Remotely Powered Two-Wire Cooperative Sensors for Biopotential Imaging Wearables
Abstract
1. Introduction
2. State-of-the-Art Cooperative Sensors
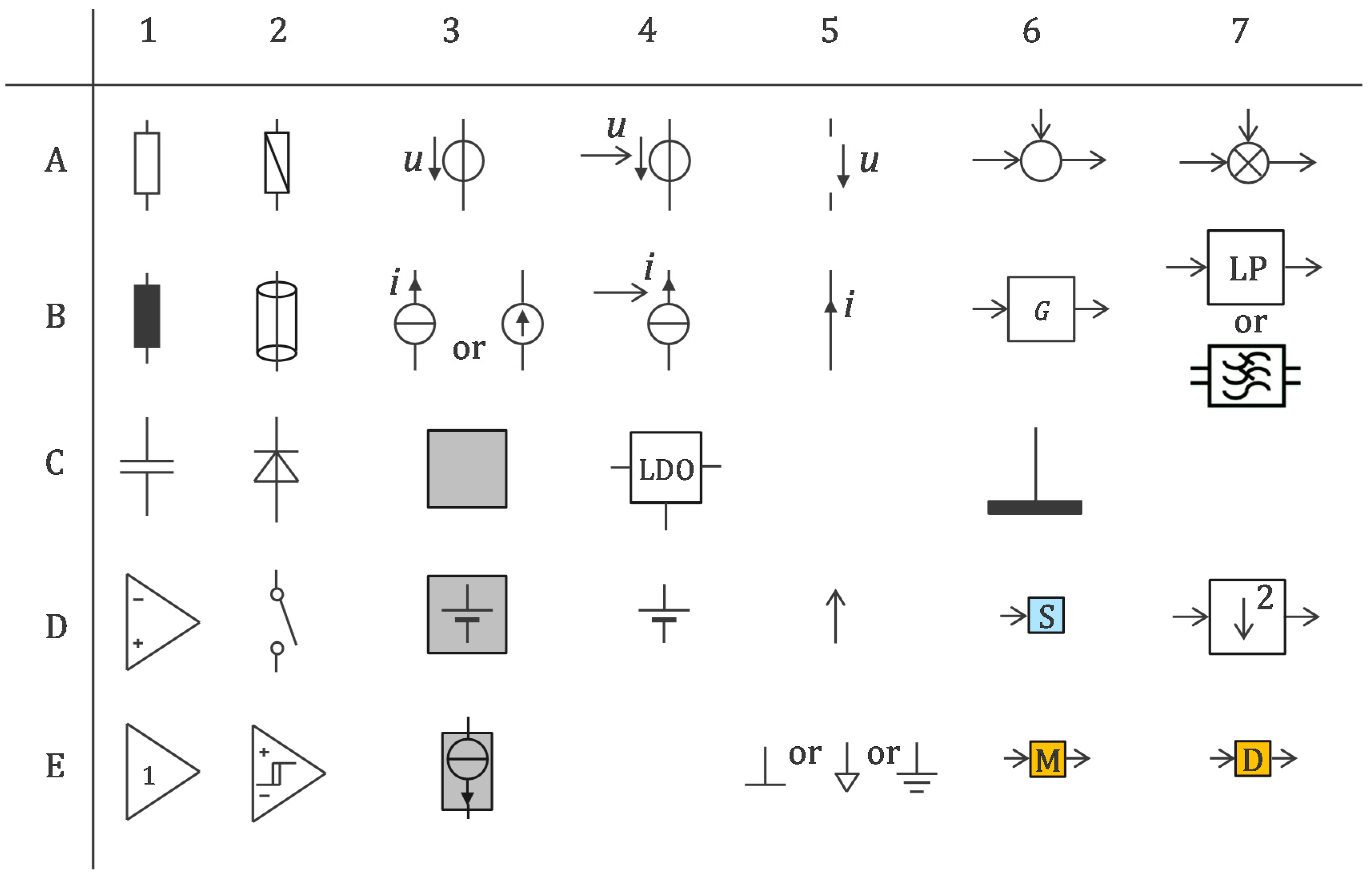
2.1. Basic Circuit and Their Interconnections
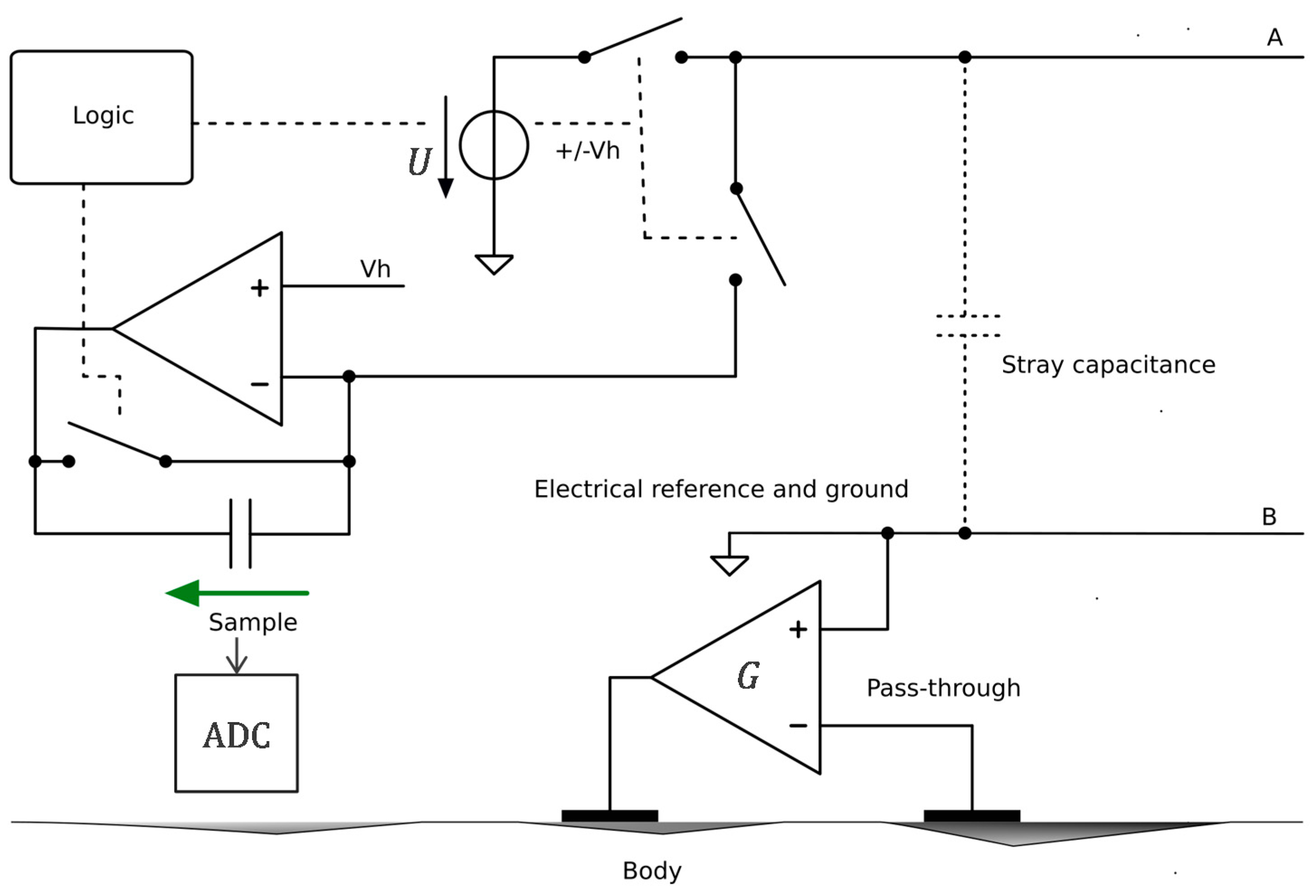
2.2. Floating Supply and Bootstrapping
3. Method
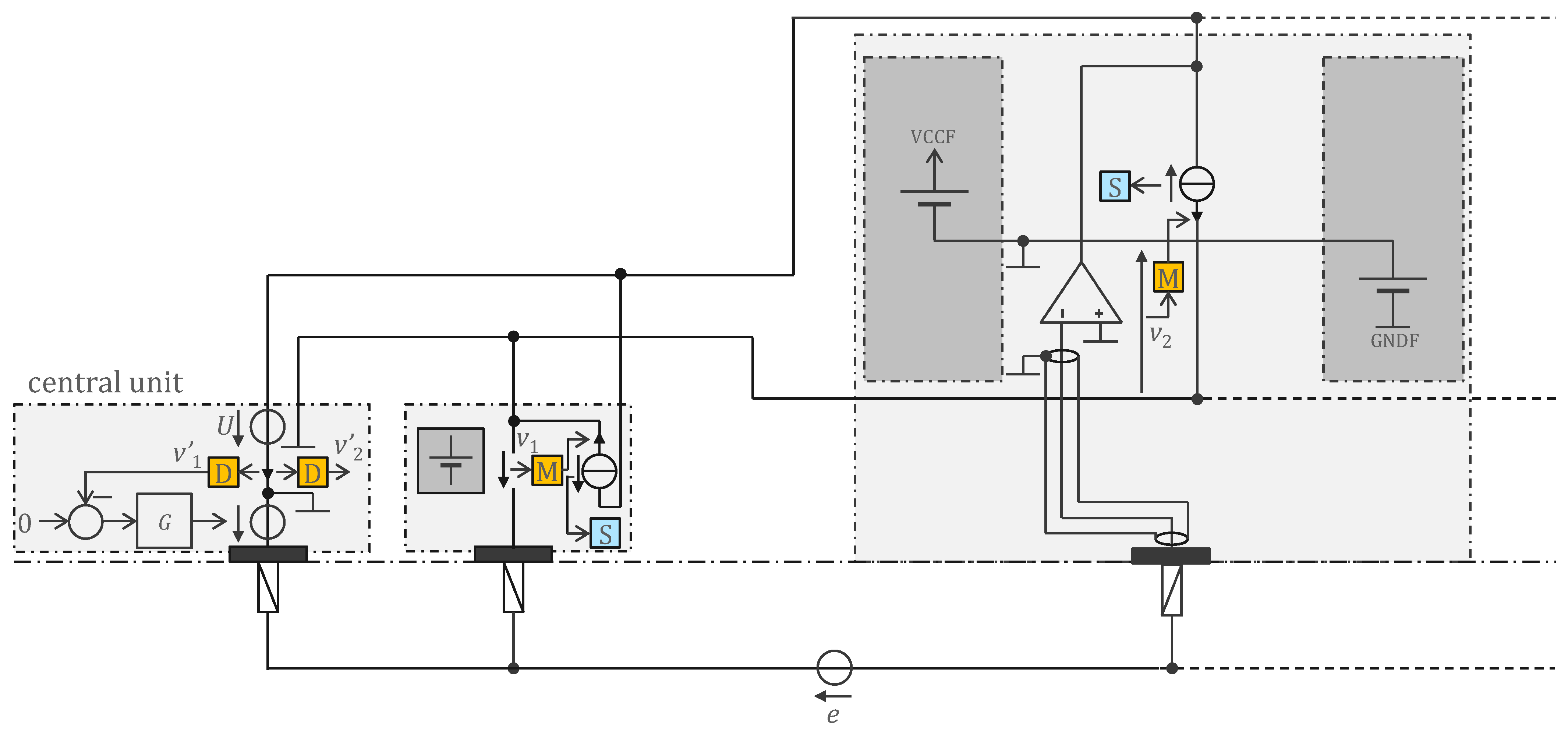
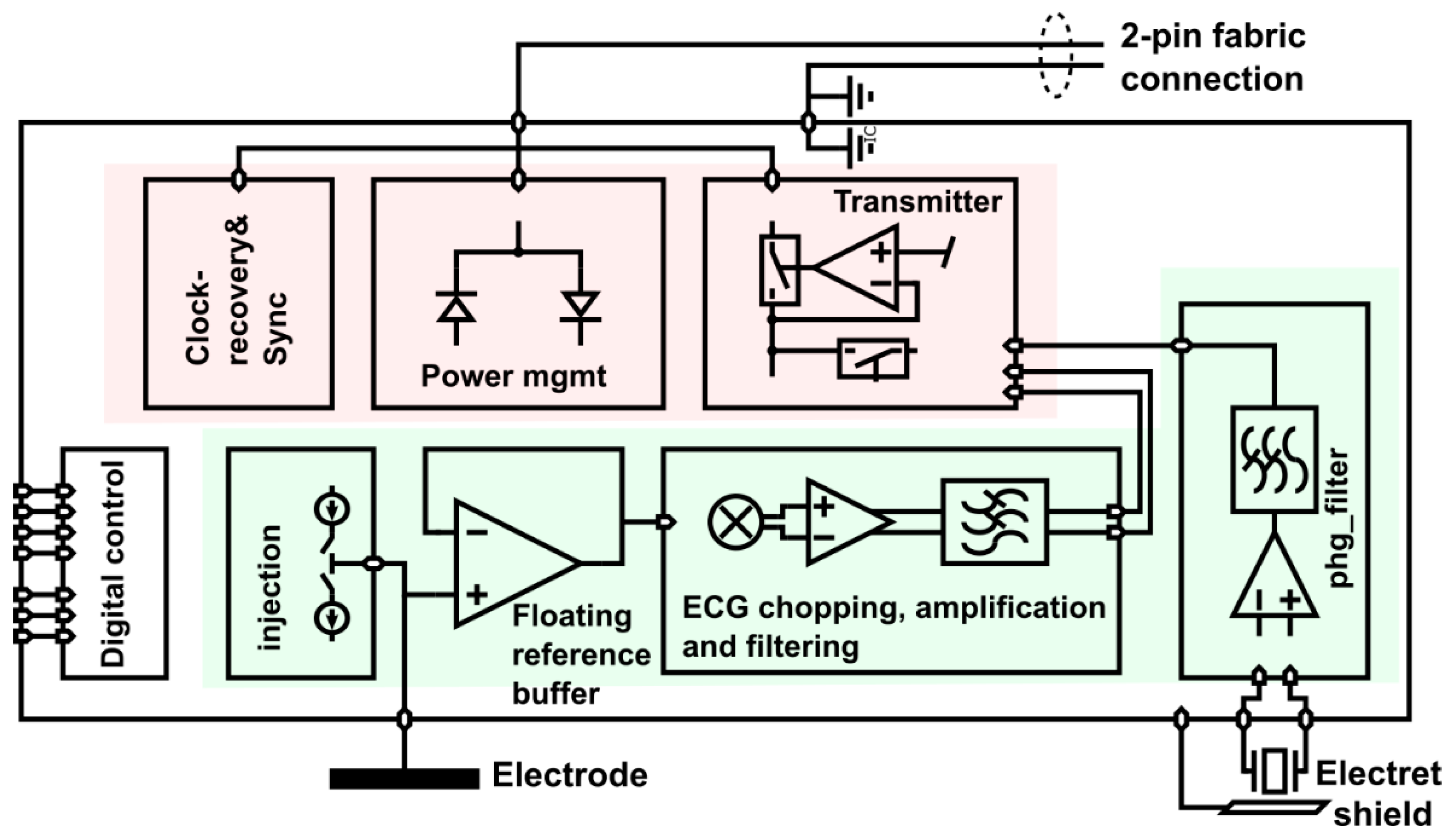
3.1. Legacy Approach with 500 Hz Powering and Off-the-Shelf Components
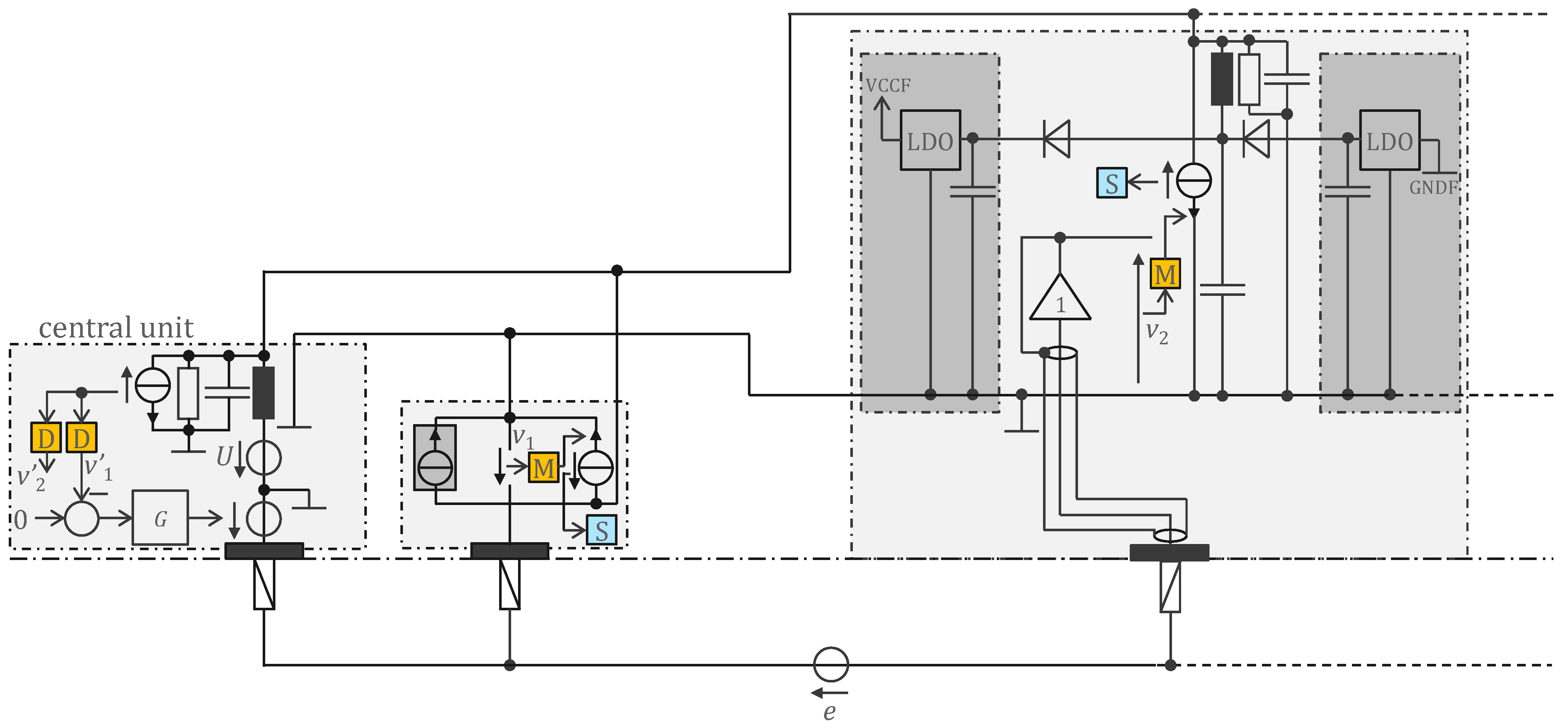
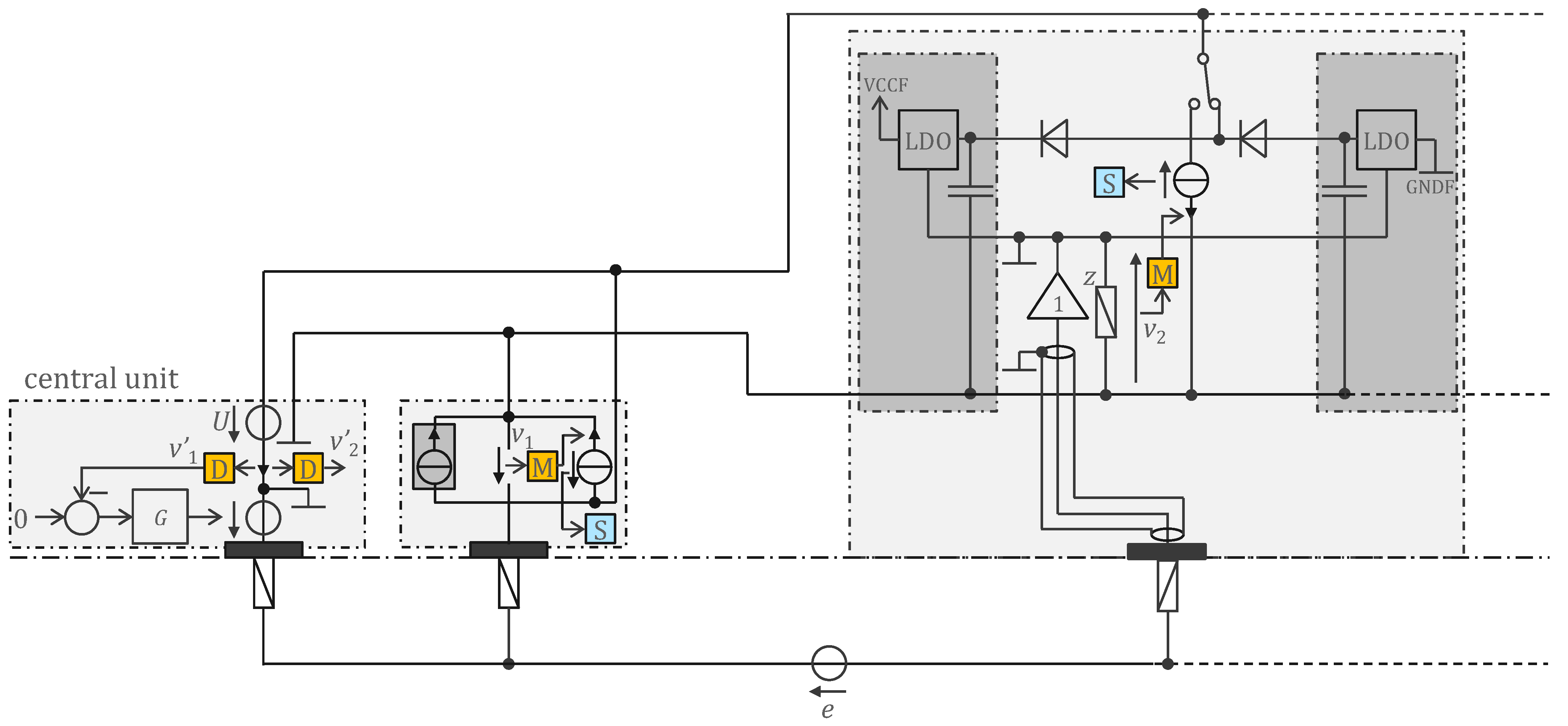
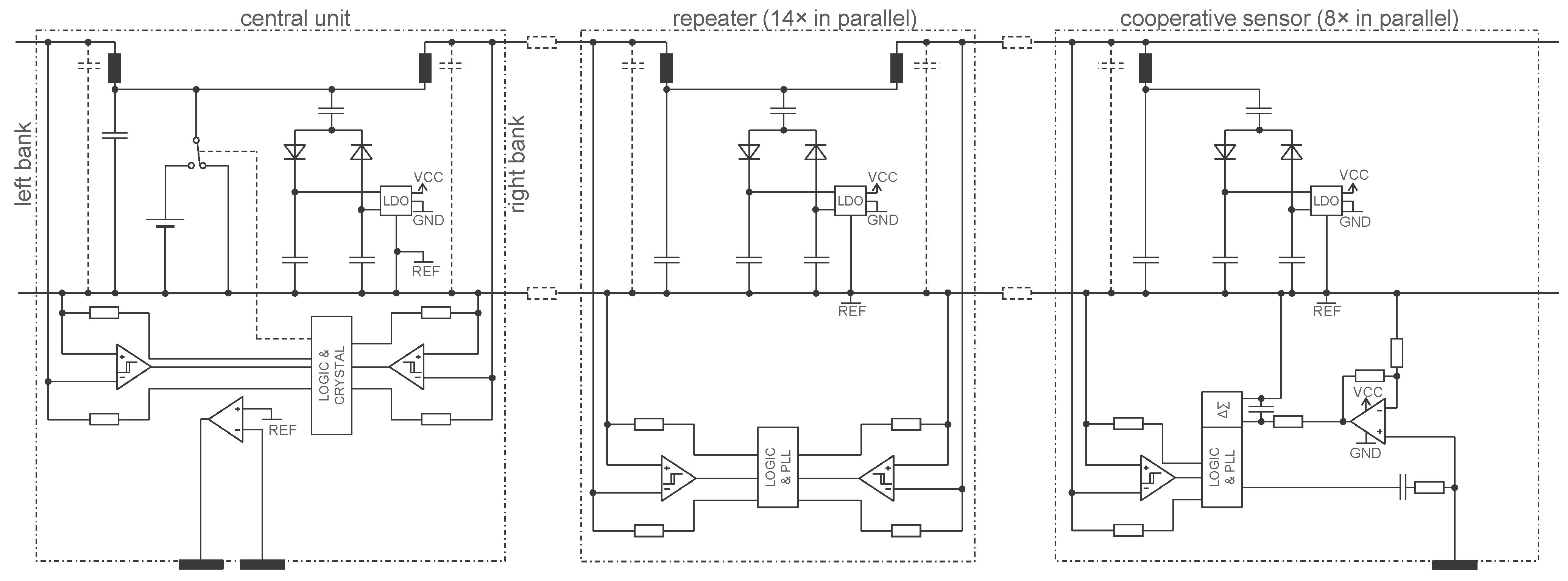
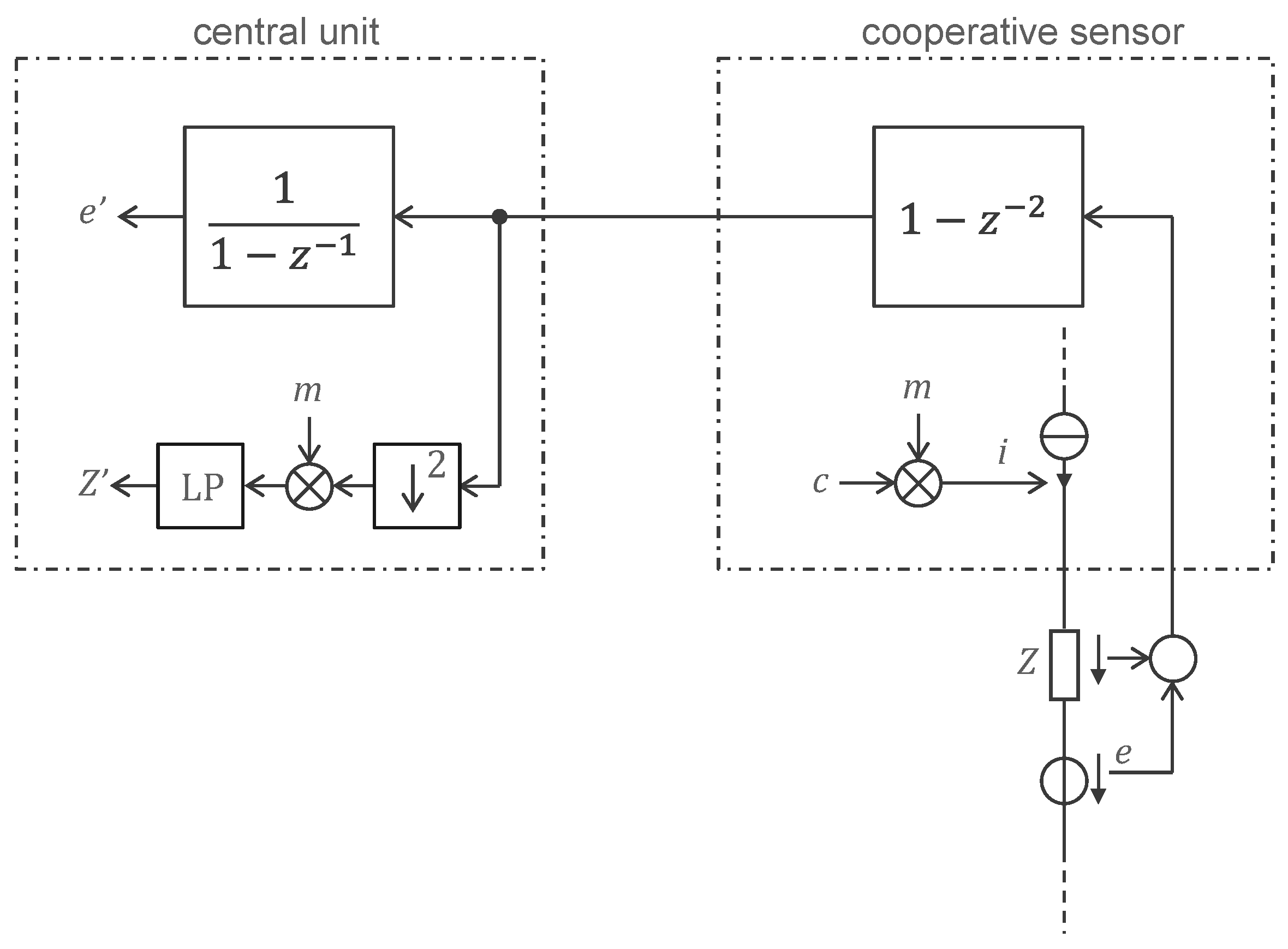
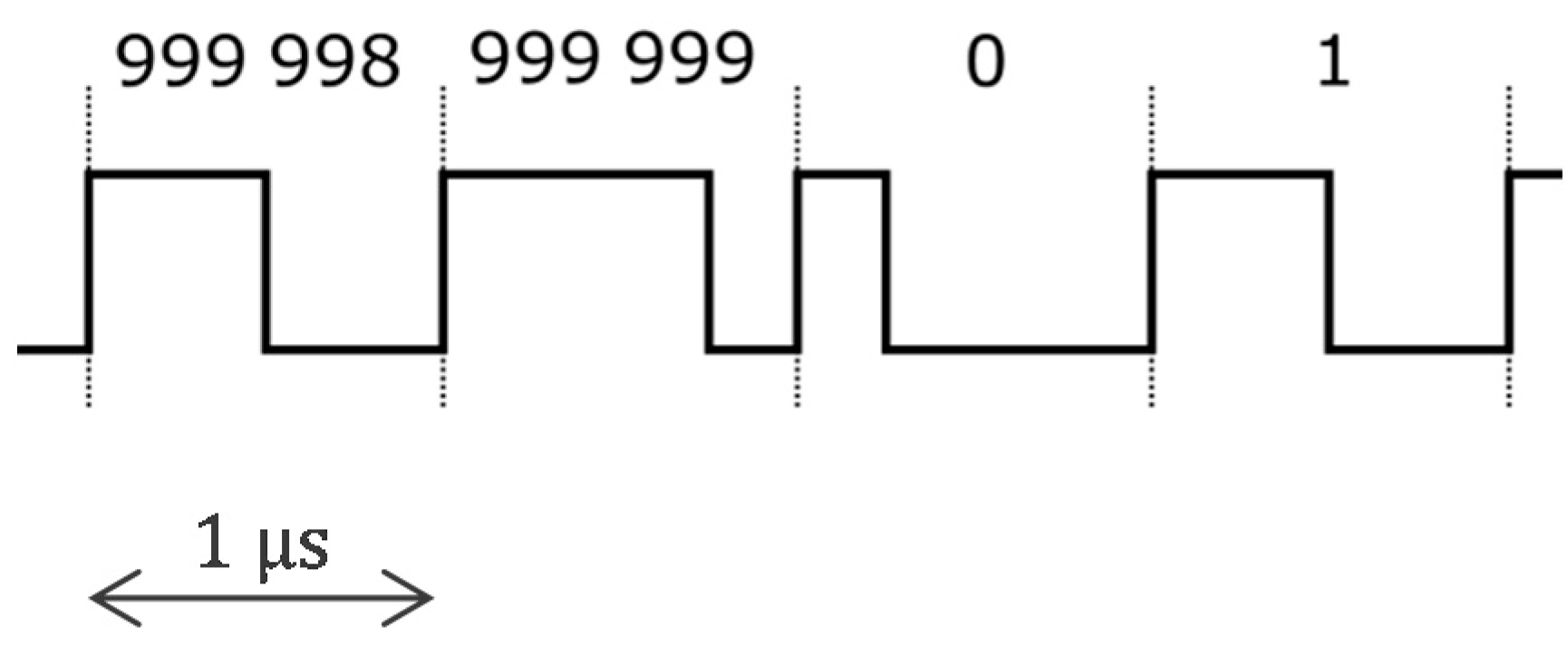
3.2. Approach Addressing the Safety Issue with Powering at 1 MHz and ASIC for 250 Sensors
3.3. Comparison to Existing Work
4. Results
4.1. Legacy Approach with 500 Hz Powering and Off-the-Shelf Components
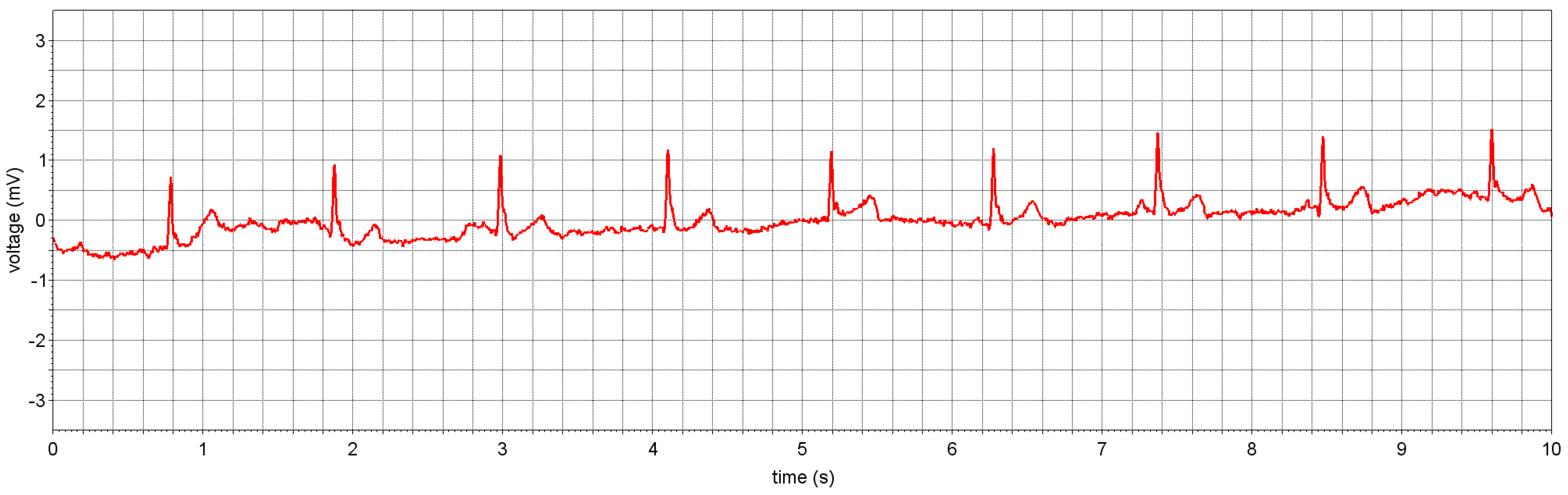
4.1.1. ECG Study Prototype
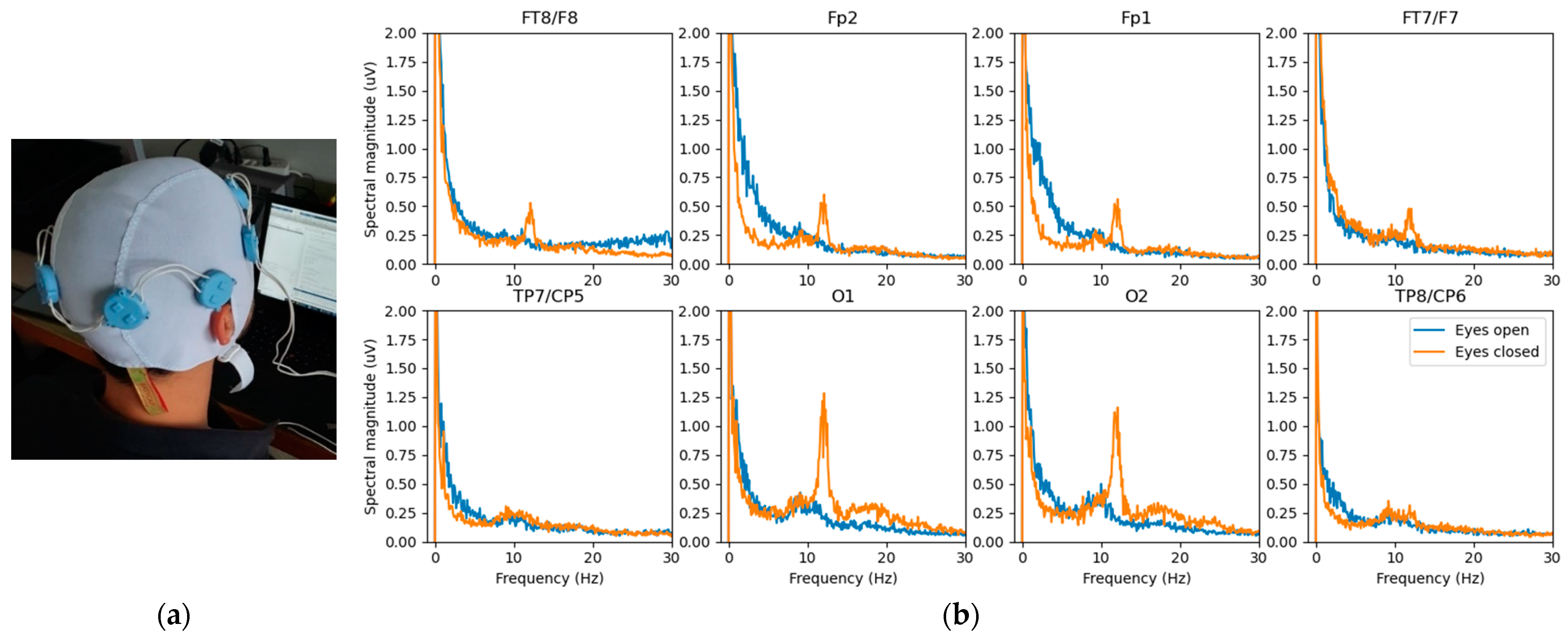
4.1.2. EEG Study Prototype
4.2. Approach Addressing the Safety Issue with Powering at 1 MHz and ASIC for 250 Sensors
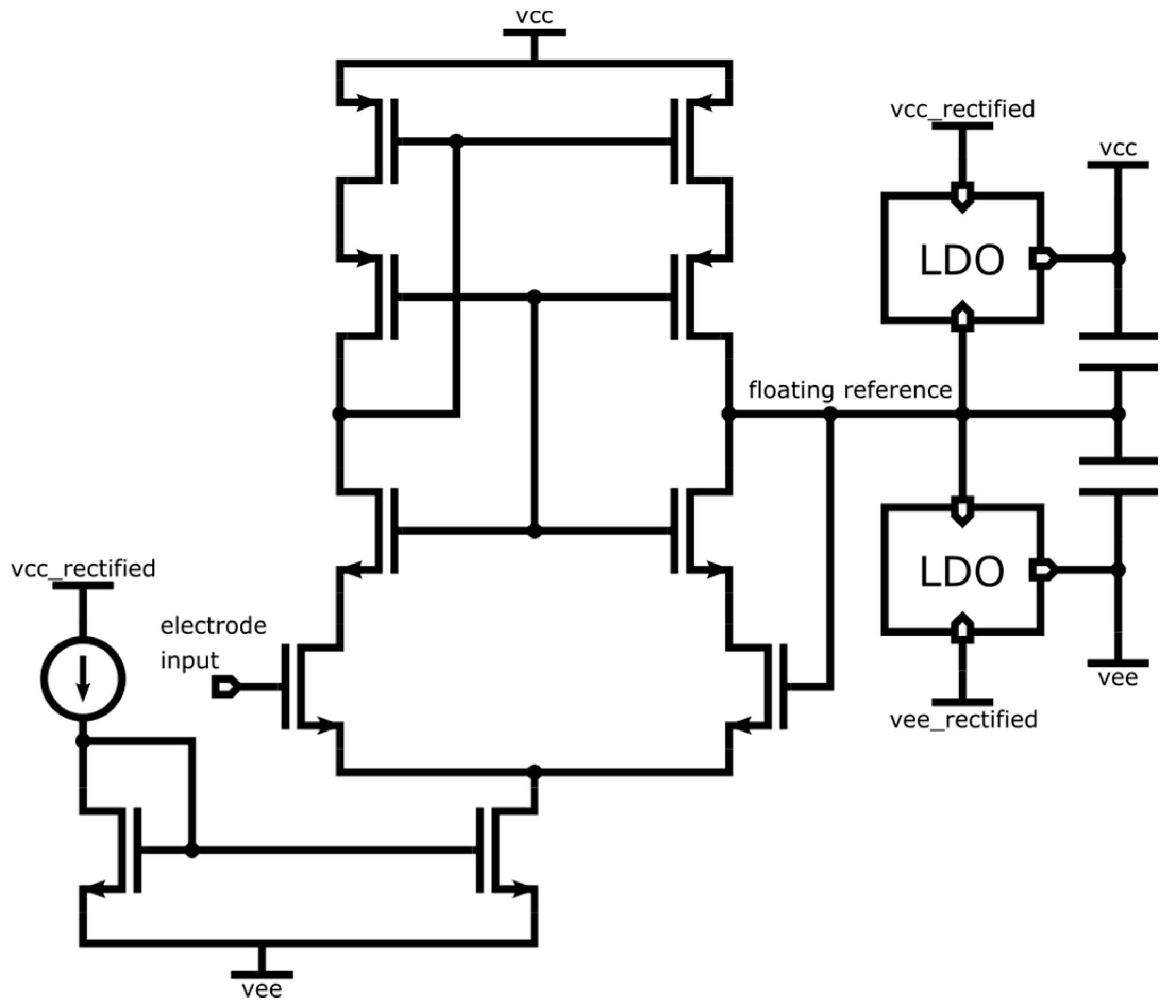
4.2.1. ASIC Architecture
4.2.2. Central Unit Architecture
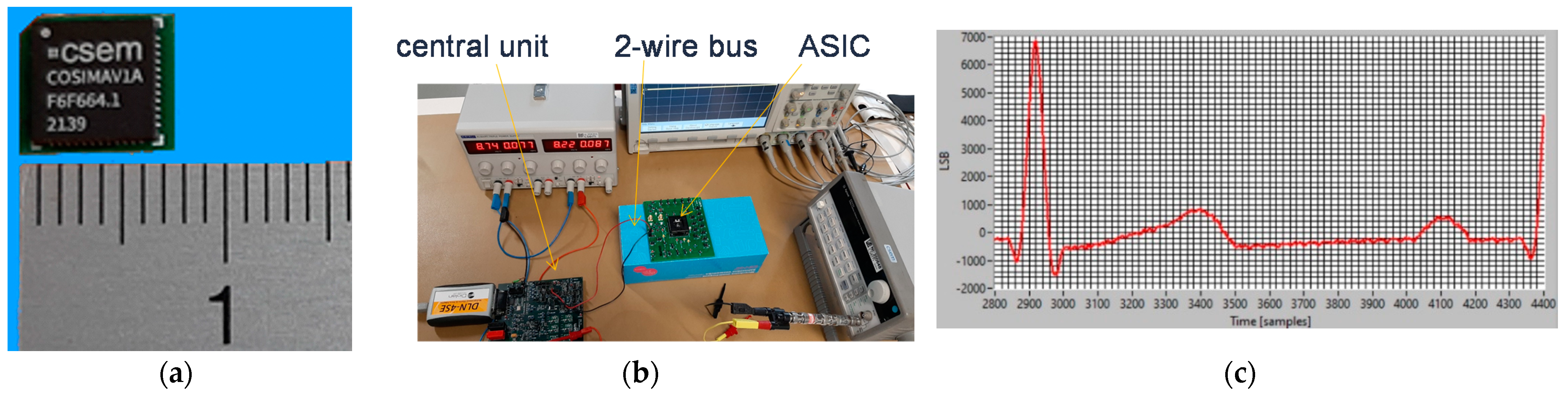
4.2.3. Implementation Results
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Webster, J.G.; Clark, J.W. (Eds.) Medical Instrumentation: Application and Design, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Boehm, A.; Yu, X.; Neu, W.; Leonhardt, S.; Teichmann, D. A Novel 12-Lead ECG T-Shirt with Active Electrodes. Electronics 2016, 5, 75. [Google Scholar] [CrossRef]
- Guerrero, F.N.; Spinelli, E.M. A Two-Wired Ultra-High Input Impedance Active Electrode. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Zipp, P.; Ahrens, H. A model of bioelectrode motion artefact and reduction of artefact by amplifier input stage design. J. Biomed. Eng. 1979, 1, 273–276. [Google Scholar] [CrossRef]
- Meziane, N.; Webster, J.G.; Attari, M.; Nimunkar, A.J. Dry electrodes for electrocardiography. Physiol. Meas. 2013, 34, R47–R69. [Google Scholar] [CrossRef] [PubMed]
- Godin, D.T.; Parker, P.A.; Scott, R.N. Noise characteristics of stainless-steel surface electrodes. Med. Biol. Eng. Comput. 1991, 29, 585–590. [Google Scholar] [CrossRef] [PubMed]
- IEC 60601-1; Medical Electrical Equipment—Part 1: General Requirements for Basic Safety and Essential Performance. IEC: Geneva, Switzerland, 2020.
- IEC 60601-2-25; Medical Electrical Equipment—Part 2–25: Particular Requirements for the Basic SAFETY and essential Performance of Electrocardiographs. IEC: Geneva, Switzerland, 2011.
- IEC 60601-2-47; Medical Electrical Equipment—Part 2–47: Particular Requirements for the Basic Safety and Essential Performance of Ambulatory Electrocardiographic Systems. IEC: Geneva, Switzerland, 2012.
- Pereira, H.; Niederer, S.; Rinaldi, C.A. Electrocardiographic imaging for cardiac arrhythmias and resynchronization therapy. EP Eur. 2020, 22, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Gevins, A.; Smith, M.E.; McEvoy, L.K.; Leong, H.; Le, J. Electroencephalographic imaging of higher brain function. Phil. Trans. R. Soc. Lond. B 1999, 354, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, H.; van der Smagt, P. iEMG: Imaging electromyography. J. Electromyogr. Kinesiol. 2016, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chételat, O. Synchronization and Communication Bus for Biopotential and Bioimpedance Measurement Systems. EP Patent 2567657 B1, 13 March 2013. [Google Scholar]
- Chételat, O.; Correvon, M. Measurement Device for Measuring Bio-Impedance and/or a Bio-Potential of a Human or Animal Body. US Patent 2015/0173677 B2, 25 June 2015. [Google Scholar]
- Rapin, M.; Regamey, Y.-J.; Chételat, O. Common-mode rejection in the measurement of wearable ECG with cooperative sensors. at-Automatisierungstechnik 2018, 66, 1002–1013. [Google Scholar] [CrossRef]
- Rapin, M.; Proença, M.; Braun, F.; Meier, C.; Sola, J.; Ferrario, D.; Grossenbacher, O.; Porchet, J.-A.; Chételat, O. Cooperative dry-electrode sensors for multi-lead biopotential and bioimpedance monitoring. Physiol. Meas. 2015, 36, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Rapin, M.; Wacker, J.; Chételat, O. Two-Wire Bus Combining Full Duplex Body-Sensor Network and Multilead Biopotential Measurements. IEEE Trans. Biomed. Eng. 2018, 65, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Rapin, M. A Wearable Sensor Architecture for High-Quality Measurement of Multilead ECG and Frequency-Multiplexed EIT. Ph.D. Thesis, ETH, Zürich, Switzerland, 2018. [Google Scholar] [CrossRef]
- Chételat, O.; Carós, J.M.S.i. Floating front-end amplifier and one-wire measuring device. US Patent 8427181 B2, 16 September 2009. [Google Scholar]
- Guermandi, M.; Bigucci, A.; Scarselli, E.F.; Guerrieri, R. EEG Acquisition System Based on Active Electrodes with Common-mode Interference Suppression by Driving Right Leg Circuit. In Proceedings of the Engineering in Medicine and Biology Society (EMBC), 2015 37th Annual International Conference of the IEEE, Milan, Italy, 25–29 August 2015; pp. 3169–3172. Available online: http://ieeexplore.ieee.org/abstract/document/7319065/ (accessed on 10 October 2017).
- Ko, W.H. Active Electrodes for EEG and Evoked Potential. In Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Vol.20 Biomedical Engineering towards the Year 2000 and Beyond (Cat. No. 98CH36286), Hong Kong, China, 1 November 1998; Volume 4, pp. 2221–2224. [Google Scholar] [CrossRef]
- Rowlandson, G.I.; Xue, J.Q.; Henderson, R.E.; Rao, C.S. System and Apparatus for Collecting Physiological Signals from a Plurality of Electrodes. US Patent 8326394 B2, 4 December 2012. [Google Scholar]
- Prot, P.; Frouin, P.-Y. Textile Device for Measuring the Electro-Physiological Activity of a Subject. US Patent 2019/0380613 A1, 19 December 2019. [Google Scholar]
- Xu, J.; Yazicioglu, R.F.; Hoof, C.V.; Makinwa, K. Low Power active Electrode ICs for Wearable EEG Acquisition; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Chételat, O.; Bonnal, B.; Fivaz, A. Remotely Powered Cooperative Sensor Device. US Patent 2021/0169543 A1, 10 June 2021. [Google Scholar]
- IEC 60601-2-26; Medical Electrical Equipment—Part 2–26: Particular Requirements for the Basic Safety and Essential Performance of Electroencephalographs. IEC: Geneva, Switzerland, 2012.


| Technique/Features | [Ref], Section | Comment |
|---|---|---|
| Conventional star arrangement | Not suitable for wearables with many electrodes | |
| Passive electrodes, shielded cables | [1] | Widespread |
| Active electrodes, two-wire cables | [2,3,20,21] | Well-known in the literature, but little used |
| Parallel bus arrangement | Scalable (connector size independent of nb. of electr.) | |
| Bus with more than 2 wires | [22] | Not easily flexible, stretchable, breathable, washable |
| Two-wire bus (cooperative sensors) | Section 2 | Simplest connection |
| Locally powered Bootstrapping | Section 2.1 Section 2.2 | Easy to comply with safety (medical standards) Suitable for dry electrodes |
| Remotely powered | Section 3/Section 4 | Sensors can be miniaturized |
| No monitoring of leakage currents No bootstrapping | Section 3.1/Section 4.1 | Requires reliable waterproof double insulation Not ideal for dry electrodes |
| Monitorable leakage currents Bootstrapping | Section 3.2/Section 4.2 | Suitably flexible, stretchable, breathable, washable Suitable for dry electrodes |
| Sensor | Peak-to-Peak Noise over 10 s (µV) | Rms Noise (µV) |
|---|---|---|
| 1 | (potential reference) | (potential reference) |
| 2 | 4.01 | 0.59 |
| 3 | 4.65 | 0.60 |
| 4 | 5.18 | 0.66 |
| 5 | 4.90 | 0.64 |
| 6 | 4.88 | 0.66 |
| 7 | 5.41 | 0.67 |
| 8 | 4.40 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chételat, O.; Rapin, M.; Bonnal, B.; Fivaz, A.; Wacker, J.; Sporrer, B. Remotely Powered Two-Wire Cooperative Sensors for Biopotential Imaging Wearables. Sensors 2022, 22, 8219. https://doi.org/10.3390/s22218219
Chételat O, Rapin M, Bonnal B, Fivaz A, Wacker J, Sporrer B. Remotely Powered Two-Wire Cooperative Sensors for Biopotential Imaging Wearables. Sensors. 2022; 22(21):8219. https://doi.org/10.3390/s22218219
Chicago/Turabian StyleChételat, Olivier, Michaël Rapin, Benjamin Bonnal, André Fivaz, Josias Wacker, and Benjamin Sporrer. 2022. "Remotely Powered Two-Wire Cooperative Sensors for Biopotential Imaging Wearables" Sensors 22, no. 21: 8219. https://doi.org/10.3390/s22218219
APA StyleChételat, O., Rapin, M., Bonnal, B., Fivaz, A., Wacker, J., & Sporrer, B. (2022). Remotely Powered Two-Wire Cooperative Sensors for Biopotential Imaging Wearables. Sensors, 22(21), 8219. https://doi.org/10.3390/s22218219




