Simultaneous Absorbance and Fluorescence Measurements Using an Inlaid Microfluidic Approach
Abstract
:1. Introduction
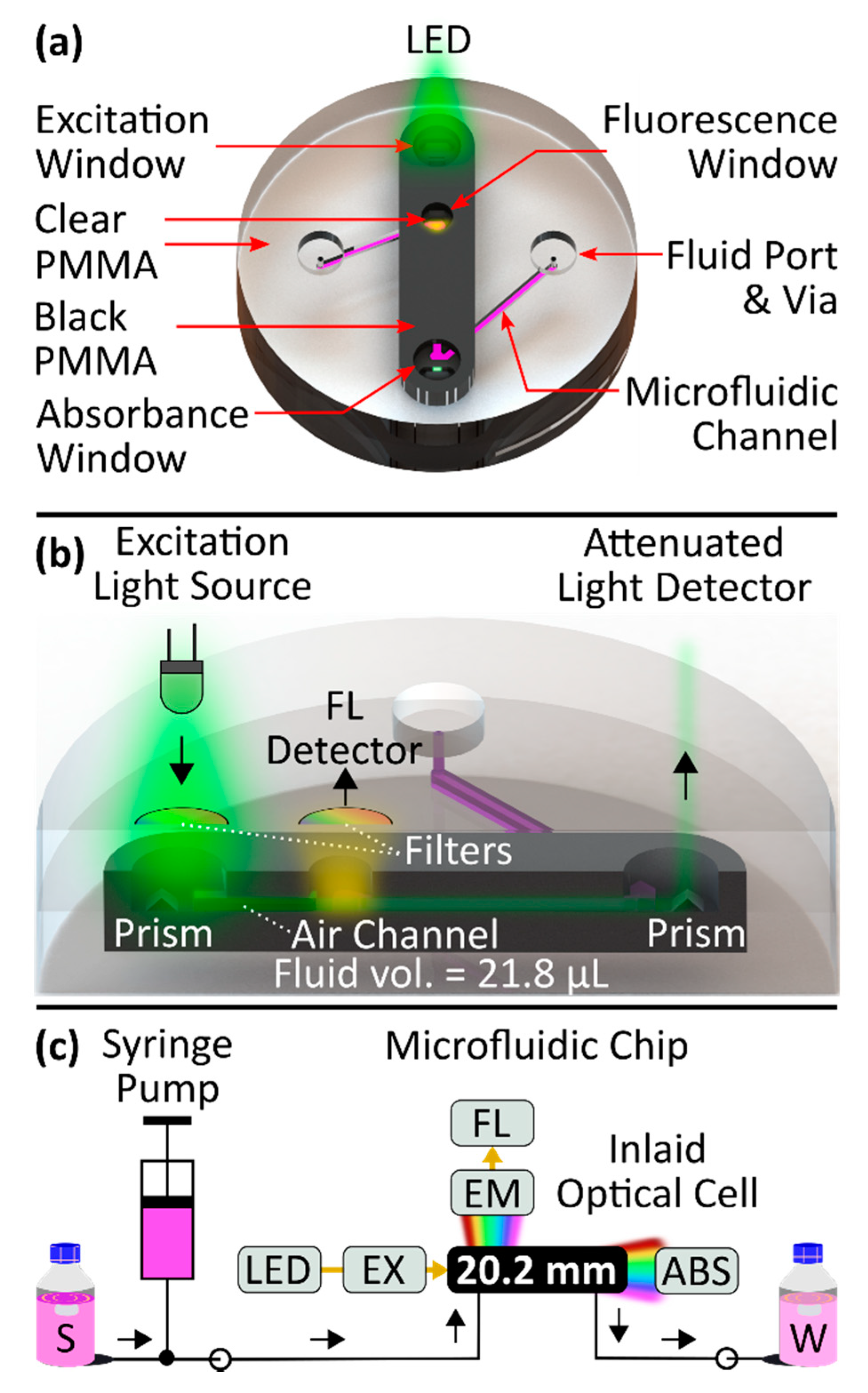
Novel Flow Cell Design
2. Materials and Methods
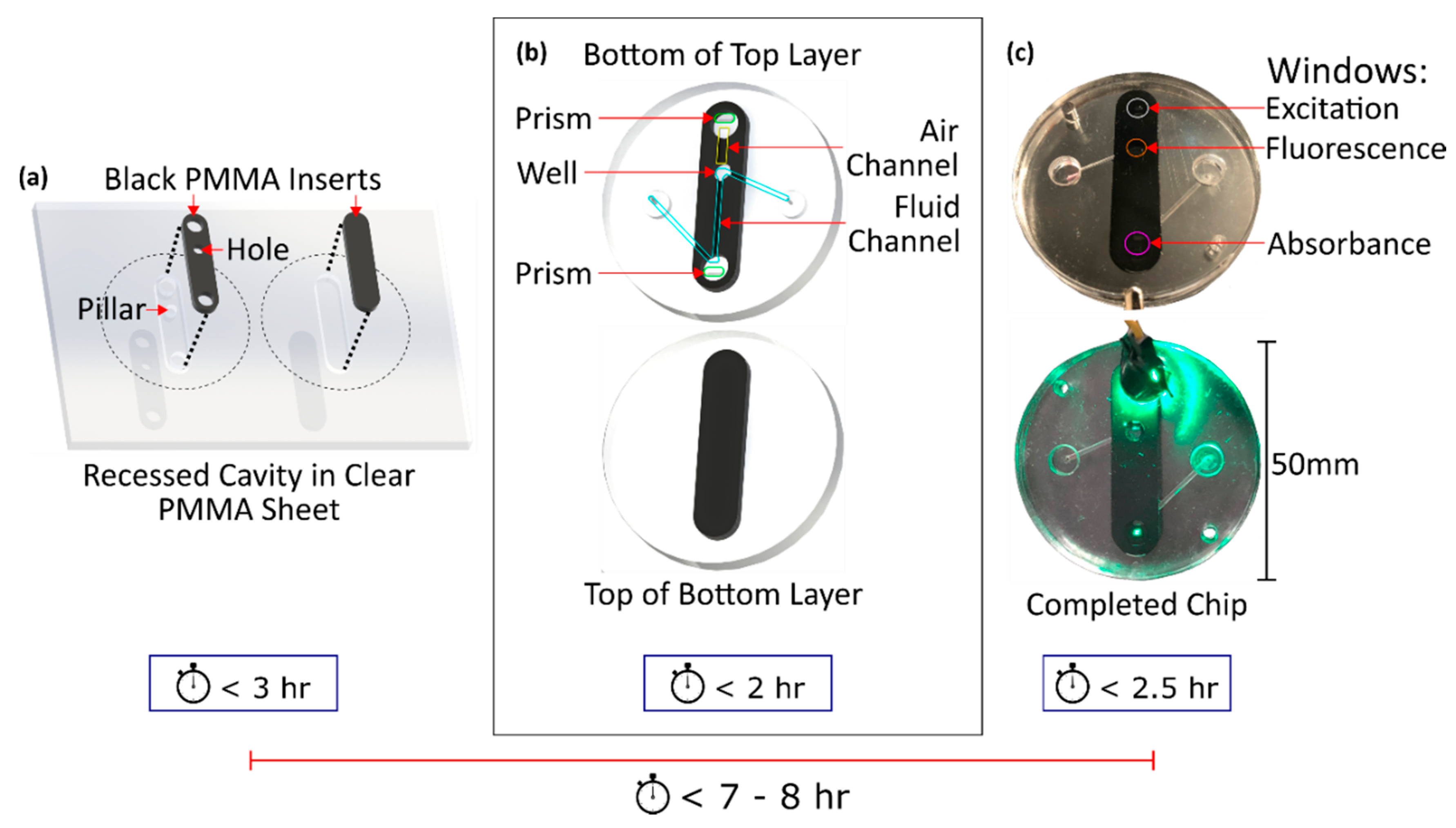
2.1. Chip Fabrication
2.2. Chemistry
2.3. Experimental Setup
2.4. Analytical
3. Results and Discussion
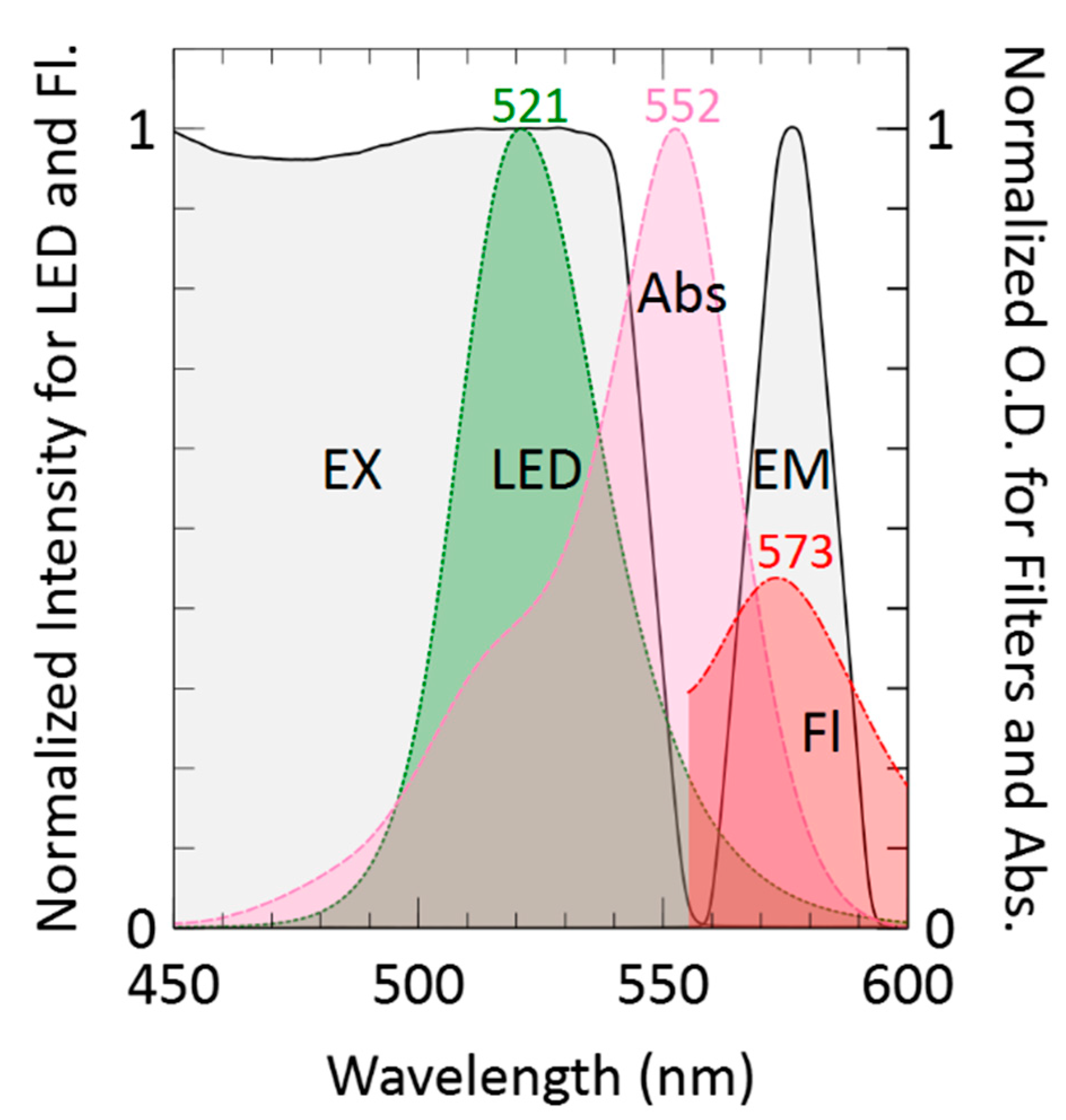
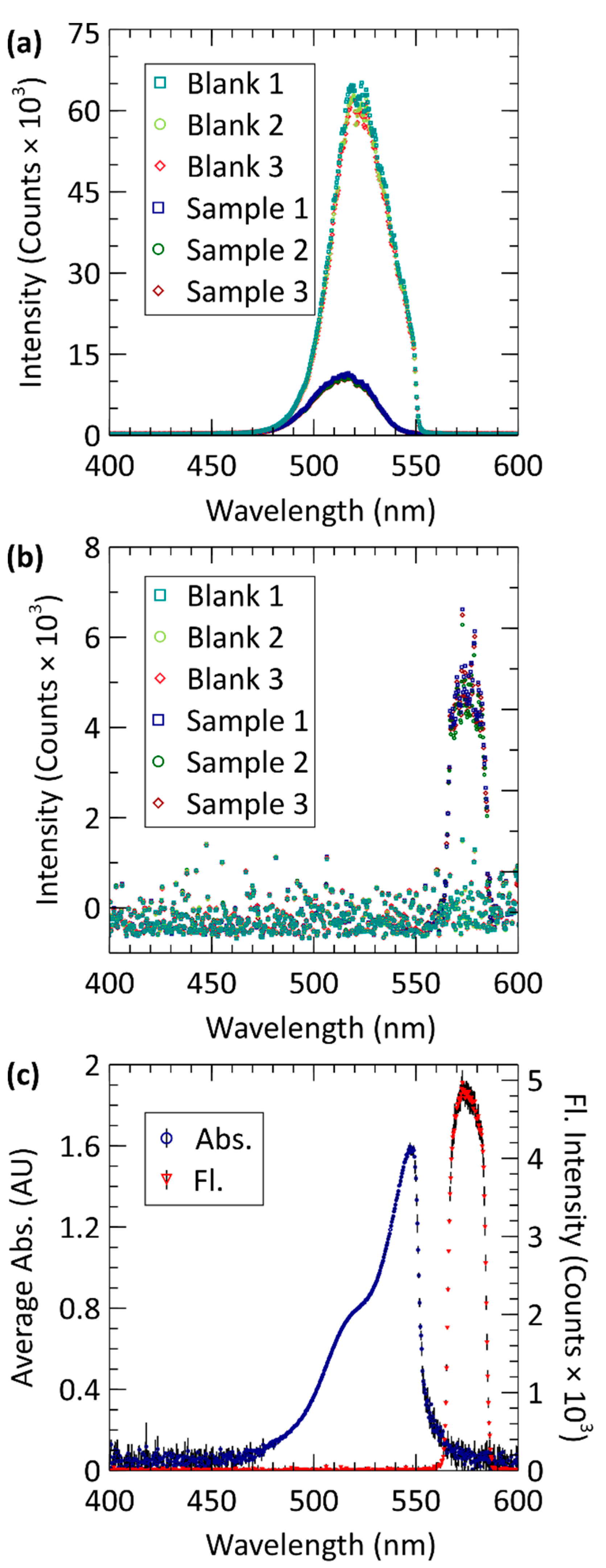
3.1. Absorbance and Fluorescence Spectra
3.2. Calibration
3.3. Detection Limits
3.4. Precision
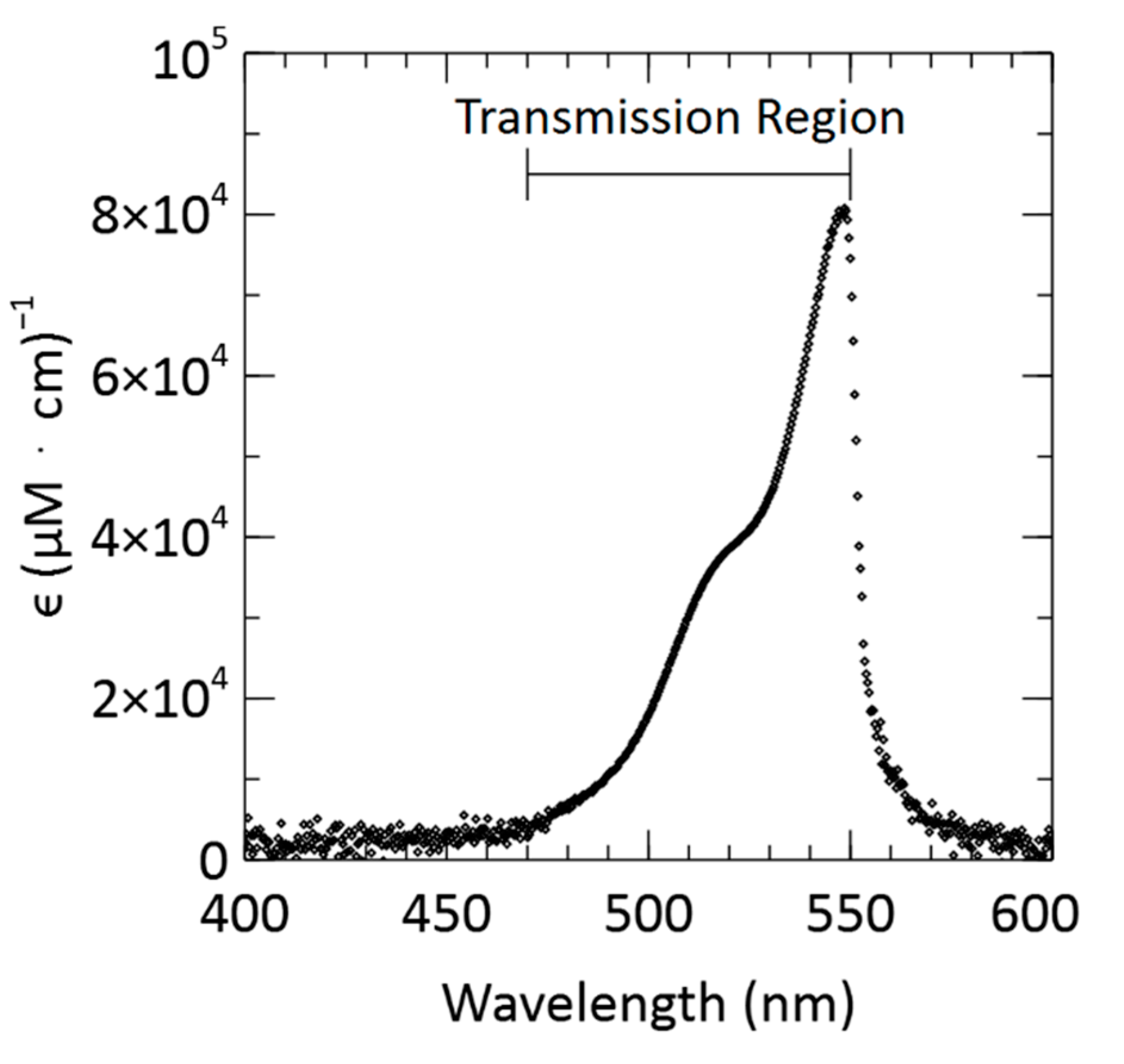
3.5. Attenuation Coefficient
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaywant, S.A.; Arif, K.M. A Comprehensive Review of Microfluidic Water Quality Monitoring Sensors. Sensors 2019, 19, 4781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanfar, M.F.; Al-Faqheri, W.; Al-Halhouli, A. Low Cost Lab on Chip for the Colorimetric Detection of Nitrate in Mineral Water Products. Sensors 2017, 17, 2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaton, A.D.; Cardwell, C.L.; Thomas, R.S.; Sieben, V.J.; Legiret, F.-E.; Waugh, E.M.; Statham, P.J.; Mowlem, M.C.; Morgan, H. Lab-on-Chip Measurement of Nitrate and Nitrite for In Situ Analysis of Natural Waters. Environ. Sci. Technol. 2012, 46, 9548–9556. [Google Scholar] [CrossRef] [PubMed]
- Grand, M.M.; Clinton-Bailey, G.S.; Beaton, A.D.; Schaap, A.M.; Johengen, T.H.; Tamburri, M.N.; Connelly, D.P.; Mowlem, M.C.; Achterberg, E.P. A Lab-On-Chip Phosphate Analyzer for Long-term In Situ Monitoring at Fixed Observatories: Optimization and Performance Evaluation in Estuarine and Oligotrophic Coastal Waters. Front. Mar. Sci. 2017, 4, 255. [Google Scholar] [CrossRef] [Green Version]
- Legiret, F.-E.; Sieben, V.J.; Woodward, E.M.S.; Bey, S.K.A.K.; Mowlem, M.C.; Connelly, D.P.; Achterberg, E.P. A high performance microfluidic analyser for phosphate measurements in marine waters using the vanadomolybdate method. Talanta 2013, 116, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Clinton-Bailey, G.S.; Grand, M.M.; Beaton, A.D.; Nightingale, A.M.; Owsianka, D.R.; Slavik, G.J.; Connelly, D.P.; Cardwell, C.L.; Mowlem, M.C. A Lab-on-Chip Analyzer for in situ Measurement of Soluble Reactive Phosphate: Improved Phosphate Blue Assay and Application to Fluvial Monitoring. Environ. Sci. Technol. 2017, 51, 9989–9995. [Google Scholar] [CrossRef]
- Tokel, O.; Yildiz, U.H.; Inci, F.; Durmus, N.G.; Ekiz, O.O.; Turker, B.; Cetin, C.; Rao, S.; Sridhar, K.; Natarajan, N.; et al. Portable Microfluidic Integrated Plasmonic Platform for Pathogen Detection. Sci. Rep. 2015, 5, 9152. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, Y.; Wei, J.; Xu, J.; Wang, Y.; Zheng, G. Paper-based three-dimensional microfluidic device for monitoring of heavy metals with a camera cell phone. Anal. Bioanal. Chem. 2014, 406, 2799–2807. [Google Scholar] [CrossRef]
- Chen, W.; Fang, X.; Li, H.; Cao, H.; Kong, J. A Simple Paper-Based Colorimetric Device for Rapid Mercury(II) Assay. Sci. Rep. 2016, 6, 31948. [Google Scholar] [CrossRef]
- Fan, C.; He, S.; Liu, G.; Wang, L.; Song, S. A portable and power-free microfluidic device for rapid and sensitive lead (Pb2+) detection. Sensors 2012, 12, 9467–9475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gencoglu, A.; Minerick, A.R. Electrochemical detection techniques in micro- and nanofluidic devices. Microfluid. Nanofluidics 2014, 17, 781–807. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Zeino, A.; Umoren, S.A. Electrochemical noise (EN) technique: Review of recent practical applications to corrosion electrochemistry research. J. Adhes. Sci. Technol. 2019, 33, 1453–1496. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nuriman; Huskens, J.; Verboom, W. Optical sensing systems for microfluidic devices: A review. Anal. Chim. Acta 2007, 601, 141–155. [Google Scholar] [CrossRef]
- Lijing, Z.; Zakoldaev, R.A.; Sergeev, M.M.; Veiko, V.P. Fluorescent Bulk Waveguide Sensor in Porous Glass: Concept, Fabrication, and Testing. Nanomaterials 2020, 10, 2169. [Google Scholar] [CrossRef]
- Lapointe, J.; Parent, F.; de Lima Filho, E.S.; Loranger, S.; Kashyap, R. Toward the integration of optical sensors in smartphone screens using femtosecond laser writing. Opt. Lett. 2015, 40, 5654–5657. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Hu, S.; Peng, Y. Applications of fiber-optic biochemical sensor in microfluidic chips: A review. Biosens. Bioelectron. 2020, 166, 112447. [Google Scholar] [CrossRef]
- Mogensen, K.B.; Kutter, J.P. Optical detection in microfluidic systems. Electrophoresis 2009, 30, S92–S100. [Google Scholar] [CrossRef]
- Okubo, K.; Uchiyamada, K.; Asakawa, K.; Suzuki, H. Silicon nitride directional coupler interferometer for surface sensing. Opt. Eng. 2017, 56, 017101. [Google Scholar] [CrossRef]
- Adams, M.L.; Quake, S.; Scherer, A. On-chip absorption and fluorescence spectroscopy with polydimethylsiloxane (PDMS) microfluidic flow channels. In Proceedings of the 2nd Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology, Cat. No.02EX578, Madison, WI, USA, 2–4 May 2002; pp. 369–373. [Google Scholar]
- Psaltis, D.; Quake, S.R.; Yang, C. Developing optofluidic technology through the fusion of microfluidics and optics. Nature 2006, 442, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, P.D.; Ordeig, O.; Mogensen, K.B.; Kutter, J.P. Electrophoresis microchip with integrated waveguides for simultaneous native UV fluorescence and absorbance detection. Electrophoresis 2009, 30, 4172–4178. [Google Scholar] [CrossRef]
- Hengoju, S.; Wohlfeil, S.; Munser, A.S.; Boehme, S.; Beckert, E.; Shvydkiv, O.; Tovar, M.; Roth, M.; Rosenbaum, M.A. Optofluidic detection setup for multi-parametric analysis of microbiological samples in droplets. Biomicrofluidics 2020, 14, 024109. [Google Scholar] [CrossRef]
- Yang, T.; Stavrakis, S.; deMello, A. A High-Sensitivity, Integrated Absorbance and Fluorescence Detection Scheme for Probing Picoliter-Volume Droplets in Segmented Flows. Anal. Chem. 2017, 89, 12880–12887. [Google Scholar] [CrossRef]
- Yamada, Y.; Hanawa, F.; Kitoh, T.; Maruno, T. Low-loss and stable fiber-to-waveguide connection utilizing UV curable adhesive. IEEE Photonics Technol. Lett. 1992, 4, 906–908. [Google Scholar] [CrossRef]
- Floquet, C.F.A.; Sieben, V.J.; Milani, A.; Joly, E.P.; Ogilvie, I.R.G.; Morgan, H.; Mowlem, M.C. Nanomolar detection with high sensitivity microfluidic absorption cells manufactured in tinted PMMA for chemical analysis. Talanta 2011, 84, 235–239. [Google Scholar] [CrossRef]
- Milani, A.; Statham, P.; Mowlem, M.; Connelly, D. Development and application of a microfluidic in-situ analyzer for dissolved Fe and Mn in natural waters. Talanta 2015, 136, 15–22. [Google Scholar] [CrossRef]
- Luy, E.A.; Morgan, S.C.; Creelman, J.J.; Murphy, B.J.; Sieben, V.J. Inlaid microfluidic optics: Absorbance cells in clear devices applied to nitrite and phosphate detection. J. Micromech. Microeng. 2020, 30, 15. [Google Scholar] [CrossRef]
- Sieben, V.J.; Floquet, C.F.A.; Ogilvie, I.R.G.; Mowlem, M.C.; Morgan, H. Microfluidic colourimetric chemical analysis system: Application to nitrite detection. Anal. Methods 2010, 2, 484–491. [Google Scholar] [CrossRef]
- Rushworth, C.M.; Jones, G.; Fischlechner, M.; Walton, E.; Morgan, H. On-chip cavity-enhanced absorption spectroscopy using a white light-emitting diode and polymer mirrors. Lab Chip 2015, 15, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.M.; Harris, A.; Ryan, J.P.; Roman, B.; Marin, R., III; Jensen, S.; Everlove, C.; Birch, J.; Dzenitis, J.M.; Pargett, D.; et al. Underwater Application of Quantitative PCR on an Ocean Mooring. PLoS ONE 2011, 6, e22522. [Google Scholar] [CrossRef] [Green Version]
- Macintyre, H.; Lawrenz, E.; Richardson, T. Taxonomic Discrimination of Phytoplankton by Spectral Fluorescence. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Suggett, D.J., Prášil, O., Borowitzka, M.A., Eds.; Springer: Dordrecht, The Netherlands, 1970; pp. 129–169. ISBN 978-90-481-9267-0. [Google Scholar]
- MacIntyre, H.L.; Cullen, J.J.; Whitsitt, T.J.; Petri, B. Enumerating viable phytoplankton using a culture-based Most Probable Number assay following ultraviolet-C treatment. J. Appl. Phycol. 2018, 30, 1073–1094. [Google Scholar] [CrossRef] [Green Version]
- Macintyre, H.; Cullen, J. Classification of phytoplankton cells as live or dead using the vital stains fluorescein diacetate and 5-chloromethylfluorescein diacetate (FDA and CMFDA). J. Phycol. 2016, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morançais, M.; Mouget, J.-L.; Dumay, J. Chapter 7-Proteins and Pigments. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 145–175. ISBN 978-0-12-811405-6. [Google Scholar]
- Kok, J.W.K.; Yeo, D.C.J.; Leong, S.C.Y. Growth, pigment, and chromophoric dissolved organic matter responses of tropical Chattonella subsalsa (Raphidophyceae) to nitrogen enrichment. Phycol. Res. 2019, 67, 134–144. [Google Scholar] [CrossRef]
- Para, J.; Coble, P.G.; Charrière, B.; Tedetti, M.; Fontana, C.; Sempéré, R. Fluorescence and absorption properties of chromophoric dissolved organic matter (CDOM) in coastal surface waters of the northwestern Mediterranean Sea, influence of the Rhône River. Biogeosciences 2010, 7, 4083–4103. [Google Scholar] [CrossRef] [Green Version]
- Nelson, N.B.; Gauglitz, J.M. Optical Signatures of Dissolved Organic Matter Transformation in the Global Ocean. Front. Mar. Sci. 2016, 2, 118. [Google Scholar] [CrossRef] [Green Version]
- Birdwell, J.E.; Engel, A.S. Characterization of dissolved organic matter in cave and spring waters using UV–Vis absorbance and fluorescence spectroscopy. Org. Geochem. 2010, 41, 270–280. [Google Scholar] [CrossRef]
- Xiaoling, Z.; Gaofang, Y.; Nanjing, Z.; Ruifang, Y.; Jianguo, L.; Wenqing, L. Chromophoric dissolved organic matter influence correction of algal concentration measurements using three-dimensional fluorescence spectra. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2019, 210, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Grumann, M.; Moser, I.; Steigert, J.; Riegger, L.; Geipel, A.; Kohn, C.; Urban, G.; Zengerle, R.; Ducree, J. Optical beam guidance in monolithic polymer chips for miniaturized colorimetric assays. In Proceedings of the 18th IEEE International Conference on Micro Electro Mechanical Systems, MEMS 2005, Miami Beach, FL, USA, 30 January–3 February 2005; pp. 108–111. [Google Scholar]
- Ogilvie, I.R.G.; Sieben, V.; Floquet, C.F.A.; Zmijan, R.; Mowlem, M.; Morgan, H. Solvent processing of PMMA and COC chips for bonding devices with optical quality surfaces. In Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences 2010, MicroTAS 2010, Groningen, The Netherlands, 3–7 October 2010; Volume 2, pp. 1244–1246. [Google Scholar]
- Beija, M.; Afonso, C.A.M.; Martinho, J.M.G. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 2009, 38, 2410–2433. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, A. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Murphy, B.; Luy, E.; Panzica, K.; Johnson, G.; Sieben, V. An Energy Efficient Thermally Regulated Optical Spectroscopy Cell for Lab-on-Chip Devices: Applied to Nitrate Detection. Micromachines 2021, 12, 861. [Google Scholar] [CrossRef]
- Pais, A.; Banerjee, A.; Klotzkin, D.; Papautsky, I. High-sensitivity, disposable lab-on-a-chip with thin-film organic electronics for fluorescence detection. Lab. Chip 2008, 8, 794–800. [Google Scholar] [CrossRef]
- Banerjee, A.; Shuai, Y.; Dixit, R.; Papautsky, I.; Klotzkin, D. Concentration dependence of fluorescence signal in a microfluidic fluorescence detector. J. Lumin. 2010, 130, 1095–1100. [Google Scholar] [CrossRef]
- Lepot, M.; Momplot, A.; Lipeme Kouyi, G.; Bertrand-Krajewski, J.-L. Rhodamine WT tracer experiments to check flow measurements in sewers. Flow Meas. Instrum. 2014, 40, 28–38. [Google Scholar] [CrossRef]
- Carroll, M.; Chigounis, D.; Gilbert, S.; Gundersen, K.; Hayashi, K.; Janzen, C.; Johengen, T.; Koles, T.; Laurier, F.; McKissack, T.; et al. Performance Verification Statement for the TURNER Designs CYCLOPS-7 fluorometer. Alliance Coast. Technol. 2005, 37. [Google Scholar] [CrossRef]
- Miyaki, K.; Guo, Y.; Shimosaka, T.; Nakagama, T.; Nakajima, H.; Uchiyama, K. Fabrication of an integrated PDMS microchip incorporating an LED-induced fluorescence device. Anal. Bioanal. Chem. 2005, 382, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Yang, Y.; Chen, H. A Unique Class of Near-Infrared Functional Fluorescent Dyes with Carboxylic-Acid-Modulated Fluorescence ON/OFF Switching: Rational Design, Synthesis, Optical Properties, Theoretical Calculations, and Applications for Fluorescence Imaging in Living Animals. J. Am. Chem. Soc. 2012, 134, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Lindsey, J. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for use in PhotochemCAD. Photochem. Photobiol. 2017, 94, 290–327. [Google Scholar] [CrossRef] [Green Version]
- Ogilvie, I.R.G.; Sieben, V.; Cortese, B.; Mowlem, M.; Morgan, H. Chemically resistant microfluidic valves from Viton (R) membranes bonded to COC and PMMA. Lab. Chip 2011, 11, 2455–2459. [Google Scholar] [CrossRef] [PubMed]
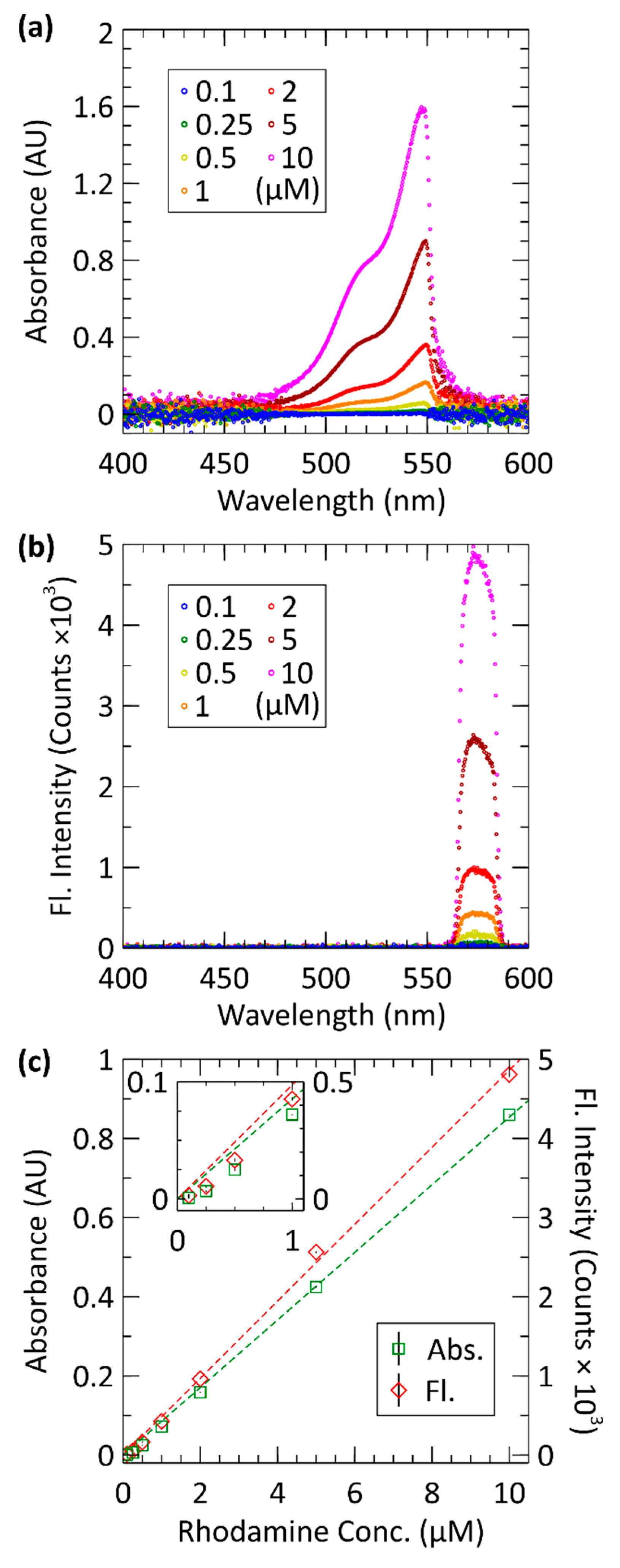
| Conc. (µM) | Absorbance (AU) | Fluorescence (Counts) |
|---|---|---|
| 0.1 | 0.0009 ± 0.0008 | 15 ± 7 |
| 0.25 | 0.0067 ± 0.003 | 54 ± 14 |
| 0.5 | 0.0250 ± 0.003 | 166 ± 14 |
| 1 | 0.0720 ± 0.0002 | 426 ± 8.4 |
| 2 | 0.1587 ± 0.004 | 963 ± 4.4 |
| 5 | 0.425 ± 0.008 | 2563 ± 45 |
| 10 | 0.8597 ± 0.004 | 4806 ± 125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Creelman, J.J.; Luy, E.A.; Beland, G.C.H.; Sonnichsen, C.; Sieben, V.J. Simultaneous Absorbance and Fluorescence Measurements Using an Inlaid Microfluidic Approach. Sensors 2021, 21, 6250. https://doi.org/10.3390/s21186250
Creelman JJ, Luy EA, Beland GCH, Sonnichsen C, Sieben VJ. Simultaneous Absorbance and Fluorescence Measurements Using an Inlaid Microfluidic Approach. Sensors. 2021; 21(18):6250. https://doi.org/10.3390/s21186250
Chicago/Turabian StyleCreelman, Joshua J., Edward A. Luy, Gabryelle C. H. Beland, Colin Sonnichsen, and Vincent J. Sieben. 2021. "Simultaneous Absorbance and Fluorescence Measurements Using an Inlaid Microfluidic Approach" Sensors 21, no. 18: 6250. https://doi.org/10.3390/s21186250
APA StyleCreelman, J. J., Luy, E. A., Beland, G. C. H., Sonnichsen, C., & Sieben, V. J. (2021). Simultaneous Absorbance and Fluorescence Measurements Using an Inlaid Microfluidic Approach. Sensors, 21(18), 6250. https://doi.org/10.3390/s21186250






