Classification between Normal and Cancerous Human Urothelial Cells by Using Micro-Dimensional Electrochemical Impedance Spectroscopy Combined with Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Cell Preparation
2.2. Methods
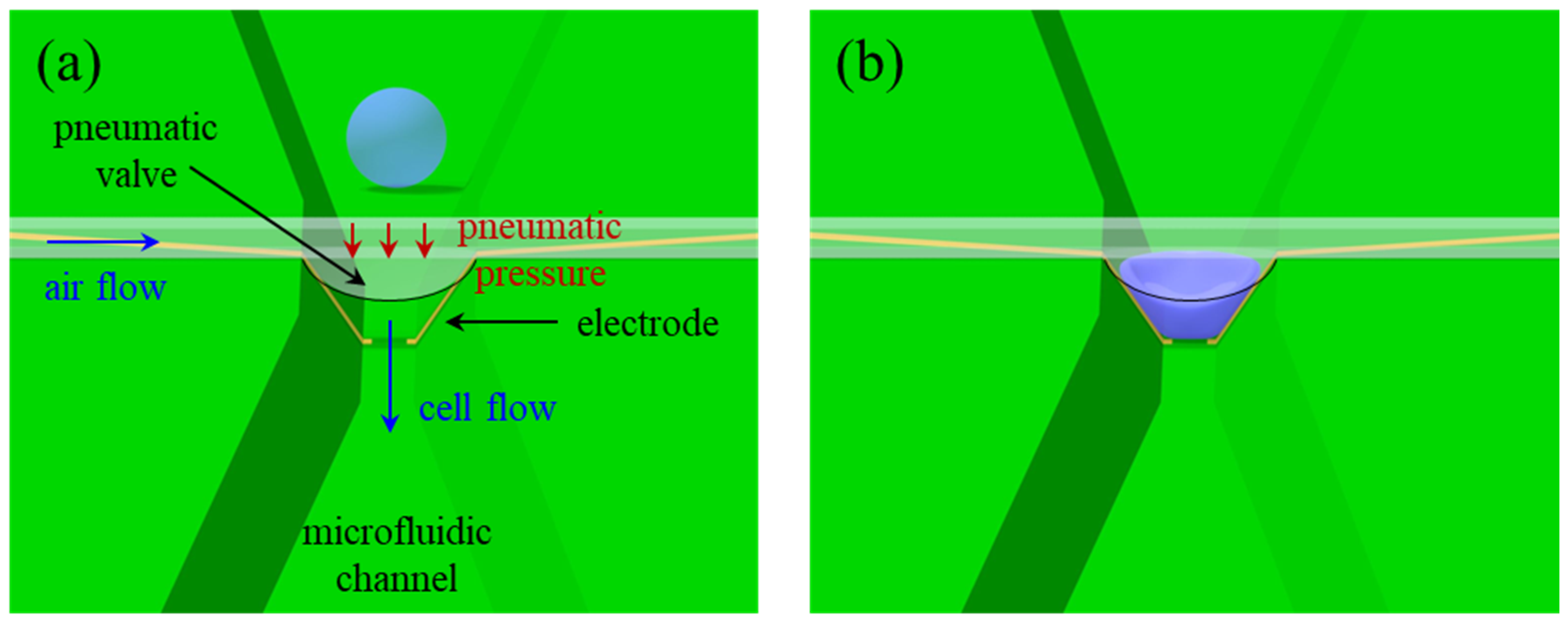
2.2.1. Device Configuration
2.2.2. Device Fabrication
2.2.3. Experiment Setup
2.2.4. Data Analysis
3. Results and Discussion
3.1. Electrochemical Impedance of Normal and Cancer Urothelial Cell Lines
3.2. Machine Learning

| LR | KNN | DT | RF | SVM | BPNN * | |
|---|---|---|---|---|---|---|
| Optimization method | Grid search | Grid search | Grid search | Grid search | Grid search | BO |
| Best Hyper -parameters | Regularization parameter: 0.17 | Number of nearest neighbors: 4 | Maximum depth: 5 | Maximum depth: 7 | Regularization parameter: 2.12 | Batch size: 27 |
| Number of estimators: 50 | Kernel parameter: 0.19 | Learning rate: 0.0004 | ||||
| Epoch: 329 | ||||||
| Optimization time (seconds) | 19.7 | 1.1 | 0.4 | 275.6 | 383.6 | 200.1 |
| Best cross-validation accuracy | 0.713 | 0.824 | 0.898 | 0.951 | 0.909 | 0.905 |

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y.; Bivalacqua, T.J.; Downs, T.; Huang, W.; Jones, J.; Kamat, A.M.; Konety, B.; Malmström, P.-U.; McKiernan, J.; O’Donnell, M. Blue light flexible cystoscopy with hexaminolevulinate in non-muscle-invasive bladder cancer: Review of the clinical evidence and consensus statement on optimal use in the USA—Update 2018. Nat. Rev. Urol. 2019, 16, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, B.W.; Burger, M.; Lotan, Y.; Solsona, E.; Stief, C.G.; Sylvester, R.J.; Witjes, J.A.; Zlotta, A.R. Recurrence and progression of disease in non–muscle-invasive bladder cancer: From epidemiology to treatment strategy. Eur. Urol. 2009, 56, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Van Der Aa, M.N.; Steyerberg, E.W.; Sen, E.F.; Zwarthoff, E.C.; Kirkels, W.J.; Van Der Kwast, T.H.; Essink-Bot, M.L. Patients’ perceived burden of cystoscopic and urinary surveillance of bladder cancer: A randomized comparison. BJU Int. 2008, 101, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Carmack, A.J.; Soloway, M.S. The diagnosis and staging of bladder cancer: From RBCs to TURs. Urology 2006, 67, 3–8. [Google Scholar] [CrossRef]
- Hajdinjak, T. UroVysion FISH test for detecting urothelial cancers: Meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol. Oncol. 2008, 26, 646–651. [Google Scholar] [CrossRef]
- Vrooman, O.P.; Witjes, J.A. Urinary markers in bladder cancer. Eur. Urol. 2008, 53, 909–916. [Google Scholar] [CrossRef]
- Park, Y.; Kim, H.W.; Yun, J.; Seo, S.; Park, C.-J.; Lee, J.Z.; Lee, J.-H. Microelectrical impedance spectroscopy for the differentiation between normal and cancerous human urothelial cell lines: Real-time electrical impedance measurement at an optimal frequency. BioMed Res. Int. 2016, 2016, 8748023. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Zhang, Y.; Wang, J.; Stimming, U.; Lee, A.A. Identifying degradation patterns of lithium ion batteries from impedance spectroscopy using machine learning. Nat. Commun. 2020, 11, 1706. [Google Scholar] [CrossRef]
- Varvara, S.; Berghian-Grosan, C.; Bostan, R.; Ciceo, R.L.; Salarvand, Z.; Talebian, M.; Raeissi, K.; Izquierdo, J.; Souto, R.M. Experimental characterization, machine learning analysis and computational modelling of the high effective inhibition of copper corrosion by 5-(4-pyridyl)-1,3,4-oxadiazole-2-thiol in saline environment. Electrochim. Acta 2021, 398, 139282. [Google Scholar] [CrossRef]
- Daassi-Gnaba, H.; Oussar, Y.; Merlan, M.; Ditchi, T.; Géron, E.; Holé, S. Wood moisture content prediction using feature selection techniques and a kernel method. Neurocomputing 2017, 237, 79–91. [Google Scholar] [CrossRef]
- Durante, G.; Becari, W.; Lima, F.A.; Peres, H.E. Electrical impedance sensor for real-time detection of bovine milk adulteration. IEEE Sens. J. 2015, 16, 861–865. [Google Scholar] [CrossRef]
- Kirchner, E.; Bienefeld, C.; Schirra, T.; Moltschanov, A. Predicting the Electrical Impedance of Rolling Bearings Using Machine Learning Methods. Machines 2022, 10, 156. [Google Scholar] [CrossRef]
- Daliri, M.R. Combining extreme learning machines using support vector machines for breast tissue classification. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Helwan, A.; Idoko, J.B.; Abiyev, R.H. Machine learning techniques for classification of breast tissue. Procedia Comput. Sci. 2017, 120, 402–410. [Google Scholar] [CrossRef]
- Murphy, E.K.; Mahara, A.; Khan, S.; Hyams, E.S.; Schned, A.R.; Pettus, J.; Halter, R.J. Comparative study of separation between ex vivo prostatic malignant and benign tissue using electrical impedance spectroscopy and electrical impedance tomography. Physiol. Meas. 2017, 38, 1242. [Google Scholar] [CrossRef]
- Schütt, J.; Sandoval Bojorquez, D.I.; Avitabile, E.; Oliveros Mata, E.S.; Milyukov, G.; Colditz, J.; Delogu, L.G.; Rauner, M.; Feldmann, A.; Koristka, S. Nanocytometer for smart analysis of peripheral blood and acute myeloid leukemia: A pilot study. Nano Lett. 2020, 20, 6572–6581. [Google Scholar] [CrossRef]
- D’Orazio, M.; Reale, R.; De Ninno, A.; Brighetti, M.A.; Mencattini, A.; Businaro, L.; Martinelli, E.; Bisegna, P.; Travaglini, A.; Caselli, F. Electro-Optical Classification of Pollen Grains via Microfluidics and Machine Learning. IEEE Trans. Biomed. Eng. 2021, 69, 921–931. [Google Scholar] [CrossRef]
- Honrado, C.; Salahi, A.; Adair, S.J.; Moore, J.H.; Bauer, T.W.; Swami, N.S. Automated biophysical classification of apoptotic pancreatic cancer cell subpopulations by using machine learning approaches with impedance cytometry. Lab Chip 2022, 22, 3708–3720. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, Z.; Chai, H.; He, W.; Huang, L.; Wang, W. Neural network-enhanced real-time impedance flow cytometry for single-cell intrinsic characterization. Lab Chip 2022, 22, 240–249. [Google Scholar] [CrossRef]
- Honrado, C.; McGrath, J.S.; Reale, R.; Bisegna, P.; Swami, N.S.; Caselli, F. A neural network approach for real-time particle/cell characterization in microfluidic impedance cytometry. Anal. Bioanal. Chem. 2020, 412, 3835–3845. [Google Scholar] [CrossRef]
- Han, A.; Yang, L.; Frazier, A.B. Quantification of the heterogeneity in breast cancer cell lines using whole-cell impedance spectroscopy. Clin. Cancer Res. 2007, 13, 139–143. [Google Scholar] [CrossRef]
- Kang, G.; Yoo, S.K.; Kim, H.-I.; Lee, J.-H. Differentiation between normal and cancerous cells at the single cell level using 3-D electrode electrical impedance spectroscopy. IEEE Sens. J. 2011, 12, 1084–1089. [Google Scholar] [CrossRef]
- Kang, G.; Kim, Y.-j.; Moon, H.-s.; Lee, J.-W.; Yoo, T.-K.; Park, K.; Lee, J.-H. Discrimination between the human prostate normal cell and cancer cell by using a novel electrical impedance spectroscopy controlling the cross-sectional area of a microfluidic channel. Biomicrofluidics 2013, 7, 044126. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, D.; Li, H.; Luo, Y.; Deng, B.; Huang, S.-B.; Chiu, T.-K.; Wu, M.-H.; Long, R.; Hu, H. A microfluidic system enabling continuous characterization of specific membrane capacitance and cytoplasm conductivity of single cells in suspension. Biosens. Bioelectron. 2013, 43, 304–307. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, D.; Luo, Y.; Li, H.; Deng, B.; Huang, S.-B.; Chiu, T.-K.; Wu, M.-H.; Long, R.; Hu, H. A microfluidic system for cell type classification based on cellular size-independent electrical properties. Lab Chip 2013, 13, 2272–2277. [Google Scholar] [CrossRef]
- Zheng, Y.; Shojaei-Baghini, E.; Wang, C.; Sun, Y. Microfluidic characterization of specific membrane capacitance and cytoplasm conductivity of singlecells. Biosens. Bioelectron. 2013, 42, 496–502. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, M.; Chen, D.; Zhao, X.; Xue, C.; Hao, R.; Yue, W.; Wang, J.; Chen, J. Single-cell electrical phenotyping enabling the classification of mouse tumor samples. Sci. Rep. 2016, 6, 19487. [Google Scholar] [CrossRef]
- Petchakup, C.; Li, K.H.H.; Hou, H.W. Advances in single cell impedance cytometry for biomedical applications. Micromachines 2017, 8, 87. [Google Scholar] [CrossRef]
- Hong, J.-L.; Lan, K.-C.; Jang, L.-S. Electrical characteristics analysis of various cancer cells using a microfluidic device based on single-cell impedance measurement. Sens. Actuators B Chem. 2012, 173, 927–934. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, R.; Zong, X. A microchip integrating cell array positioning with in situ single-cell impedance measurement. Analyst 2015, 140, 6571–6578. [Google Scholar] [CrossRef]
- Jang, L.-S.; Wang, M.-H. Microfluidic device for cell capture and impedance measurement. Biomed. Microdevices 2007, 9, 737–743. [Google Scholar] [CrossRef]
- Tsai, S.L.; Wang, M.H.; Chen, M.K.; Jang, L.S. Analytical and numerical modeling methods for electrochemical impedance analysis of single cells on coplanar electrodes. Electroanalysis 2014, 26, 389–398. [Google Scholar] [CrossRef]
- Huang, S.-B.; Zhao, Y.; Chen, D.; Lee, H.-C.; Luo, Y.; Chiu, T.-K.; Wang, J.; Chen, J.; Wu, M.-H. A clogging-free microfluidic platform with an incorporated pneumatically driven membrane-based active valve enabling specific membrane capacitance and cytoplasm conductivity characterization of single cells. Sens. Actuators B Chem. 2014, 190, 928–936. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, Y.; Tan, Q.; Zhang, Y.L.; Li, J.; Geddie, W.R.; Jewett, M.A.; Sun, Y. A microfluidic device for simultaneous electrical and mechanical measurements on single cells. Biomicrofluidics 2011, 5, 014113. [Google Scholar] [CrossRef]
- Frankowski, M.; Simon, P.; Bock, N.; El-Hasni, A.; Schnakenberg, U.; Neukammer, J. Simultaneous optical and impedance analysis of single cells: A comparison of two microfluidic sensors with sheath flow focusing. Eng. Life Sci. 2015, 15, 286–296. [Google Scholar] [CrossRef]
- Spencer, D.; Caselli, F.; Bisegna, P.; Morgan, H. High accuracy particle analysis using sheathless microfluidic impedance cytometry. Lab Chip 2016, 16, 2467–2473. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Huang, Y.-W.; Wu, Y.-T. System-level biochip for impedance sensing and programmable manipulation of bladder cancer cells. Sensors 2011, 11, 11021–11035. [Google Scholar] [CrossRef]
- Keshtkar, A.; Keshtkar, A.; Smallwood, R.H. Electrical impedance spectroscopy and the diagnosis of bladder pathology. Physiol. Meas. 2006, 27, 585. [Google Scholar] [CrossRef]
- Wilkinson, B.; Smallwood, R.; Keshtar, A.; Lee, J.; Hamdy, F. Electrical impedance spectroscopy and the diagnosis of bladder pathology: A pilot study. J. Urol. 2002, 168, 1563–1567. [Google Scholar] [CrossRef]
- Keshtkar, A.; Salehnia, Z.; Keshtkar, A.; Shokouhi, B. Bladder cancer detection using electrical impedance technique (Tabriz Mark 1). Pathol. Res. Int. 2012, 2012, 470101. [Google Scholar] [CrossRef]
- Močkus, J. On Bayesian methods for seeking the extremum. In Optimization Techniques, Proceedings of the IFIP Technical Conference, Novosibirsk, Russia, 1–7 July 1974; Springer: Berlin/Heidelberg, Germany, 1975; pp. 400–404. [Google Scholar]
- Itakura, K.; Saito, Y.; Suzuki, T.; Kondo, N.; Hosoi, F. Estimation of citrus maturity with fluorescence spectroscopy using deep learning. Horticulturae 2018, 5, 2. [Google Scholar] [CrossRef]
- Schackart III, K.E.; Yoon, J.-Y. Machine learning enhances the performance of bioreceptor-free biosensors. Sensors 2021, 21, 5519. [Google Scholar] [CrossRef]






| LR | KNN | DT | RF | SVM | BPNN | |
|---|---|---|---|---|---|---|
| Accuracy | 0.771 | 0.854 | 0.896 | 0.917 * | 0.917 * | 0.896 |
| Sensitivity | 0.851 | 1.000 * | 0.929 | 0.929 | 0.964 | 0.958 |
| Precision | 0.774 | 0.800 | 0.897 | 0.929 * | 0.900 | 0.852 |
| Specificity | 0.650 | 0.650 | 0.850 | 0.900 * | 0.850 | 0.833 |
| F1-score | 0.814 | 0.889 | 0.912 | 0.929 | 0.931 * | 0.902 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.-J.; Kim, K.; Kim, H.W.; Park, Y. Classification between Normal and Cancerous Human Urothelial Cells by Using Micro-Dimensional Electrochemical Impedance Spectroscopy Combined with Machine Learning. Sensors 2022, 22, 7969. https://doi.org/10.3390/s22207969
Jeong H-J, Kim K, Kim HW, Park Y. Classification between Normal and Cancerous Human Urothelial Cells by Using Micro-Dimensional Electrochemical Impedance Spectroscopy Combined with Machine Learning. Sensors. 2022; 22(20):7969. https://doi.org/10.3390/s22207969
Chicago/Turabian StyleJeong, Ho-Jung, Kihyun Kim, Hyeon Woo Kim, and Yangkyu Park. 2022. "Classification between Normal and Cancerous Human Urothelial Cells by Using Micro-Dimensional Electrochemical Impedance Spectroscopy Combined with Machine Learning" Sensors 22, no. 20: 7969. https://doi.org/10.3390/s22207969
APA StyleJeong, H.-J., Kim, K., Kim, H. W., & Park, Y. (2022). Classification between Normal and Cancerous Human Urothelial Cells by Using Micro-Dimensional Electrochemical Impedance Spectroscopy Combined with Machine Learning. Sensors, 22(20), 7969. https://doi.org/10.3390/s22207969





