Electrogastrogram-Derived Features for Automated Sickness Detection in Driving Simulator
Abstract
:1. Introduction
1.1. Rationale for Introduction of New EGG-Based Parameters
1.2. Noise Effect on EGG-Based Parameters
1.3. Aims of the Study
- We present an extended list of EGG-based features for nausea assessment following pertinent reasoning for their calculation.
- We report on the sensitivity/robustness of the proposed EGG-based parameters to different levels of SNRs and the noise effect on nausea detection.
2. Materials and Methods
2.1. Available EGG Data and Recording Procedure
- Baseline measurement before the driving simulation.
- Driving simulation in autonomous vehicle.
- EGG measurement while resting after driving simulation.

2.2. EGG Preprocessing and Creation of Semi-Synthetic EGG Dataset
2.3. Automated Procedure for EGG-Based Features Extraction
2.4. Statistical Analysis and Machine Learning Approach
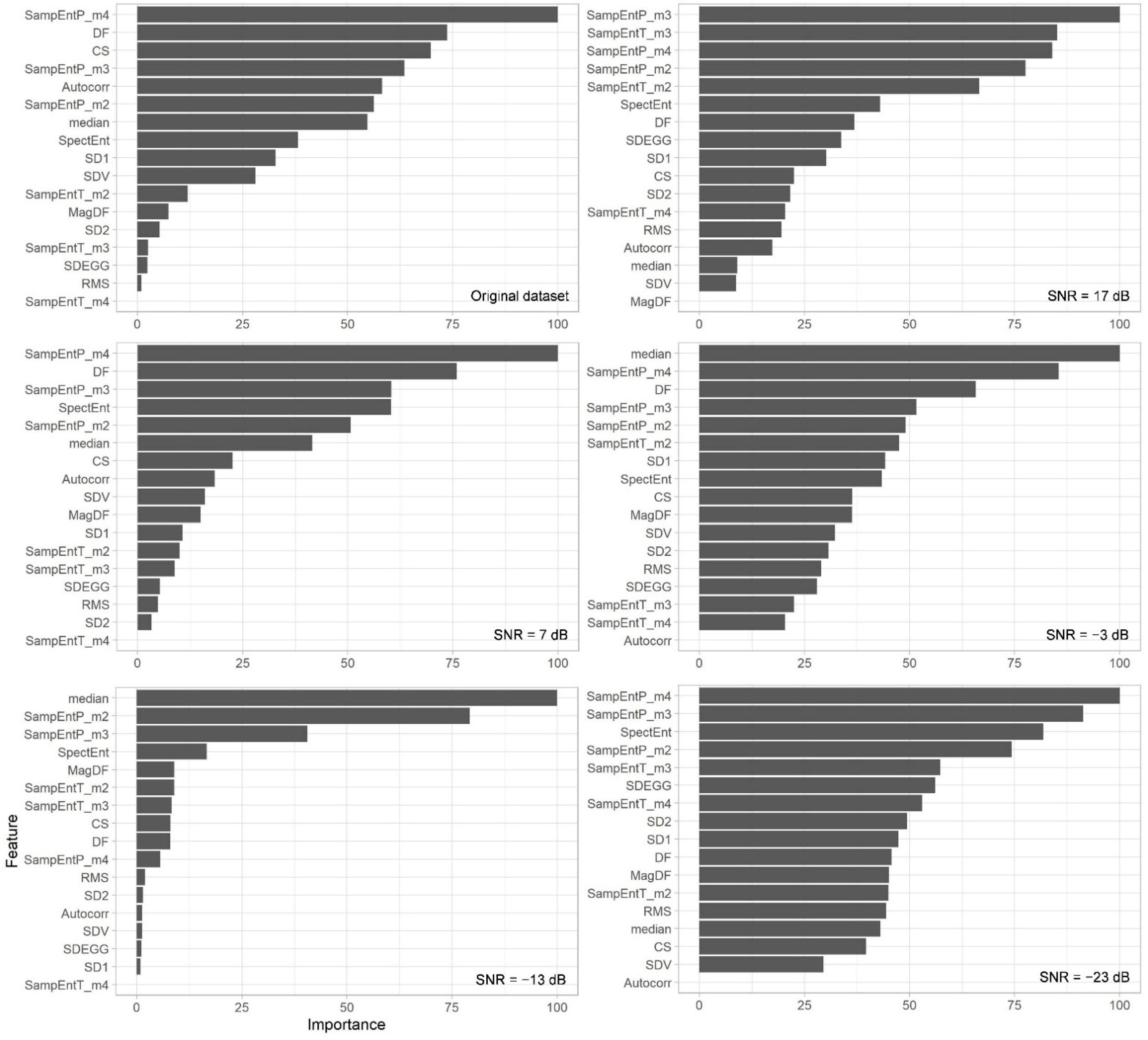
3. Results
4. Discussion
4.1. Effect of Noise on EGG-Based Parameters
4.2. Effect of Noise on Random Forest Classifier for Nausea Detection
4.3. Effect of Noise on Detection of Nausea through Statistical Tests
4.4. Limitations of the Study
- We use a discrete set of predefined SNRs, and one should note that the actual SNRs were much higher, as our data were already contaminated with noises and artifacts. Despite the linear Butterworth filtering applied in the preprocessing stage, the noise with overlapping frequency content probably remains present in the semi-synthetic EGG dataset. Future efforts towards the generation of synthetic noises would provide a firm basis for exact SNR contamination and more reliable analysis.
- It should also be noted that sample entropy scaling parameter r is kept constant at the 0.15 of the noiseless data standard deviation. This value was determined empirically based on the recommendations [24]. Adjusting this value for different SNRs may have a further effect on the results and should be investigated in the future.
- We apply procedures for automatic feature calculation. However, a guided visual observation and manual corrections are still considered a gold standard for the evaluation of EGG-based parameters especially in cases of excessive noises [10,70,71]. We use visual inspection only for channel selection. Despite this drawback, we obtained promising results in nausea assessment by both statistical and ML approaches.
- We select the embedded dimension m for sample entropy calculation empirically. For future selection and discussion on embedding dimension selection, one may look at outstanding reasoning by Matilla-García et al. [72].
- We did not apply unimodal or multi-modal machine learning algorithms, and we do not provide comparison of existing machine learning techniques as in [67].
- Our method is applied only for nausea occurrence. Further customization of presented EGG-based parameters and complementary approach by RF and statistical analysis should yield at assessment of sickness levels similarly as in [67].
- The dataset used for the analysis contains more male than female participants. However, we do not consider this to be a major drawback of our study, as we were not interested in the differences between the genders but focused on the relationships between the occurrence of nausea, the EGG parameters, and noise. Moreover, a systematic review performed by Grassini and Laumann [73] showed conflicting results in published studies focused on determining sex differences in experiencing simulator sickness.
- We did not use multi biomarkers for the assessment of sickness occurrence as our focus was solely on the direct assessment of gastric activity. However, future studies should be focused on a promising heterogeneous approach as, for example, suggested by Dennison et al. [67].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, J.O.; Goodenough, R.R.; Crisler, M.C.; Klein, N.D.; Alley, R.L.; Koon, B.L.; Logan, W.C., Jr.; Ogle, J.H.; Tyrrell, R.A.; Wills, R.F. Simulator sickness during driving simulation studies. Accid. Anal. Prev. 2010, 42, 788–796. [Google Scholar] [CrossRef]
- Lucas, G.; Kemeny, A.; Paillot, D.; Colombet, F. A simulation sickness study on a driving simulator equipped with a vibration platform. Transp. Res. Part F Traffic Psychol. Behav. 2020, 68, 15–22. [Google Scholar]
- Wang, J.; Liang, H.N.; Monteiro, D.; Xu, W.; Xiao, J. Real-time Prediction of Simulator Sickness in Virtual Reality Games. IEEE Trans. Games 2022. [Google Scholar] [CrossRef]
- Gruden, T.; Popović, N.B.; Stojmenova, K.; Jakus, G.; Miljković, N.; Tomažič, S.; Sodnik, J. Electrogastrography in Autonomous Vehicles—An Objective Method for Assessment of Motion Sickness in Simulated Driving Environments. Sensors 2021, 21, 550. [Google Scholar] [CrossRef]
- Classen, S.; Bewernitz, M.; Shechtman, O. Driving simulator sickness: An evidence-based review of the literature. Am. J. Occup. Ther. 2011, 65, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Dużmańska, N.; Strojny, P.; Strojny, A. Can simulator sickness be avoided? A review on temporal aspects of simulator sickness. Front. Psychol. 2018, 9, 2132. [Google Scholar]
- Aykent, B.; Merienne, F.; Guillet, C.; Paillot, D.; Kemeny, A. Motion sickness evaluation and comparison for a static driving simulator and a dynamic driving simulator. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2014, 228, 818–829. [Google Scholar]
- Dennison, M.S.; Krum, D.M. Unifying research to address motion sickness. In Proceedings of the 2019 IEEE Conference on Virtual Reality and 3D User Interfaces (VR), Osaka, Japan, 23–27 March 2019; pp. 1858–1859. [Google Scholar]
- Smyth, J.; Birrell, S.; Woodman, R.; Jennings, P. Exploring the utility of EDA and skin temperature as individual physiological correlates of motion sickness. Appl. Ergon. 2021, 92, 103315. [Google Scholar] [CrossRef]
- Popović, N.B.; Miljković, N.; Stojmenova, K.; Jakus, G.; Prodanov, M.; Sodnik, J. Lessons Learned: Gastric Motility Assessment During Driving Simulation. Sensors 2019, 19, 3175. [Google Scholar] [CrossRef] [Green Version]
- John, B. Pupil diameter as a measure of emotion and sickness in VR. In Proceedings of the 11th ACM Symposium on Eye Tracking Research & Applications, Denver, CO, USA, 25–28 June 2019; pp. 1–3. [Google Scholar]
- Park, S.; Mun, S.; Ha, J.; Kim, L. Non-contact measurement of motion sickness using pupillary rhythms from an infrared camera. Sensors 2021, 21, 4642. [Google Scholar] [CrossRef]
- Wachler, B.S.B.; Krueger, R.R. Agreement and repeatability of pupillometry using videokeratography and infrared devices. J. Cataract Refract. Surg. 2000, 26, 35–40. [Google Scholar]
- Koohestani, A.; Nahavandi, D.; Asadi, H.; Kebria, P.M.; Khosravi, A.; Alizadehsani, R.; Nahavandi, S. A Knowledge Discovery in Motion Sickness: A Comprehensive Literature Review. IEEE Access 2019, 7, 85755–85770. [Google Scholar] [CrossRef]
- Laviola, J.J. A discussion of cybersickness in virtual environments. ACM Sigchi Bull. 2000, 32, 47–56. [Google Scholar] [CrossRef]
- Crampton, G.H. Motion and Space Sickness; CRC Press: Boca Raton, FL, USA, 1990; ISBN 978-0-8493-4703-0. [Google Scholar]
- Golding, J.F. Motion sickness. In Handbook of Clinical Neurology; Furman, J.M., Lempert, T., Eds.; Neuro-Otology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 137, pp. 371–390. [Google Scholar]
- Davis, S.; Nesbitt, K.; Nalivaiko, E. Comparing the onset of cybersickness using the Oculus Rift and two virtual roller coasters. In Proceedings of the 11th Australasian Conference on Interactive Entertainment (IE 2015), Sydney, Australia, 27–30 January 2015. [Google Scholar]
- Iskander, J.; Attia, M.; Saleh, K.; Nahavandi, D.; Abobakr, A.; Mohamed, S.; Asadi, H.; Hossny, M.; Lim, C.P.; Hossny, M. From car sickness to autonomous car sickness: A review. Transp. Res. Part F Traffic Psychol. Behav. 2019, 62, 716–726. [Google Scholar]
- Mühlbacher, D.; Tomzig, M.; Reinmueller, K.; Rittger, L. Methodological Considerations Concerning Motion Sickness Investigations during Automated Driving. Information 2020, 11, 265. [Google Scholar]
- Koch, K.L. Gastric dysrhythmias: A potential objective measure of nausea. Exp. Brain Res. 2014, 232, 2553–2561. [Google Scholar] [CrossRef]
- Wolpert, N.; Rebollo, I.; Tallon-Baudry, C. Electrogastrography for psychophysiological research: Practical considerations, analysis pipeline, and normative data in a large sample. Psychophysiology 2020, 57, e13599. [Google Scholar]
- Delgado-Bonal, A. Quantifying the randomness of the stock markets. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Delgado-Bonal, A.; Marshak, A. Approximate entropy and sample entropy: A comprehensive tutorial. Entropy 2019, 21, 541. [Google Scholar] [CrossRef] [Green Version]
- Fele-Žorž, G.; Kavšek, G.; Novak-Antolič, Ž.; Jager, F. A comparison of various linear and non-linear signal processing techniques to separate uterine EMG records of term and pre-term delivery groups. Med. Biol. Eng. Comput. 2008, 46, 911–922. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Liang, Z.; Voss, L.J.; Sleigh, J.W. Analysis of depth of anesthesia with Hilbert–Huang spectral entropy. Clin. Neurophysiol. 2008, 119, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, A.; Subramaniam, R.; Srivastava, A.; Prabhakar, H.; Kalaivani, M.; Paranjape, S. Spectral entropy monitoring for adults and children undergoing general anaesthesia. Cochrane Database Syst. Rev. 2016, 3. [Google Scholar] [CrossRef]
- Schrumpf, F.; Sturm, M.; Bausch, G.; Fuchs, M. Derivation of the respiratory rate from directly and indirectly measured respiratory signals using autocorrelation. Curr. Dir. Biomed. Eng. 2016, 2, 241–245. [Google Scholar] [CrossRef]
- Rangayyan, R.M. Filtering for removal of artifacts. In Biomedical Signal Analysis; IEEE: Piscataway, NJ, USA, 2015; pp. 91–231. [Google Scholar] [CrossRef]
- Mintchev, M.P.; Stickel, A.; Bowes, K.L. Dynamics of the level of randomness in gastric electrical activity. Dig. Dis. Sci. 1998, 43, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Palaniswami, M.; Kamen, P. Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Trans. Biomed. Eng. 2001, 48, 1342–1347. [Google Scholar]
- Yarkoni, T.; Westfall, J. Choosing prediction over explanation in psychology: Lessons from machine learning. Perspect. Psychol. Sci. 2017, 12, 1100–1122. [Google Scholar] [CrossRef]
- Yin, J.; Chen, J.D. Electrogastrography: Methodology, validation and applications. J. Neurogastroenterol. Motil. 2013, 19, 5. [Google Scholar]
- Nervtech Simuation Technologies. Available online: https://www.nervtech.com (accessed on 20 September 2022).
- Vengust, M.; Možina, D.; Pušenjak, N.; Zevnik, L.; Sodnik, J.; Kaluža, B.; Tavčar, A. NERVteh 4DOF motion car driving simulator. In Proceedings of the 6th International Conference on Automotive User Interfaces and Interactive Vehicular Applications, Seattle, WA, USA, 17–19 September 2014; pp. 1–6. [Google Scholar]
- Fanatec ClubSport Pedals V3. Available online: https://fanatec.com/eu-en/pedals/clubsport-pedals-v3 (accessed on 20 September 2022).
- Fanatec ClubSport Wheel Base V2.5. Available online: https://fanatec.com/eu-en/racing-wheels-wheel-bases/wheel-bases/clubsport-wheel-base-v2.5 (accessed on 20 September 2022).
- AV Simulation SCANeR Studio. Available online: https://www.avsimulation.com/scanerstudio/ (accessed on 20 September 2022).
- Oculus Rift Oculus. Available online: https://www.oculus.com/rift/ (accessed on 20 September 2022).
- Kinkade, R.G.; Wheaton, G.R. Training device design. In Human Engineering Guide to Equipment Design; Van Cott, H.P., Kinkade, R.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1972; pp. 668–699. [Google Scholar]
- Hays, R.T. Simulator Fidelity: A Concept Paper; Army Research Inst for the Behavioral and Social Sciences: Alexandria, VA, USA, 1980. [Google Scholar]
- SAE Levels of Driving Automation™ Refined for Clarity and International Audience. Available online: https://www.sae.org/blog/sae-j3016-update (accessed on 20 September 2022).
- Riezzo, G.; Russo, F.; Indrio, F. Electrogastrography in adults and children: The strength, pitfalls, and clinical significance of the cutaneous recording of the gastric electrical activity. BioMed Res. Int. 2013, 2013, 282757. [Google Scholar] [CrossRef]
- Popović, N.; Miljković, N.; Popović, M.B. Simple gastric motility assessment method with a single-channel electrogastrogram. Biomed. Tech. Eng. 2018, 64, 177–185. [Google Scholar] [CrossRef]
- Universal Interface Module UIM100C. Available online: https://www.biopac.com/product/universal-interface-module/ (accessed on 20 September 2022).
- Jovanović, N.; Popović, N.B.; Miljković, N. Combined approach for automatic and robust calculation of dominant frequency of electrogastrogram. arXiv 2020, arXiv:2009.09023. [Google Scholar]
- Chang, F.Y. Electrogastrography: Basic knowledge, recording, processing and its clinical applications. J. Gastroenterol. Hepatol. 2005, 20, 502–516. [Google Scholar] [PubMed]
- Koch, K.L.; Stern, R.M. Handbook of Electrogastrography; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boljanić, T.; Miljković, N.; Lazarević, L.B.; Knežević, G.; Milašinović, G. Relationship between electrocardiogram-based features and personality traits: Machine learning approach. Ann. Noninvasive Electrocardiol. 2022, 27, e12919. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C.R. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. 2021. Available online: https://www.r-project.org (accessed on 20 September 2022).
- Wickham, H.; Francois, R.; Henry, L.; Müller, K.; Dplyr, A. Grammar of Data Manipulation. R Found. Stat. Comput., Vienna. R Package Version 0.4.3. 2015. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 20 September 2022).
- Meyer, D.; Dimitriadou, E.; Hornik, K. Misc Functions of the Department of Statistics, Probability Theory Group (e1071), TU Wien. R Package Version 1–7. 2019. Available online: https://CRAN.R-project.org/package=e1071 (accessed on 20 September 2022).
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; Team, R.C. Package ‘Caret.’. R J. 2020, 223, 7. [Google Scholar]
- Paluszynska, A.; Biecek, P.; Jiang, Y.; Jiang, M.Y. Package ‘randomForestExplainer’. Explaining and visualizing random forests in terms of variable importance. 2020. Available online: https://CRAN.R-project.org/package=randomForestExplainer (accessed on 20 September 2022).
- Hadley, W. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Torchiano, M. Effsize: Efficient Effect Size Computation. R Package Version 0.8.1. 2020. Available online: https://CRAN.R-project.org/package=effsize (accessed on 20 September 2022). [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Jakus, G.; Sodnik, J.; Miljković, N. NadicaSm/Statistical-Analysis-and-Machine-Learning-for-EGG-based-Nausea-Detection: V1 (Version v1). Version V1. Zenodo 2022. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Liaw, A.; Wiener, M. Classification and Regression by Random Forest. R News 2002, 2, 18–22. [Google Scholar]
- Schwalbe Lehtihet, O.; Åryd, V.A. Comparison of Performance and Noise Resistance of Different Machine Learning Classifiers on Gaussian Clusters; KTH Royal Institute of Technology, School of Electrical Engineering and Computer Science: Stockholm, Sweden, 2021. [Google Scholar]
- Ishii, S.; Ljunggren, D.A. Comparative Analysis of Robustness to Noise in Machine Learning Classifiers; KTH Royal Institute of Technology, School of Electrical Engineering and Computer Science: Stockholm, Sweden, 2021. [Google Scholar]
- Wong, T.T. Performance evaluation of classification algorithms by k-fold and leave-one-out cross validation. Pattern Recognit. 2015, 48, 2839–2846. [Google Scholar]
- Arlot, S.; Celisse, A. A survey of cross-validation procedures for model selection. Stat. Surv. 2010, 4, 40–79. [Google Scholar] [CrossRef]
- Verhagen, M.A.; Van Schelven, L.J.; Samsom, M.; Smout, A.J. Pitfalls in the analysis of electrogastrographic recordings. Gastroenterology 1999, 117, 453–460. [Google Scholar] [PubMed]
- Dennison, M., Jr.; D’Zmura, M.; Harrison, A.; Lee, M.; Raglin, A. Improving motion sickness severity classification through multi-modal data fusion. In Artificial Intelligence and Machine Learning for Multi-Domain Operations Applications; SPIE: Bellingham, WA, USA, 2019; Volume 11006, pp. 277–286. [Google Scholar]
- Strobl, C.; Boulesteix, A.-L.; Kneib, T.; Augustin, T.; Zeileis, A. Conditional variable importance for random forests. BMC Bioinform. 2008, 9, 307. [Google Scholar] [CrossRef]
- Toloşi, L.; Lengauer, T. Classification with correlated features: Unreliability of feature ranking and solutions. Bioinformatics 2011, 27, 1986–1994. [Google Scholar] [CrossRef] [Green Version]
- Komorowski, D. EGG DWPack: System for multi-channel electrogastrographic signals recording and analysis. J. Med. Syst. 2018, 42, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, P.; O’Grady, G.; Paskaranandavadivel, N.; Tang, S.J.; Abell, T.; Cheng, L.K. High-resolution mapping of hyperglycemia-induced gastric slow wave dysrhythmias. J. Neurogastroenterol. Motil. 2019, 25, 276. [Google Scholar] [CrossRef] [Green Version]
- Matilla-García, M.; Morales, I.; Rodríguez, J.M.; Ruiz Marín, M. Selection of embedding dimension and delay time in phase space reconstruction via symbolic dynamics. Entropy 2021, 23, 221. [Google Scholar] [CrossRef]
- Grassini, S.; Laumann, K. Are modern head-mounted displays sexist? A systematic review on gender differences in HMD-mediated virtual reality. Front. Psychol. 2020, 11, 1604. [Google Scholar] [CrossRef]

| Feature | Explanation | Unit | References |
|---|---|---|---|
| RMS (Root Mean Square) | RMS of the amplitude of EGG selected segment | μV | [4,10] |
| median | Median frequency of PSD of selected EGG segment | cpm (cycles per minute) | |
| DF (Dominant Frequency) | Dominant frequency of PSD of selected EGG segment | cpm | [4,10,48] |
| MagDF | Magnitude of DF in PSD of selected EGG segment | mV2/Hz | [4,10,48] |
| CS (Crest Factor) | CS of PSD of selected EGG segments | / | [4,10] |
| SDV (Spectral Variation Distribution) | PSD with magnitude higher than 25% of DF | % | [4] |
| SampEntT (Sample Entropy of Time Series) | Embedding dimensions m = 2, 3, and 4 | / | Introduced here and inspired by [49] |
| SampEntP (Sample Entropy of PSD) | Embedding dimensions m = 2, 3, and 4 | / | |
| SpectEnt (Spectral Entropy) | / | / | |
| Autocorr (Autocorrelation zero-crossing) | The first lag of autocorrelation function of EGG at which autocorrelation equals 0 | S | Introduced here and inspired by [46] |
| SD1 | Transverse line of the Poincaré plot in the perpendicular direction. A Poincaré plot presents a scatter plot of the current EGG sample in relation to the prior EGG sample. | μV | Introduced here and inspired by [31,50] |
| SD2 | Longitudinal line of the Poincaré plot in the perpendicular direction. | μV | |
| SDEGG | Standard deviation of EGG samples obtained from the SD1 and SD2. | μV |
| Features | SNR = 17 dB | SNR = 7 dB | SNR = −3 dB | SNR = −13 dB | SNR = −23 dB |
|---|---|---|---|---|---|
| RMS | V = 321, p < 0.001, Cdelta = 0.021 | V = 77, p < 0.001, Cdelta = 0.092 | V = 0, p < 0.001, Cdelta = 0.344 | V = 0, p < 0.001, Cdelta = 0.733 | V = 0, p < 0.001, Cdelta = 0.951 |
| median | V = 188.5, p = 0.750, Cdelta = 0.002 | t = −1.761, df = 67, p = 0.083, Cd = −0.060 | t = −2.852, df = 67, p = 0.006, Cd = −0.265 | t = −4.5567, df = 67, p < 0.001, Cd = −0.652 | V = 460, p < 0.001, Cdelta = −0.457 |
| MagDF | V = 739, p = 0.008, Cdelta = −0.007 | V = 812, p = 0.028, Cdelta = −0.031 | V = 206, p < 0.001, Cdelta = −0.254 | V = 0, p < 0.001, Cdelta = −0.653 | V = 0, p < 0.001, Cdelta = −0.919 |
| DF | V = 64, p = 0.476, Cdelta = 0.007 | V = 240, p = 0.461, Cdelta = −0.061 | V = 508.5, p = 0.730, Cdelta = −0.034 | V = 647, p = 0.009, Cdelta = −0.253 | V = 845, p = 0.045, Cdelta = −0.176 |
| CS | V = 1591, p = 0.011, Cdelta = 0.031 | V = 1688, p = 0.002, Cdelta = 0.098 | V = 1872, p < 0.001, Cdelta = 0.246 | V = 2108, p < 0.001, Cdelta = 0.533 | V = 2136, p < 0.001, Cdelta = 0.546 |
| SDV | V = 495, p = 0.342, Cdelta = −0.012 | V = 335, p < 0.001, Cdelta = −0.135 | V = 344.5, p < 0.001, Cdelta = −0.344 | V = 173.5, p < 0.001, Cdelta = −0.674 | V = 97.5, p < 0.001, Cdelta = −0.764 |
| SampEntT_m2 | V = 920, p = 0.46, Cdelta = 0.031 | V = 610, p = 0.256, Cdelta = −0.052 | V = 1213, p = 0.249, Cdelta = 0.085 | V = 1918, p < 0.001, Cdelta = 0.413 | V = 2330, p < 0.001, Cdelta = 0.754 |
| SampEntT_m3 | V = 626.5, p = 0.350, Cdelta = 0.067 | V = 444, p = 0.556, Cdelta = −0.018 | V = 787, p = 0.083, Cdelta = 0.136 | V = 1666, p < 0.001, Cdelta = 0.410 | V = 2285, p < 0.001, Cdelta = 0.682 |
| SampEntT_m4 | V = 254, p = 0.914, Cdelta = 0.039 | V = 221, p = 0.948, Cdelta = 0.018 | V = 437, p = 0.338, Cdelta = 0.101 | V = 1598, p < 0.001, Cdelta = 0.419 | V = 2209, p < 0.001, Cdelta = 0.671 |
| SampEntP_m2 | V = 1289, p = 0.480, Cdelta = 0.030 | V = 1349, p = 0.284, Cdelta = 0.040 | V = 1278, p = 0.523, Cdelta = −0.006 | V = 1373, p = 0.223, Cdelta = 0.030 | V = 1221, p = 0.772, Cdelta = 0.008 |
| SampEntP_m3 | V = 1306, p = 0.418, Cdelta = 0.015 | V = 1252, p = 0.631, Cdelta = 0.006 | V = 1275, p = 0.535, Cdelta = −0.013 | V = 1369, p = 0.232, Cdelta = 0.083 | V = 1086, p = 0.597, Cdelta = −0.042 |
| SampEntP_m4 | V = 1293, p = 0.465, Cdelta = 0.008 | V = 1344, p = 0.297, Cdelta = −0.009 | V = 1417, p = 0.137, Cdelta = 0.020 | V = 1385, p = 0.196, Cdelta = 0.047 | V = 955, p = 0.184, Cdelta = −0.154 |
| SpectEnt | V = 727, p = 0.006, Cdelta = −0.019 | V = 340, p < 0.001, Cdelta = −0.154 | V = 180, p < 0.001, Cdelta = −0.416 | V = 156, p < 0.001, Cdelta = −0.695 | V = 161, p < 0.001, Cdelta = −0.724 |
| Autocorr | V = 30, p = 0.351, Cdelta = 0.024 | V = 27, p = 0.608, Cdelta = 0.027 | V = 540, p < 0.001, Cdelta = 0.245 | V = 1066, p < 0.001, Cdelta = 0.492 | V = 1215, p < 0.001, Cdelta = 0.557 |
| SD1 | V = 205, p < 0.001, Cdelta = −0.021 | V = 19, p < 0.001, Cdelta = −0.096 | V = 0, p < 0.001, Cdelta = −0.384 | V = 0, p < 0.001, Cdelta = −0.766 | V = 0, p < 0.001, Cdelta = −0.958 |
| SD2 | V = 327, p < 0.001, Cdelta = −0.022 | V = 80, p < 0.001, Cdelta = −0.093 | V = 0, p < 0.001, Cdelta = −0.345 | V = 0, p < 0.001, Cdelta = −0.733 | V = 0, p < 0.001, Cdelta = −0.951 |
| SDEGG | V = 321, p < 0.001, Cdelta = −0.021 | V = 76, p < 0.001, Cdelta = −0.093 | V = 0, p < 0.001, Cdelta = −0.344 | V = 0, p < 0.001, Cdelta = −0.733 | V = 0, p < 0.001, Cdelta = −0.951 |
| Evaluation Classifier Metrics | Original Dataset | Noisy Data | ||||
| SNR = 17 dB | SNR = 7 dB | SNR = −3 dB | SNR = −13 dB | SNR = −23 dB | ||
| Kappa | 0.452 | 0.452 | 0.301 | 0.452 | −0.214 | −0.097 |
| 95% CI | (0.636, 0.985) | (0.636, 0.985) | (0.566, 0.962) | (0.636, 0.985) | (0.383, 0.858) | (0.501, 0.932) |
| Accuracy | 0.882 | 0.882 | 0.823 | 0.882 | 0.647 | 0.765 |
| Sensitivity | 1.000 | 1.000 | 0.929 | 1.000 | 0.786 | 0.929 |
| Specificity | 0.333 | 0.333 | 0.333 | 0.333 | 0 | 0 |
| Precision | 0.875 | 0.875 | 0.867 | 0.875 | 0.786 | 0.812 |
| Recall | 1.000 | 1.000 | 0.929 | 1.000 | 0.786 | 0.929 |
| AUC (training) | 0.616 | 0.616 | 0.616 | 0.616 | 0.616 | 0.616 |
| AUC (test) | 0.667 | 0.667 | 0.631 | 0.667 | 0.393 | 0.464 |
| Evaluation Classifier Metrics | Noisy Test Data | ||||
|---|---|---|---|---|---|
| SNR = 17 dB | SNR = 7 dB | SNR = −3 dB | SNR = −13 dB | SNR = −23 dB | |
| Kappa | 0.452 | 0.452 | 0.452 | 0 | 0 |
| 95% CI | (0.636, 0.985) | (0.636, 0.985) | (0.636, 0.985) | (0.566, 0.962) | (0.566, 0.962) |
| Accuracy | 0.882 | 0.882 | 0.882 | 0.823 | 0.823 |
| Sensitivity | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Specificity | 0.333 | 0.333 | 0.333 | 0 | 0 |
| Precision | 0.875 | 0.875 | 0.875 | 0.823 | 0.823 |
| Recall | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| AUC (training) | 0.616 | 0.616 | 0.616 | 0.616 | 0.616 |
| AUC (test) | 0.667 | 0.667 | 0.667 | 0.500 | 0.500 |
| Features | Original | SNR = 17 dB | SNR = 7 dB | SNR = −3 dB | SNR = −13 dB | SNR = −23 dB |
|---|---|---|---|---|---|---|
| RMS | W = 245, p = 0.145, Cdelta = −0.270 | W = 247, p = 0.154, Cdelta = −0.265 | W = 243, p = 0.137, Cdelta = −0.277 | W = 256, p = 0.201, Cdelta = −0.238 | W = 297, p = 0.536, Cdelta = −0.116 | W = 299, p = 0.557, Cdelta = −0.110 |
| median | t = −0.8408, df = 19.075, p = 0.411, Cd = −0.232 | W = 268, p = 0.277, Cdelta = −0.202 | t = −1.7105, df = 18.665, p = 0.104, Cd = −0.480 | t = −1.7556, df = 14.249, p = 0.101, Cd = −0.641 | t = −2.97, df = 18.279, p = 0.008, Cd = −0.846 | W = 246, p = 0.150, Cdelta = −0.268 |
| MagDF | W = 261, p = 0.231, Cdelta = −0.223 | W = 263, p = 0.243, Cdelta = −0.217 | W = 263, p = 0.243, Cdelta = −0.217 | W = 263, p = 0.243, Cdelta = −0.217 | W = 301, p = 0.579, Cdelta = −0.104 | W = 317, p = 0.766, Cdelta = −0.056 |
| DF | W = 319.5, p = 0.796, Cdelta = −0.049 | W = 293.5, p = 0.498, Cdelta = −0.126 | W = 245.5, p = 0.147, Cdelta = −0.269 | W = 191, p = 0.020, Cdelta = −0.431 | W = 296.5, p = 0.530, Cdelta = −0.117 | W = 315.5, p = 0.747, Cdelta = −0.061 |
| CS | W = 469, p = 0.033, Cdelta = 0.396 | W = 468, p = 0.034, Cdelta = 0.393 | W = 408, p = 0.250, Cdelta = 0.214 | W = 377, p = 0.515, Cdelta = 0.122 | W = 343, p = 0.917, Cdelta = 0.021 | W = 385, p = 0.435, Cdelta = 0.146 |
| SDV | t = −2.7527, df = 36.441, p = 0.009, Cd = −0.559 | W = 252, p = 0.179, Cdelta = −0.250 | W = 266, p = 0.263 2, Cdelta = −0.208 | W = 353, p = 0.791, Cdelta = 0.050 | W = 287.5, p = 0.439, Cdelta = −0.144 | W = 308.5, p = 0.663, Cdelta = −0.082 |
| SampEntT_m2 | W = 421, p = 0.174, Cdelta = 0.253 | W = 486.5, p = 0.016, Cdelta = 0.448 | W = 443.5, p = 0.085, Cdelta = 0.320 | W = 411.5, p = 0.228, Cdelta = 0.225 | W = 393.5, p = 0.359, Cdelta = 0.171 | W = 372, p = 0.568, Cdelta = 0.107 |
| SampEntT_m3 | W = 415.5, p = 0.198, Cdelta = 0.237 | W = 475, p = 0.025, Cdelta = 0.414 | W = 440, p = 0.091, Cdelta = 0.309 | W = 406.5, p = 0.259, Cdelta = 0.210 | W = 421, p = 0.174, Cdelta = 0.253 | W = 388, p = 0.407, Cdelta = 0.155 |
| SampEntT_m4 | W = 420, p = 0.136, Cdelta = 0.250 | W = 459.5, p = 0.034, Cdelta = 0.367 | W = 446, p = 0.054, Cdelta = 0.327 | W = 399.5, p = 0.293, Cdelta = 0.189 | W = 382, p = 0.464, Cdelta = 0.137 | W = 370, p = 0.590, Cdelta = 0.101 |
| SampEntP_m2 | W = 413, p = 0.218, Cdelta = 0.229 | W = 438, p = 0.102, Cdelta = 0.303 | W = 406, p = 0.263, Cdelta = 0.208 | W = 420, p = 0.179, Cdelta = 0.250 | W = 504, p = 0.007, Cdelta = 0.500 | W = 256, p = 0.201, Cdelta = −0.238 |
| SampEntP_m3 | W = 408, p = 0.250, Cdelta = 0.214 | W = 404, p = 0.277, Cdelta = 0.202 | W = 401, p = 0.299, Cdelta = 0.193 | W = 404, p = 0.277, Cdelta = 0.202 | W = 525, p = 0.002, Cdelta = 0.562 | W = 273, p = 0.315, Cdelta = −0.187 |
| SampEntP_m4 | W = 410, p = 0.237, Cdelta = 0.220 | W = 416, p = 0.201, Cdelta = 0.238 | W = 442, p = 0.090, Cdelta = 0.315 | W = 466, p = 0.037, Cdelta = 0.387 | W = 427, p = 0.145, Cdelta = 0.271 | W = 339, p = 0.968, Cdelta = 0.009 |
| SpectEnt | W = 172, p = 0.008, Cdelta = −0.488 | W = 175, p = 0.010, Cdelta = −0.479 | W = 160, p = 0.005, Cdelta = −0.524 | W = 226, p = 0.078, Cdelta = −0.327 | t = −2.032, df = 17.237, p = 0.058, Cd = −0.606 | t = −2.055, df = 17.409, p = 0.055, Cd = −0.608 |
| Autocorr | W = 439, p = 0.084, Cdelta = 0.306 | W = 438.5, p = 0.082, Cdelta = 0.305 | W = 447.5, p = 0.060, Cdelta = 0.332 | W = 446.5, p = 0.047, Cdelta = 0.329 | W = 399, p = 0.261, Cdelta = 0.187 | W = 369, p = 0.548, Cdelta = 0.098 |
| SD1 | W = 232, p = 0.096, Cdelta = −0.309 | W = 232, p = 0.096, Cdelta = −0.309 | W = 231, p = 0.093, Cdelta = −0.312 | W = 249, p = 0.164, Cdelta = −0.259 | W = 285, p = 0.417, Cdelta = −0.152 | W = 294, p = 0.504, Cdelta = −0.125 |
| SD2 | W = 245, p = 0.145, Cdelta = −0.271 | W = 246, p = 0.150, Cdelta = −0.268 | W = 243, p = 0.137, Cdelta = −0.277 | W = 257, p = 0.207, Cdelta = −0.235 | W = 298, p = 0.546, Cdelta = −0.113 | W = 299, p = 0.557, Cdelta = −0.110 |
| SDEGG | W = 245, p = 0.145, Cdelta = −0.271 | W = 247, p = 0.154, Cdelta = −0.265 | W = 243, p = 0.137, Cdelta = −0.277 | W = 256, p = 0.201, Cdelta = −0.238 | W = 297, p = 0.536, Cdelta = −0.116 | W = 299, p = 0.557, Cdelta = −0.110 |
| Feature | Proportions of Reported Nausea Corresponding to the Selected Feature (Low/High) | Proportions of Regular EGG Corresponding to the Selected Features (Low/High) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Original | SNR = 17 dB | SNR = 7 dB | SNR = −3 dB | SNR = −13 dB | SNR = −23 dB | Original | SNR = 17 dB | SNR = 7 dB | SNR = −3 dB | SNR = −13 dB | SNR = −23 dB | |
| SampEntT_m2 | 0.58/0.25 | 0.58/0.17 | 0.50/0.25 | 0.42/0.25 | 0.67/0.08 | 0.92/0.00 | 0.27/0.30 | 0.21/0.25 | 0.23/0.32 | 0.25/0.12 | 0.70/0.07 | 0.93/0.00 |
| SampEntT_m3 | 0.50/0.42 | 0.75/0.25 | 0.67/0.33 | 0.67/0.33 | 0.75/0.17 | 0.92/0.00 | 0.37/0.61 | 0.39/0.55 | 0.41/0.59 | 0.48/0.43 | 0.84/0.09 | 0.98/0.02 |
| SampEntT_m4 | 0.33/0.58 | 0.50/0.50 | 0.42/0.58 | 0.50/0.50 | 0.67/0.33 | 0.92/0.08 | 0.30/0.70 | 0.25/0.71 | 0.29/0.70 | 0.36/0.62 | 0.70/0.25 | 0.95/0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakus, G.; Sodnik, J.; Miljković, N. Electrogastrogram-Derived Features for Automated Sickness Detection in Driving Simulator. Sensors 2022, 22, 8616. https://doi.org/10.3390/s22228616
Jakus G, Sodnik J, Miljković N. Electrogastrogram-Derived Features for Automated Sickness Detection in Driving Simulator. Sensors. 2022; 22(22):8616. https://doi.org/10.3390/s22228616
Chicago/Turabian StyleJakus, Grega, Jaka Sodnik, and Nadica Miljković. 2022. "Electrogastrogram-Derived Features for Automated Sickness Detection in Driving Simulator" Sensors 22, no. 22: 8616. https://doi.org/10.3390/s22228616
APA StyleJakus, G., Sodnik, J., & Miljković, N. (2022). Electrogastrogram-Derived Features for Automated Sickness Detection in Driving Simulator. Sensors, 22(22), 8616. https://doi.org/10.3390/s22228616







