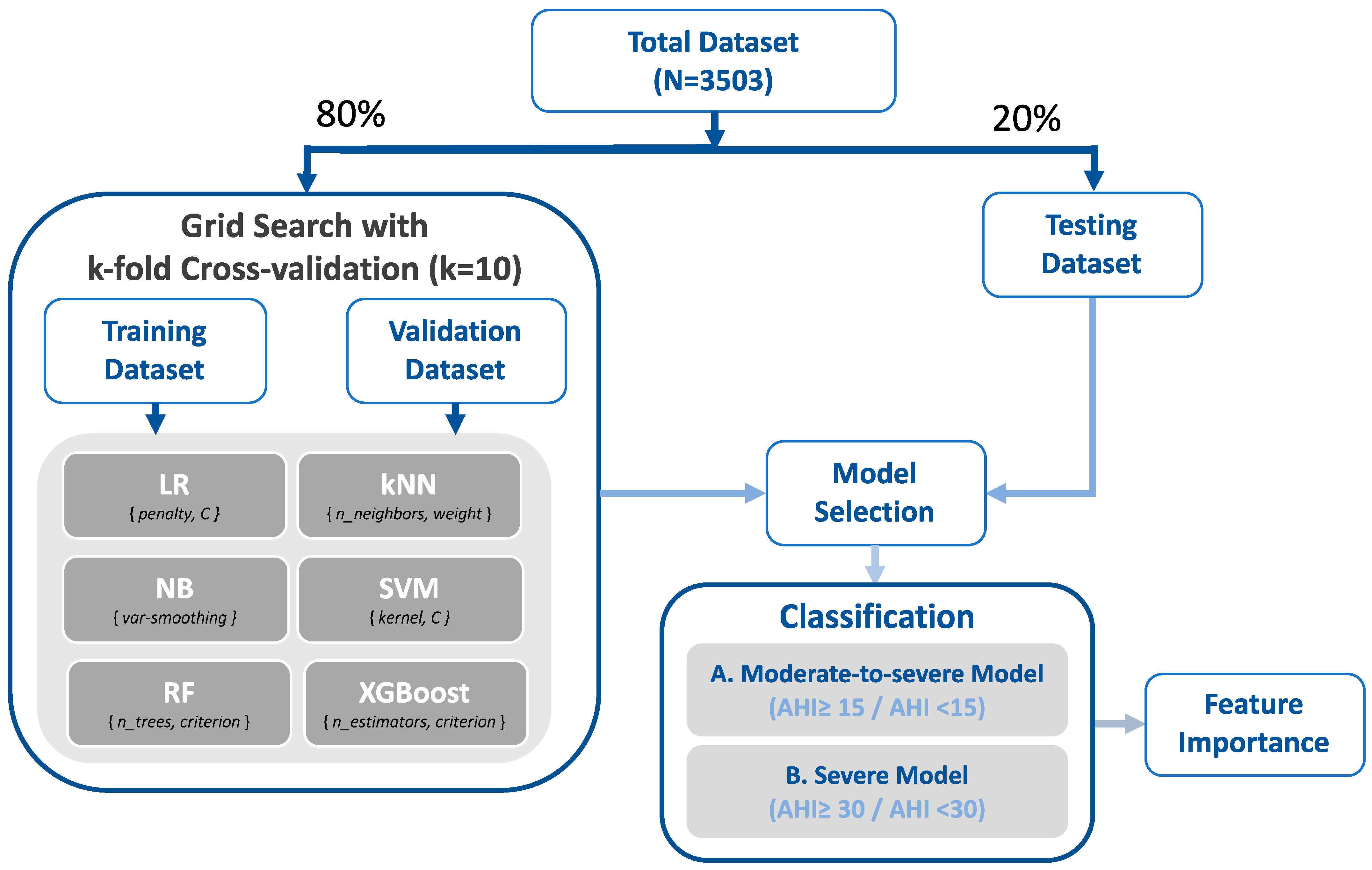
2.6. Machine Learning Approaches
3. Results
3.1. Characterization of Enrolled Participants
3.2. Sleep Parameters
3.3. Sleep Quality Index and Anthropometric Features
3.4. Validation Performance of Machine Learning Approaches
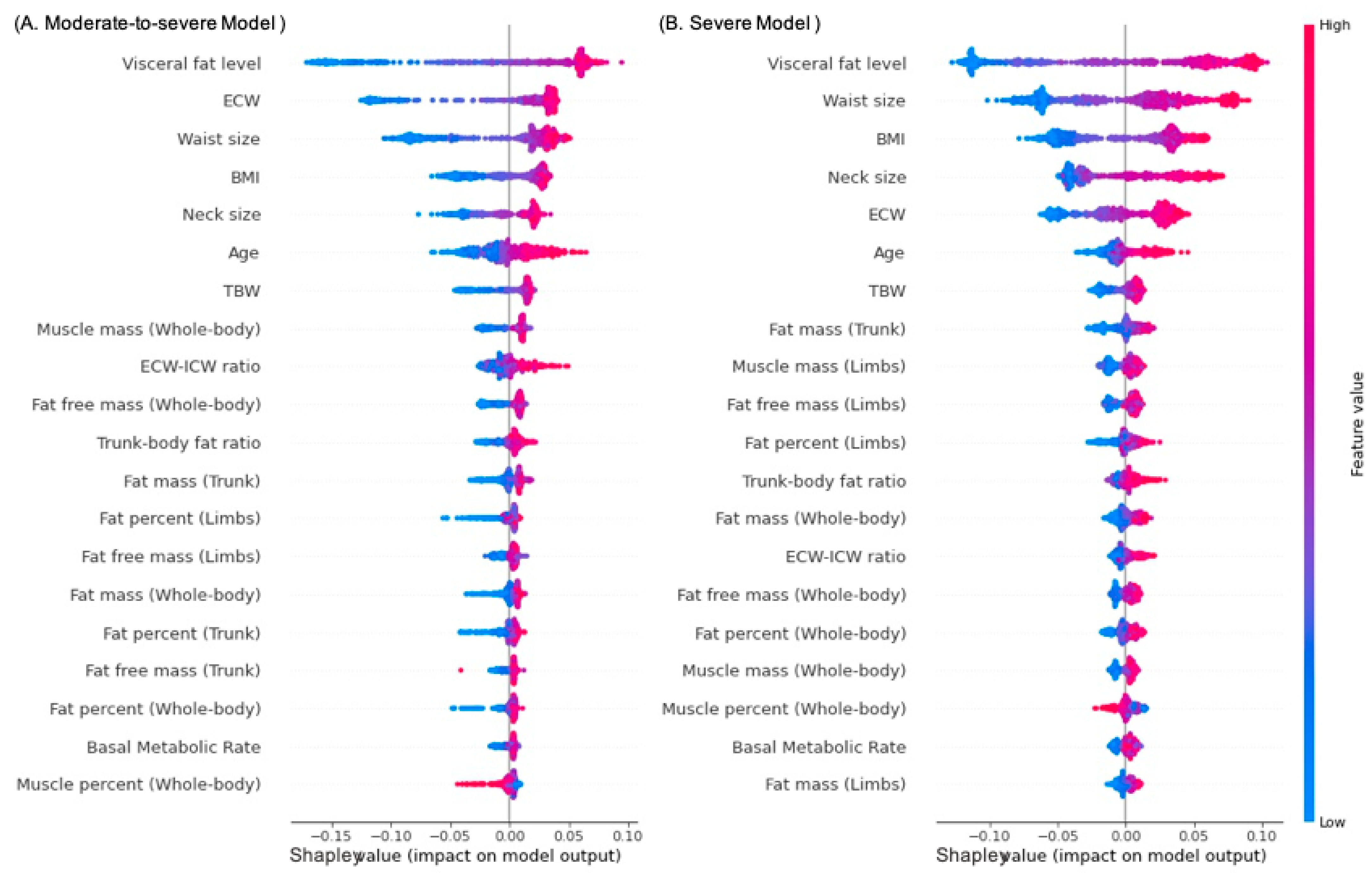
3.5. Accuracy Performance and Feature Importance
3.6. Supplementary
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maspero, C.; Giannini, L.; Galbiati, G.; Rosso, G.; Farronato, G. Obstructive sleep apnea syndrome: A literature review. Minerva Stomatol. 2015, 64, 97–109. [Google Scholar]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and management of obstructive sleep apnea: A review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.; Strange, C.; Bachman, D. Neurocognitive impairment in obstructive sleep apnea. Chest 2012, 141, 1601–1610. [Google Scholar] [CrossRef]
- Quan, S.; Gillin, J.C.; Littner, M.; Shepard, J. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. editorials. Sleep 1999, 22, 662–689. [Google Scholar] [CrossRef] [PubMed]
- Flemons, W.W.; Littner, M.R.; Rowley, J.A.; Gay, P.; Anderson, W.M.; Hudgel, D.W.; McEvoy, R.D.; Loube, D.I. Home diagnosis of sleep apnea: A systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest 2003, 124, 1543–1579. [Google Scholar] [CrossRef]
- Amra, B.; Rahmati, B.; Soltaninejad, F.; Feizi, A. Screening questionnaires for obstructive sleep apnea: An updated systematic review. Oman Med. J. 2018, 33, 184. [Google Scholar] [CrossRef]
- Nerfeldt, P.; Aoki, F.; Friberg, D. Polygraphy vs. polysomnography: Missing osas in symptomatic snorers—A reminder for clinicians. Sleep Breath. 2014, 18, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Gupta, S.S.; Sabharwal, N.; Meghrajani, V.; Sharma, S.; Kamholz, S.; Kupfer, Y. A comprehensive review of obstructive sleep apnea. Sleep Sci. 2021, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Ernst, G.; Bosio, M.; Salvado, A.; Dibur, E.; Nigro, C.; Borsini, E. Difference between apnea-hypopnea index (AHI) and oxygen desaturation index (ODI): Proportional increase associated with degree of obesity. Sleep Breath. 2016, 20, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.M.; Patel, M.R. Waist circumference and sleep disordered breathing. Laryngoscope 2008, 118, 339–347. [Google Scholar] [CrossRef]
- Lovin, S.; Bercea, R.; Cojocaru, C.; Rusu, G.; Mihăescu, T. Body composition in obstructive sleep apneahypopnea syndrome bio-impedance reflects the severity of sleep apnea. Multidiscip. Respir. Med. 2010, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Öğretmenoğlu, O.; Süslü, A.E.; Yücel, Ö.T.; Önerci, T.M.; Şahin, A. Body fat composition: A predictive factor for obstructive sleep apnea. Laryngoscope 2005, 115, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Hnin, K.; Mukherjee, S.; Antic, N.A.; Catcheside, P.; Chai-Coetzer, C.L.; McEvoy, D.; Vakulin, A. The impact of ethnicity on the prevalence and severity of obstructive sleep apnea. Sleep Med. Rev. 2018, 41, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM scoring manual updates for 2017 (version 2.4); American Academy of Sleep Medicine: Darien, IL, USA, 2017; Volume 13, pp. 665–666. [Google Scholar]
- Abad, V.C.; Guilleminault, C. Treatment options for obstructive sleep apnea. Curr. Treat. Options Neurol. 2009, 11, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Pontes, F.J.; Amorim, G.; Balestrassi, P.P.; Paiva, A.; Ferreira, J.R. Design of experiments and focused grid search for neural network parameter optimization. Neurocomputing 2016, 186, 22–34. [Google Scholar] [CrossRef]
- Sundararajan, M.; Najmi, A. In The many Shapley values for model explanation. In Proceedings of the International Conference on Machine Learning, Virtual, 13–18 July 2020. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Zhou, J.; Li, E.; Wei, H.; Li, C.; Qiao, Q.; Armaghani, D.J. Random forests and cubist algorithms for predicting shear strengths of rockfill materials. Appl. Sci. 2019, 9, 1621. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Kuan, Y.-C.; Hsu, W.-H.; Lin, Y.-T.; Hsu, C.-R.; Lo, K.; Hsu, W.-H.; Majumdar, A.; Liu, Y.-S.; Hsu, S.-M. Differentiation model for insomnia disorder and the respiratory arousal threshold phenotype in obstructive sleep apnea in the taiwanese population based on oximetry and anthropometric features. Diagnostics 2021, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Dasariraju, S.; Huo, M.; McCalla, S. Detection and classification of immature leukocytes for diagnosis of acute myeloid leukemia using random forest algorithm. Bioengineering 2020, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Böhning, N.; Schultheiss, B.; Eilers, S.; Penzel, T.; Böhning, W.; Schmittendorf, E. Comparability of pulse oximeters used in sleep medicine for the screening of OSA. Physiol. Meas. 2010, 31, 875. [Google Scholar] [CrossRef]
- Pretto, J.J.; Roebuck, T.; Beckert, L.; Hamilton, G. Clinical use of pulse oximetry: Official guidelines from the Thoracic Society of Australia and New Zealand. Respirology 2014, 19, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Baty, F.; Boesch, M.; Widmer, S.; Annaheim, S.; Fontana, P.; Camenzind, M.; Rossi, R.M.; Schoch, O.D.; Brutsche, M.H. Classification of sleep apnea severity by electrocardiogram monitoring using a novel wearable device. Sensors 2020, 20, 286. [Google Scholar] [CrossRef]
- Hanif, U.; Leary, E.B.; Schneider, L.D.; Paulsen, R.R.; Morse, A.M.; Blackman, A.; Schweitzer, P.K.; Kushida, C.A.; Liu, S.Y.; Jennum, P. Estimation of apnea-hypopnea index using deep learning on 3-D craniofacial scans. IEEE J. Biomed. Health Inform. 2021, 25, 4185–4194. [Google Scholar] [CrossRef]
- Kuan, Y.-C.; Hong, C.-T.; Chen, P.-C.; Liu, W.-T.; Chung, C.-C. Logistic regression and artificial neural network-based simple predicting models for obstructive sleep apnea by age, sex, and body mass index. Math. Biosci. Eng. 2022, 19, 11409–11421. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Liu, W.-T.; Lin, Y.-T.; Lin, S.-Y.; Houghton, R.; Hsu, W.-H.; Wu, D.; Lee, H.-C.; Wu, C.-J.; Li, L.Y.J. Machine learning approaches for screening the risk of obstructive sleep apnea in the Taiwan population based on body profile. Inform. Health Soc. Care 2021, 1–16. [Google Scholar] [CrossRef]
- Carter, R., III; Watenpaugh, D.E. Obesity and obstructive sleep apnea: Or is it OSA and obesity? Pathophysiology 2008, 15, 71–77. [Google Scholar] [CrossRef]
- Mitra, A.K.; Bhuiyan, A.R.; Jones, E.A. Association and Risk Factors for Obstructive Sleep Apnea and Cardiovascular Diseases: A Systematic Review. Diseases 2021, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Roux, F. The relationship of obesity and obstructive sleep apnea. Clin. Chest Med. 2009, 30, 455–465. [Google Scholar] [CrossRef]
- Wolk, R.; Shamsuzzaman, A.S.; Somers, V.K. Obesity, sleep apnea, and hypertension. Hypertension 2003, 42, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Topîrceanu, A.; Udrescu, L.; Udrescu, M.; Mihaicuta, S. Gender phenotyping of patients with obstructive sleep apnea syndrome using a network science approach. J. Clin. Med. 2020, 9, 4025. [Google Scholar] [CrossRef]
- Ryan, S. Adipose tissue inflammation by intermittent hypoxia: Mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J. Physiol. 2017, 595, 2423–2430. [Google Scholar] [CrossRef]
- Krüchten, R.V.; Rospleszcz, S.; Lorbeer, R.; Hasic, D.; Peters, A.; Bamberg, F.; Schulz, H.; Karrasch, S.; Schlett, C.L. Whole-Body MRI-Derived Adipose Tissue Characterization and Relationship to Pulmonary Function Impairment. Tomography 2022, 8, 46. [Google Scholar] [CrossRef]
- Santiago-Fernández, C.; Martín-Reyes, F.; Tome, M.; Gutierrez-Repiso, C.; Fernandez-Garcia, D.; Ocaña-Wilhelmi, L.; Rivas-Becerra, J.; Tatzber, F.; Pursch, E.; Tinahones, F.J. Oxidized LDL Increase the Proinflammatory Profile of Human Visceral Adipocytes Produced by Hypoxia. Biomedicines 2021, 9, 1715. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Bradley, T.; Logan, A. Pathogenesis of obstructive sleep apnoea in hypertensive patients: Role of fluid retention and nocturnal rostral fluid shift. J. Hum. Hypertens. 2015, 29, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Lanis, A.; Kerns, E.; Hu, S.L.; Bublitz, M.H.; Risica, P.; Martin, S.; Parker, J.; Millman, R.; Dworkin, L.D.; Bourjeily, G. Residual renal function affects severity of sleep apnea in peritoneal dialysis: A pilot study. Lung 2018, 196, 425–431. [Google Scholar] [CrossRef]
- Kang, S.C.; Park, K.S.; Chang, T.I.; Shin, S.K.; Kang, E.W. Sleep Apnea is Associated with Residual Kidney Function and Mortality in Patients with Peritoneal Dialysis: Prospective Cohort Study. Semin. Dial. 2022, 35, 146–153. [Google Scholar] [CrossRef]
- Kosacka, M.; Korzeniewska, A.; Jankowska, R. The evaluation of body composition, adiponectin, C-reactive protein and cholesterol levels in patients with obstructive sleep apnea syndrome. Adv. Clin. Exp. Med. 2013, 22, 817–824. [Google Scholar] [PubMed]
- Costa, E. Sousa, R.A.; dos Santos Gil, N.A. Craniofacial skeletal architecture and obstructive sleep apnoea syndrome severity. J. Cranio-Maxillofac. Surg. 2013, 41, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Raneri, J.; Hanly, P. Staging sleep in polysomnograms: Analysis of inter-scorer variability. J. Clin. Sleep Med. 2016, 12, 885–894. [Google Scholar] [CrossRef]
- Ding, L.; Chen, B.; Dai, Y.; Li, Y. A meta-analysis of the first-night effect in healthy individuals for the full age spectrum. Sleep Med. 2022, 89, 159–165. [Google Scholar] [CrossRef]
- Newell, J.; Mairesse, O.; Verbanck, P.; Neu, D. Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples. Psychiatry Res. 2012, 200, 795–801. [Google Scholar] [CrossRef]
- Duan, X.; Huang, J.; Zheng, M.; Zhao, W.; Lao, L.; Li, H.; Wang, Z.; Lu, J.; Chen, W.; Deng, H. Association of healthy lifestyle with risk of obstructive sleep apnea: A cross-sectional study. BMC Pulm. Med. 2022, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; McNicholas, W.T. Bidirectional relationships of comorbidity with obstructive sleep apnoea. Eur. Respir. Rev. 2022, 31. [Google Scholar] [CrossRef]
- Perger, E.; Mattaliano, P.; Lombardi, C. Menopause and sleep apnea. Maturitas 2019, 124, 35–38. [Google Scholar] [CrossRef]
| Categorical Variables | N-M Group (N = 951) | M Group (N = 969) | S Group (N = 1583) | Post Hoc |
|---|---|---|---|---|
| Age (years) a | 43.19 ± 13.85 | 49.48 ± 13.26 | 49.85 ± 13.04 | N-M < M ** & S ** |
| Sex (male to female ratio) b | 0.57 (348/603) | 2.32 (677/292) | 4.57 (1299/284) | N-M < M ** & S **; M < S ** |
| BMI (kg/m2) c | 23.01 ± 3.33 | 26.55 ± 4.02 | 29.44 ± 4.83 | N-M < M ** & S **; M < S ** |
| Neck size (cm) a | 33.96 ± 3.12 | 37.36 ± 3.34 | 39.85 ± 3.48 | N-M < M ** & S **; M < S ** |
| Waist size (cm) c | 80.56 ± 9.49 | 91.35 ± 9.91 | 99.21 ± 11.03 | N-M < M ** & S **; M < S ** |
| Body composition data | ||||
| Whole body | ||||
| Fat mass (kg) c | 16.58 ± 6.87 | 21.14 ± 8.72 | 25.87 ± 10.94 | N-M < M ** & S **; M < S ** |
| Muscle mass (kg) c | 42.26 ± 8.69 | 50.13 ± 9.54 | 54.68 ± 9.17 | N-M < M ** & S **; M < S ** |
| Visceral fat level c | 6.77 ± 3.35 | 11.83 ± 3.76 | 14.89 ± 3.96 | N-M < M ** & S **; M < S ** |
| Bone mass (kg) c | 2.47 ± 0.44 | 2.84 ± 0.44 | 3.05 ± 0.42 | N-M < M ** & S **; M < S ** |
| Fat-free mass (kg) c | 44.73 ± 9.1 | 52.97 ± 9.96 | 57.74 ± 9.57 | N-M < M ** & S **; M < S ** |
| Fat percentage (%) a | 26.82 ± 8.63 | 28.13 ± 8.79 | 30.22 ± 8.79 | N-M < S **; M < S ** |
| Muscle percentage (%) a | 69.15 ± 8.32 | 68.02 ± 8.48 | 66.08 ± 8.45 | N-M > S **; M > S ** |
| Basal metabolic rate (kJ) c | 5415.96 ± 1009.17 | 6320.99 ± 1138.15 | 6919.51 ± 1144.22 | N-M < M ** & S **; M < S ** |
| Physique rating c | 36.07 ± 9.89 | 29.5 ± 9.36 | 25.44 ± 8.7 | N-M > M ** & S **; M > S ** |
| Limbs | ||||
| Fat mass (kg) c | 7.46 ± 2.91 | 8.95 ± 3.81 | 10.97 ± 5.13 | N-M < M ** & S **; M < S ** |
| Fat-free mass (kg) c | 20.75 ± 4.96 | 25.1 ± 5.83 | 28.11 ± 5.92 | N-M < M ** & S **; M < S ** |
| Muscle mass (kg) c | 19.54 ± 4.67 | 23.65 ± 5.52 | 26.5 ± 5.63 | N-M < M ** & S **; M < S ** |
| Fat percentage (%) c | 26.58 ± 8.49 | 26.13 ± 8.52 | 27.42 ± 8.16 | N-M * & M ** < S |
| Trunk | ||||
| Fat mass (kg) c | 9.12 ± 4.07 | 12.19 ± 5.01 | 14.9 ± 6.04 | N-M < M ** & S **; M < S ** |
| Fat-free mass (kg) c | 23.98 ± 4.44 | 27.87 ± 4.5 | 29.62 ± 4.42 | N-M < M ** & S **; M < S ** |
| Muscle mass (kg) c | 22.72 ± 4.29 | 26.48 ± 4.38 | 28.18 ± 4.28 | N-M < M ** & S **; M < S ** |
| Fat percentage (%) a | 27.01 ± 9.09 | 29.85 ± 9.27 | 32.72 ± 9.63 | N-M < M ** & S **; M < S ** |
| Trunk to whole-body fat ratio (%) c | 54.24 ± 5.11 | 57.61 ± 3.36 | 57.85 ± 3.21 | N-M < M ** & S ** |
| Body water | ||||
| TBW (kg) a | 31.33 ± 6.09 | 36.84 ± 6.39 | 40.18 ± 6.37 | N-M < M ** & S **; M < S ** |
| ECW (kg) c | 12.89 ± 2.06 | 15.22 ± 1.93 | 16.5 ± 1.89 | N-M < M ** & S **; M < S ** |
| ICW (kg) c | 18.44 ± 4.19 | 21.62 ± 4.61 | 23.68 ± 4.63 | N-M < M ** & S **; M < S ** |
| Body water percentage (%) c | 51.25 ± 5.41 | 50.03 ± 5.35 | 48.59 ± 5.76 | N-M > M ** & S **; M > S ** |
| ECW to ICW ratio (%) c | 71.14 ± 7.88 | 71.98 ± 8.66 | 71.03 ± 8.14 | M > S * |
| Categorical Variables | N-M Group (N = 951) | M Group (N = 969) | S Group (N = 1583) | Post Hoc |
|---|---|---|---|---|
| Sleep efficiency (%) a | 75.27 ± 16.16 | 74.64 ± 16.5 | 72.5 ± 16.99 | N-M > S **; M > S ** |
| Mean SpO2 (%) b | 96.35 ± 1.44 | 95.28 ± 1.51 | 93.46 ± 2.58 | N-M > M ** & S **; M > S ** |
| Minimum SpO2 (%) b | 90.06 ± 4.7 | 84.48 ± 6.04 | 77.14 ± 8.64 | N-M > M ** & S **; M > S ** |
| WASO (min) b | 54.1 ± 46.93 | 62.91 ± 50.0 | 73.98 ± 53.48 | N-M < M ** & S **; M < S ** |
| Total sleep time (min) b | 276.4 ± 60.66 | 273.7 ± 60.72 | 264.96 ± 62.5 | N-M > S **; M > S ** |
| Sleep stage (% of SPT) | ||||
| Wake b | 16.64 ± 14.7 | 18.96 ± 15.42 | 22.06 ± 16.18 | N-M < M ** & S **; M < S ** |
| NREM b | 71.26 ± 12.59 | 69.33 ± 12.87 | 67.83 ± 13.84 | N-M > M ** & S **; M > S * |
| REM a | 12.03 ± 6.9 | 11.68 ± 6.47 | 10.1 ± 6.3 | N-M > S **; M > S ** |
| Sleep quality index (events/h) | ||||
| AHI b | 7.81 ± 4.3 | 21.68 ± 4.24 | 56.5 ± 21.18 | N-M < M ** & S **; M < S ** |
| ODI b | 4.02 ± 3.65 | 14.81 ± 6.46 | 48.84 ± 23.44 | N-M < M ** & S **; M < S ** |
| Snoring index b | 99.73 ± 158.35 | 216.41 ± 214.94 | 315.69 ± 220.36 | N-M < M ** & S **; M < S ** |
| Arousal index b | 13.81 ± 7.79 | 18.27 ± 9.28 | 32.67 ± 18.13 | N-M < M ** & S **; M < S ** |
| Categorical Variable | Sleep Quality Index (events/h) | |||
|---|---|---|---|---|
| AHI | ODI | Snoring Index | Arousal Index | |
| Age (years) | 0.11 ** | 0.08 ** | 0.06 ** | 0.09 ** |
| Sex (male/female) | 0.33 ** | 0.30 ** | 0.17 ** | 0.21 ** |
| BMI (kg/m2) | 0.57 ** | 0.59 ** | 0.4 ** | 0.3 ** |
| Neck size (cm) | 0.59 ** | 0.58 ** | 0.38 ** | 0.36 ** |
| Waist size (cm) | 0.61 ** | 0.61 ** | 0.41 ** | 0.35 ** |
| Body composition | ||||
| Whole body | ||||
| Fat mass (kg) | 0.45 ** | 0.47 ** | 0.32 ** | 0.23 ** |
| Muscle mass (kg) | 0.46 ** | 0.45 ** | 0.29 ** | 0.28 ** |
| Visceral fat level | 0.64 ** | 0.62 ** | 0.41 ** | 0.36 ** |
| Bone mass (kg) | 0.47 ** | 0.47 ** | 0.31 ** | 0.28 ** |
| Fat-free mass (kg) | 0.47 ** | 0.45 ** | 0.29 ** | 0.28 ** |
| Fat percentage (%) | 0.22 ** | 0.24 ** | 0.18 ** | 0.08 ** |
| Muscle percentage (%) | −0.21 ** | −0.23 ** | −0.17 ** | −0.08 ** |
| Basal metabolic rate (kJ) | 0.49 ** | 0.49 ** | 0.31 ** | 0.28 ** |
| Physique rating | −0.41 ** | −0.42 ** | −0.3 ** | −0.2 ** |
| Limbs | ||||
| Fat mass (kg) | 0.42 ** | 0.44 ** | 0.3 ** | 0.22 ** |
| Fat-free mass (kg) | 0.48 ** | 0.48 ** | 0.3 ** | 0.28 ** |
| Muscle mass (kg) | 0.48 ** | 0.48 ** | 0.3 ** | 0.28 ** |
| Fat percentage (%) | 0.11 ** | 0.14 ** | 0.12 ** | 0.03 |
| Trunk | ||||
| Fat mass (kg) | 0.46 ** | 0.47 ** | 0.32 ** | 0.23 ** |
| Fat-free mass (kg) | 0.4 ** | 0.38 ** | 0.24 ** | 0.25 ** |
| Muscle mass (kg) | 0.4 ** | 0.38 ** | 0.24 ** | 0.25 ** |
| Fat percentage (%) | 0.29 ** | 0.31 ** | 0.22 ** | 0.13 ** |
| Trunk to body fat ratio (%) | 0.22 ** | 0.19 ** | 0.13 ** | 0.12 ** |
| Body water | ||||
| TBW (kg) | 0.49 ** | 0.48 ** | 0.32 ** | 0.29 ** |
| ECW (kg) | 0.58 ** | 0.56 ** | 0.38 ** | 0.34 ** |
| ICW (kg) | 0.43 ** | 0.43 ** | 0.28 ** | 0.26 ** |
| Body water percent (%) | −0.24 ** | −0.26 ** | −0.17 ** | −0.1 ** |
| ECW to ICW ratio (%) | −0.05 * | −0.05 ** | −0.03 * | −0.04 * |
| Categorical Variables | LR | kNN | NB | SVM | RF | XGBoost |
|---|---|---|---|---|---|---|
| Moderate to severe OSA model | AHI ≥ 15 (N = 2039)/AHI < 15 (N = 763) | |||||
| Precision | 91.4 ± 2.22 | 86.46 ± 1.77 | 90.3 ± 2.84 | 83.1 ± 0.79 | 86.72 ± 1.93 | 86.75 ± 1.01 |
| Recall | 82.44 ± 2.91 | 90.24 ± 1.93 | 78.47 ± 2.96 | 97.11 ± 1.45 | 94.07 ± 1.99 | 91.96 ± 2.71 |
| Accuracy | 81.55 ± 2.77 | 82.59 ± 2.05 | 78.16 ± 3.29 | 83.51 ± 0.61 | 85.19 ± 2.86 | 83.94 ± 2.52 |
| F1 score | 86.66 ± 2.07 | 88.29 ± 1.38 | 83.94 ± 2.44 | 89.55 ± 0.43 | 90.24 ± 1.88 | 89.27 ± 1.78 |
| AUC | 90.03 ± 2.67 | 83.43 ± 3.64 | 87.41 ± 3.27 | 89.86 ± 2.02 | 90.41 ± 2.44 | 88.64 ± 1.74 |
| Severe OSA model | AHI ≥ 30 (N = 1268)/AHI < 30 (N = 1534) | |||||
| Precision | 68.29 ± 1.22 | 67.08 ± 0.84 | 68.9 ± 1.07 | 71.32 ± 0.16 | 70.67 ± 2.86 | 70.3 ± 0.7 |
| Recall | 73.66 ± 1.26 | 68.45 ± 1.91 | 72.16 ± 1.67 | 70.58 ± 0.24 | 76.57 ± 3.39 | 73.34 ± 0.83 |
| Accuracy | 72.59 ± 0.99 | 70.52 ± 0.88 | 72.66 ± 1.04 | 73.84 ± 0.04 | 75.95 ± 2.24 | 73.91 ± 0.48 |
| F1 score | 70.86 ± 1.02 | 67.75 ± 1.19 | 70.49 ± 1.25 | 70.95 ± 0.04 | 73.44 ± 2.28 | 71.79 ± 0.46 |
| AUC | 80.87 ± 0.36 | 75.97 ± 0.64 | 80.8 ± 0.5 | 83.13 ± 0.17 | 83.24 ± 1.69 | 81.59 ± 0.71 |
| Categorical Variables | Moderate-to-Severe OSA Model | Severe OSA Model |
|---|---|---|
| AHI ≥ 15 (N = 513); AHI < 15 (N = 188) | AHI ≥30 (N = 315); AHI < 30 (N = 386) | |
| Precision % | 85.74 | 67.72 |
| Recall % | 94.93 | 74.6 |
| Accuracy % | 84.74 | 72.61 |
| F1 Score % | 90.1 | 71.0 |
| AUC, % (95% CI) | 89.58 (87.44–92.01) | 80.07 (77.05–82.80) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-Y.; Huang, H.-T.; Cheng, H.-C.; Wang, J.; Duh, P.-J.; Hsu, W.-H.; Stettler, M.; Kuan, Y.-C.; Lin, Y.-T.; Hsu, C.-R.; et al. Screening for Obstructive Sleep Apnea Risk by Using Machine Learning Approaches and Anthropometric Features. Sensors 2022, 22, 8630. https://doi.org/10.3390/s22228630
Tsai C-Y, Huang H-T, Cheng H-C, Wang J, Duh P-J, Hsu W-H, Stettler M, Kuan Y-C, Lin Y-T, Hsu C-R, et al. Screening for Obstructive Sleep Apnea Risk by Using Machine Learning Approaches and Anthropometric Features. Sensors. 2022; 22(22):8630. https://doi.org/10.3390/s22228630
Chicago/Turabian StyleTsai, Cheng-Yu, Huei-Tyng Huang, Hsueh-Chien Cheng, Jieni Wang, Ping-Jung Duh, Wen-Hua Hsu, Marc Stettler, Yi-Chun Kuan, Yin-Tzu Lin, Chia-Rung Hsu, and et al. 2022. "Screening for Obstructive Sleep Apnea Risk by Using Machine Learning Approaches and Anthropometric Features" Sensors 22, no. 22: 8630. https://doi.org/10.3390/s22228630
APA StyleTsai, C.-Y., Huang, H.-T., Cheng, H.-C., Wang, J., Duh, P.-J., Hsu, W.-H., Stettler, M., Kuan, Y.-C., Lin, Y.-T., Hsu, C.-R., Lee, K.-Y., Kang, J.-H., Wu, D., Lee, H.-C., Wu, C.-J., Majumdar, A., & Liu, W.-T. (2022). Screening for Obstructive Sleep Apnea Risk by Using Machine Learning Approaches and Anthropometric Features. Sensors, 22(22), 8630. https://doi.org/10.3390/s22228630









