Robotic Biofeedback for Post-Stroke Gait Rehabilitation: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
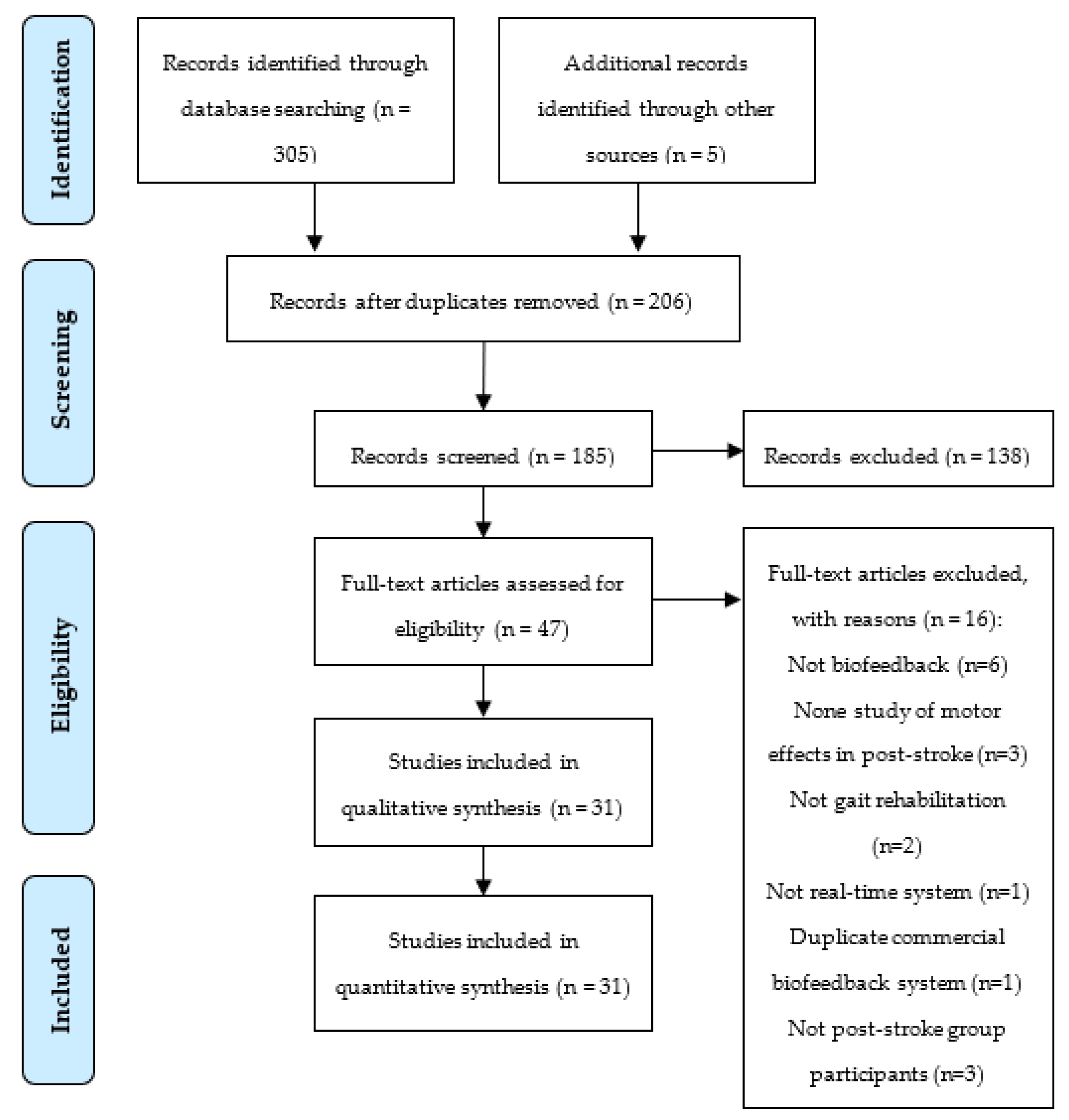
2.2. Study Selection
2.3. Data Extraction and Synthesis
2.4. Methodological Quality Assessment
3. Results
3.1. Technical Specifications
3.1.1. Sensors and Biofeedback Parameters
3.1.2. Actuators and Biofeedback Mode
3.1.3. Biofeedback Control Strategies
3.1.4. Assistive Devices Used in Adjunction with BSs
3.1.5. Physiotherapist-Oriented Sensory Cues
3.2. Clinical Specifications
3.2.1. Post-Stroke Participants
3.2.2. Study Design
3.2.3. Clinical Protocols
3.2.4. Sensor-Based and Clinical Outcomes
3.2.5. BS Effects on Post-Stroke Recovery
3.3. Methodological Quality Assessment
4. Discussion
4.1. Technical Specifications
4.2. Clinical Specifications
4.3. Future Directions and Challenges to Overcome
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 5XSST | Five Times Sit-to-Stand Test |
| 9HPT | Nine-Hole Peg Test |
| ARAT | Action Research Arm Test |
| BBS | Berg Balance Scale |
| BBT | Box and Block Test |
| BDI | Beck Depression Inventory |
| BI | Barthel Index |
| BS | biofeedback systems |
| CESD | Center for Epidemiologic Studies Depression Scale |
| DGI | Dynamic Gait Index |
| EPHPP | Effective Public Health Practice Project |
| EEG | electroencephalography |
| EMG | electromyographic |
| ESS | European Stroke Scale |
| FAC | Functional Ambulation Category |
| FES | Functional Electrical Stimulation |
| FGA | Functional Gait Assessment |
| FIM | Functional Independence Measure |
| FM | Fugl–Meyer |
| fNIRS | functional near-infrared spectroscopy |
| FRT | Functional Reach Test |
| GDS | Geriatric Depression Scale |
| HADS | Hospital Anxiety and Depression Scales |
| HAI | Hauser Ambulance Index |
| IAB | Individual Alpha-Band |
| IADL | Instrumental Activities of Daily Living |
| IMU | Inertial Measurement Unit |
| KIINCE | Kinetic Immersive Interface for Neuromuscular Coordination Enhancement |
| LOS | Limit of Stability |
| MAL | Motor Activity Log |
| MAS | Modified Ashworth Scale |
| MMT | Manual Muscle Test |
| MoCA | Montreal Cognitive Assessment |
| MRC | Medical Research Council |
| MRI | Magnetic Resonance Imaging |
| NASA-TLX | National Aeronautics and Space Administration Task Load Index |
| PP | Pin Prick |
| QCM | Questionnaire for Current Motivation |
| QUEST | Quebec User Evaluation of Satisfaction with Assistive Technology |
| RCS | Relationship Change Scale |
| SBT | Standing Balance Test |
| SMR | sensorimotor rhythm |
| SMA | supplementary motor area |
| TGA | Tinetti Gait Assessment |
| TIS | Trunk Impairment Scale |
| TUG | Timed Up and Go |
| VAS | Visual Analogue Scale |
| WBLT | Weight-Bearing Lunge Test |
| WISCI | Walking Index for Spinal Cord Injury |
References
- Luengo-Fernandez, R.; Leal, J.; Candio, P.; Violato, M.; Stroke Alliance for Europe. Economic Impact of Stroke. 2019. Available online: https://www.safestroke.eu/economic-impact-of-stroke/ (accessed on 24 April 2020).
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef]
- Wolf, S.L. Electromyographic Biofeedback Applications to Stroke Patients. Phys. Ther. 1983, 63, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wolf, S.L.; He, J. Recent developments in biofeedback for neuromotor rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- FlintRehab. Neuroplasticity After Stroke: The Single Most Important Key to Recovery. 2018. Available online: https://www.flintrehab.com/2018/neuroplasticity-after-stroke/ (accessed on 27 May 2019).
- Association for Applied Psychophysiology and Biofeedback. Standards for Performing Biofeedback. 2013. Available online: https://www.aapb.org/i4a/pages/index.cfm?pageid=3678#I (accessed on 20 March 2020).
- van Gelder, L.M.A.; Barnes, A.; Wheat, J.S.; Heller, B.W. The use of biofeedback for gait retraining: A mapping review. Clin. Biomech. 2018, 59, 159–166. [Google Scholar] [CrossRef]
- Stanton, R.; Ada, L.; Dean, C.M.; Preston, E. Biofeedback improves performance in lower limb activities more than usual therapy in people following stroke: A systematic review. J. Physiother. 2017, 63, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bowman, T.; Gervasoni, E.; Arienti, C.; Lazzarini, S.G.; Negrini, S.; Crea, S.; Cattaneo, D.; Carrozza, M.C. Wearable Devices for Biofeedback Rehabilitation: A Systematic Review and Meta-Analysis to Design Application Rules and Estimate the Effectiveness on Balance and Gait Outcomes in Neurological Diseases. Sensors 2021, 21, 3444. [Google Scholar] [CrossRef]
- Spencer, J.; Wolf, S.L.; Kesar, T.M. Biofeedback for Post-stroke Gait Retraining: A Review of Current Evidence and Future Research Directions in the Context of Emerging Technologies. Front. Neurol. 2021, 12, 637199. [Google Scholar] [CrossRef]
- Mikolajczyk, T.; Ciobanu, I.; Badea, D.I.; Iliescu, A.; Pizzamiglio, S.; Schauer, T.; Seel, T.; Seiciu, P.L.; Turner, D.L.; Berteanu, M. Advanced technology for gait rehabilitation: An overview. Adv. Mech. Eng. 2018, 10, 1–19. [Google Scholar] [CrossRef]
- Perry, J. Gait Analysis: Normal and Pathological Function; SLACK Incorporated: West Deptford, NJ, USA, 1992. [Google Scholar]
- Morone, G.; Spitoni, G.F.; de Bartolo, D.; Ghooshchy, S.G.; di Iulio, F.; Paolucci, S.; Zoccolotti, P.; Iosa, M. Rehabilitative devices for a top-down approach. Expert Rev. Med. Devices 2019, 16, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, G.; Takagi, A.; Osu, R.; Yoshioka, T.; Kawato, M.; Burdet, E. Two is better than one: Physical interactions improve motor performance in humans. Sci. Rep. 2015, 4, 3824. [Google Scholar] [CrossRef]
- Effective Public Healthcare Panacea Project. Quality Assessment Tool for Quantitative Studies. Available online: https://www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/ (accessed on 25 January 2020).
- Ma, C.Z.-H.; Zheng, Y.-P.; Lee, W.C.-C. Changes in gait and plantar foot loading upon using vibrotactile wearable biofeedback system in patients with stroke. Top. Stroke Rehabil. 2018, 25, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Genthe, K.; Schenck, C.; Eicholtz, S.; Zajac-Cox, L.; Wolf, S.; Kesar, T.M. Effects of real-time gait biofeedback on paretic propulsion and gait biomechanics in individuals post-stroke. Top. Stroke Rehabil. 2018, 25, 186–193. [Google Scholar] [CrossRef]
- Afzal, M.R.; Lee, H.; Eizad, A.; Lee, C.H.; Oh, M.-K.; Yoon, J. Effects of Vibrotactile Biofeedback Coding Schemes on Gait Symmetry Training of Individuals With Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1617–1625. [Google Scholar] [CrossRef]
- Khoo, I.-H.; Marayong, P.; Krishnan, V.; Balagtas, M.; Rojas, O.; Leyba, K. Real-time biofeedback device for gait rehabilitation of post-stroke patients. Biomed. Eng. Lett. 2017, 7, 287–298. [Google Scholar] [CrossRef]
- Nan, W.; Dias, A.P.B.; Rosa, A.C. Neurofeedback Training for Cognitive and Motor Function Rehabilitation in Chronic Stroke: Two Case Reports. Front. Neurol. 2019, 10, 800. [Google Scholar] [CrossRef]
- Arpa, S.; Ozcakir, S. Does electromyographic biofeedback improve exercise effects in hemiplegic patients? A pilot randomized controlled trial. J. Rehabil. Med. 2019, 51, 109–112. [Google Scholar] [CrossRef]
- Tamburella, F.; Moreno, J.C.; Valenzuela, D.S.H.; Pisotta, I.; Iosa, M.; Cincotti, F.; Mattia, D.; Pons, J.L.; Molinari, M. Influences of the biofeedback content on robotic post-stroke gait rehabilitation: Electromyographic vs. joint torque biofeedback. J. Neuroeng. Rehabil. 2019, 16, 95. [Google Scholar] [CrossRef]
- Day, K.A.; Cherry-Allen, K.M.; Bastian, A.J. Individualized feedback to change multiple gait deficits in chronic stroke. J. Neuroeng. Rehabil. 2019, 16, 158. [Google Scholar] [CrossRef]
- Guzik, A.; Drużbicki, M.; Kwolek, A.; Przysada, G.; Brzozowska-Magoń, A.; Wolan-Nieroda, A.; Ćwirlej-Sozańska, A.; Wiśniowska-Szurlej, A.; Wyszyńska, J. Analysis of the association between selected factors and outcomes of treadmill gait training with biofeedback in patients with chronic stroke. J. Back Musculoskelet. Rehabil. 2020, 33, 159–168. [Google Scholar] [CrossRef]
- Dost Sürücü, G.; Tezen, Ö. The effect of EMG biofeedback on lower extremity functions in hemiplegic patients. Acta Neurol. Belg. 2021, 121, 113–118. [Google Scholar] [CrossRef]
- Ochi, M.; Wada, F.; Saeki, S.; Hachisuka, K. Gait training in subacute non-ambulatory stroke patients using a full weight-bearing gait-assistance robot: A prospective, randomized, open, blinded-endpoint trial. J. Neurol. Sci. 2015, 353, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lee, J.; Lee, B.-H. Effect of an EMG–FES Interface on Ankle Joint Training Combined with Real-Time Feedback on Balance and Gait in Patients with Stroke Hemiparesis. Healthcare 2020, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-J.; Kim, J.; Roth, E.J.; Rymer, W.Z.; Wu, M. Use of Pelvic Corrective Force With Visual Feedback Improves Paretic Leg Muscle Activities and Gait Performance After Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Givon, N.; Zeilig, G.; Weingarden, H.; Rand, D. Video-games used in a group setting is feasible and effective to improve indicators of physical activity in individuals with chronic stroke: A randomized controlled trial. Clin. Rehabil. 2016, 30, 383–392. [Google Scholar] [CrossRef]
- Nagano, H.; Said, C.M.; James, L.; Begg, R.K. Feasibility of Using Foot–Ground Clearance Biofeedback Training in Treadmill Walking for Post-Stroke Gait Rehabilitation. Brain Sci. 2020, 10, 978. [Google Scholar] [CrossRef]
- Byl, N.; Zhang, W.; Coo, S.; Tomizuka, M. Clinical impact of gait training enhanced with visual kinematic biofeedback: Patients with Parkinson’s disease and patients stable post stroke. Neuropsychologia 2015, 79, 332–343. [Google Scholar] [CrossRef]
- Jung, K.-S.; Bang, H.; In, T.-S.; Cho, H.-Y. Gait training with auditory feedback improves trunk control, muscle activation and dynamic balance in patients with hemiparetic stroke: A randomized controlled pilot study. J. Back Musculoskelet. Rehabil. 2020, 33, 1–6. [Google Scholar] [CrossRef]
- Shin, J.; Chung, Y. Influence of visual feedback and rhythmic auditory cue on walking of chronic stroke patient induced by treadmill walking in real-time basis. NeuroRehabilitation 2017, 41, 445–452. [Google Scholar] [CrossRef]
- Song, G.B.; Park, E.C. Effect of virtual reality games on stroke patients’ balance, gait, depression, and interpersonal relationships. J. Phys. Ther. Sci. 2015, 27, 2057–2060. [Google Scholar] [CrossRef]
- Kim, J.-S.; Oh, D.-W. Use of real-time visual feedback during overground walking training on gait symmetry and velocity in patients with post-stroke hemiparesis: Randomized controlled, single-blind study. Int. J. Rehabil. Res. 2020, 43, 247–254. [Google Scholar] [CrossRef]
- Jung, J.; Choi, W.; Lee, S. Immediate augmented real-time forefoot weight bearing using visual feedback improves gait symmetry in chronic stroke. Technol. Health Care 2020, 28, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Mottaz, A.; Corbet, T.; Doganci, N.; Magnin, C.; Nicolo, P.; Schnider, A.; Guggisberg, A.G. Modulating functional connectivity after stroke with neurofeedback: Effect on motor deficits in a controlled cross-over study. NeuroImage Clin. 2018, 20, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Boehm, W.L.; Gruben, K.G. Development of KIINCE: A kinetic feedback-based robotic environment for study of neuromuscular coordination and rehabilitation of human standing and walking. J. Rehabil. Assist. Technol. Eng. 2018, 5, 205566831879358. [Google Scholar] [CrossRef] [PubMed]
- Tsaih, P.-L.; Chiu, M.-J.; Luh, J.-J.; Yang, Y.-R.; Lin, J.-J.; Hu, M.-H. Practice Variability Combined with Task-Oriented Electromyographic Biofeedback Enhances Strength and Balance in People with Chronic Stroke. Behav. Neurol. 2018, 2018, 7080218. [Google Scholar] [CrossRef] [PubMed]
- Schließmann, D.; Nisser, M.; Schuld, C.; Gladow, T.; Derlien, S.; Heutehaus, L.; Weidner, N.; Smolenski, U.; Rupp, R. Trainer in a pocket-proof-of-concept of mobile, real-time, foot kinematics feedback for gait pattern normalization in individuals after stroke, incomplete spinal cord injury and elderly patients. J. Neuroeng. Rehabil. 2018, 15, 44. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Kim, J.-D.; Lee, J.-H.; Cha, Y.-J. Walking and balance ability gain from two types of gait intervention in adult patients with chronic hemiplegic stroke: A pilot study. Assist. Technol. 2019, 31, 112–115. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Bae, S.-H.; Lee, S.-H.; Kim, K.-Y. Neurofeedback Training Improves the Dual-Task Performance Ability in Stroke Patients. Tohoku J. Exp. Med. 2015, 236, 81–88. [Google Scholar] [CrossRef]
- Hankinson, K.; Shaykevich, A.; Vallence, A.-M.; Rodger, J.; Rosenberg, M.; Etherton-Beer, C. A Tailored Music-Motor Therapy and Real-Time Biofeedback Mobile Phone App (‘GotRhythm’) to Promote Rehabilitation Following Stroke: A Pilot Study. Neurosci. Insights 2022, 17, 26331055221100587. [Google Scholar] [CrossRef]
- Mihara, M.; Fujimoto, H.; Hattori, N.; Otomune, H.; Kajiyama, Y.; Konaka, K.; Watanabe, Y.; Hiramatsu, Y.; Sunada, Y.; Miyai, I.; et al. Effect of Neurofeedback Facilitation on Poststroke Gait and Balance Recovery. Neurology 2021, 96, e2587–e2598. [Google Scholar] [CrossRef]
- Park, S.; Liu, C.; Sánchez, N.; Tilson, J.K.; Mulroy, S.J.; Finley, J.M. Using Biofeedback to Reduce Step Length Asymmetry Impairs Dynamic Balance in People Poststroke. Neurorehabil. Neural Repair 2021, 35, 738–749. [Google Scholar] [CrossRef]
- Skvortsov, D.V.; Kaurkin, S.N.; Ivanova, G.E. A Study of Biofeedback Gait Training in Cerebral Stroke Patients in the Early Recovery Phase with Stance Phase as Target Parameter. Sensors 2021, 21, 7217. [Google Scholar] [CrossRef]
- Lünenburger, L.; Colombo, G.; Riener, R. Biofeedback for robotic gait rehabilitation. J. Neuroeng. Rehabil. 2007, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Saichi, K.; Yasuda, K.; Kitaji, Y.; Kaibuki, N.; Iwata, H. Development and pilot clinical evaluation of a haptic-based perception-empathy biofeedback device for gait rehabilitation. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; Volume 2016, pp. 6158–6161. [Google Scholar] [CrossRef]
- Giggins, O.M.; Persson, U.; Caulfield, B. Biofeedback in rehabilitation. J. Neuroeng. Rehabil. 2013, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Saichi, K.; Kaibuki, N.; Harashima, H.; Iwata, H. Haptic-based perception-empathy biofeedback system for balance rehabilitation in patients with chronic stroke: Concepts and initial feasibility study. Gait Posture 2018, 62, 484–489. [Google Scholar] [CrossRef]
- Chan, A.H.S.; Ng, A.W.Y. Finger response times to visual, auditory and tactile modality stimuli. Lect. Notes Eng. Comput. Sci. 2012, 2196, 1449–1454. [Google Scholar]
- Afzal, M.R.; Oh, M.-K.; Lee, C.-H.; Park, Y.S.; Yoon, J. A Portable Gait Asymmetry Rehabilitation System for Individuals with Stroke Using a Vibrotactile Feedback. Biomed. Res. Int. 2015, 2015, 375638. [Google Scholar] [CrossRef]
- Klamroth-Marganska, V. Stroke Rehabilitation: Therapy Robots and Assistive Devices. Adv. Exp. Med. Biol. 2018, 1065, 579–587. [Google Scholar]
- Stoller, O.; Waser, M.; Stammler, L.; Schuster, C. Evaluation of robot-assisted gait training using integrated biofeedback in neurologic disorders. Gait Posture 2012, 35, 595–600. [Google Scholar] [CrossRef]
| Studies (Year) | Sensor | Actuator | Assistive Device | Therapist-Oriented Cue | Control (Threshold Source, Periodicity, Reinforcement) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Device | Biofeedback Parameter | Location | Device | Mode | Location | ||||
| Ma et al. (2018) [16] | 2 thin-film force sensitive resistors (FSRs) (A301, Tekscan Co., Ltd., Wood Dale IL, USA) | medial and lateral plantar forces | Paretic first and fifth metatarsal heads | 1 vibrator (XY-B1027-DX, Xiongying electronics Co., Ltd., China) | haptic | paretic wrist | NA | NA | paretic limb, gait cycle, PR |
| Genthe et al. (2018) [17] | force platforms (Bertec Corporation, Columbus, OH, USA) | anterior–posterior ground reaction force | dual-belt treadmill | screen and speaker (MotionMonitor, Illinois, USA) | visual, auditory | non-wearable | treadmill | NM | baseline, gait cycle, PR |
| Afzal et al. (2019) [18] | 4 FSRs (Tekscan, A401) | time symmetry ratio | toe, metatarsal 1, metatarsal 5, and heel of feet | vibrotactor array (6 units, Precision Microdrives 310-101) | haptic | paretic leg | NA | NA | healthy, gait cycle, PR and NR |
| Khoo et al. (2017) [19] | 6 FSRs (TekScan) | heel-strike and toe-off events, stance and swing times | 3 at the front towards the toe and 3 at the back towards the heel of feet | piezo speaker (NM), electrotactile system | auditory, haptic | waist, electrode placed on the user’s thigh on the unaffected side | NA | NM, NA | non-paretic limb, gait cycle, NR |
| Nan et al. (2019) [20] | EEG system (Compact 823, Meditron, Electromedicina Ltd.a, São Paulo, Brazil) | relative individual alpha band amplitude in the target location (Cz, Oz) | head | computer screen (NM) | visual | non-wearable | NA | NM | baseline, 2 s, PR |
| Arpa et al. (2019) [21] | EMG system (Neurotrac ETS Simplex 2005) | EMG signal | tibialis anterior and quadriceps femoris muscles | computer monitor, speaker (NM) | visual, auditory | non-wearable | NA | NM | maximum voluntary contraction, NM, PR |
| Tamburella et al. (2019) [22] | BS1: EMG system (g.tec, Austria); BS2: load cells (Lokomat) | BS1: EMG signal; BS2: weighted averages of the joint human–robot interaction torque | BS1: tibialis anterior, gastrocnemius lateralis, soleus, vastus lateralis, rectus femoris, biceps femoris of the affected leg; BS2: hip and knee joints of exoskeleton | BS1: computer screen (NM); BS2: screen (NM) | visual | non-wearable | treadmill, hip and knee exoskeleton (Lokomat) | BS1: NM; BS2: visual | BS1: healthy, gait cycle, PR and NR; BS2: NA, gait cycle |
| Day et al. (2019) [23] | Optotrak Certus optical motion capture system (Northern Digital, Waterloo, ON, Canada) | information from sagittal plane hip and knee angles was condensed to a one-dimensional summary of performance | markers attached on bilateral lower limbs | tv screen (NM) | visual | non-wearable | treadmill (Woodway, WI) | NM | healthy, gait cycle, PR and NR |
| Guzik et al. (2020) [24] | strain gauge (NM) | step length | treadmill | screen, headphones (NM) | visual, auditory | non-wearable | Gait trainer 2 treadmill (Biodex) | NM, NA | NM, gait cycle, PR |
| Surucu et al. (2021) [25] | EMG system (Electronica Pagani Italy Modular Biofeedback) | EMG signal | tibialis anterior muscle | computer screen, speaker (NM) | visual, auditory | non-wearable | NA | visual | NM, PR |
| Ochi et al. (2015) [26] | load sensors (NM) | stance phase and load amount | inserted between the sole of the foot and the foot bed of the shoe (feet) | lights (NM) | visual | non-wearable | gait-assistance robot (4 robotic arms control both thighs and legs), treadmill | NM | body weight, gait cycle, NM |
| Bae et al. (2020) [27] | web cam (NM) | participants’ ankle from the side | non-wearable | tv screen (NM) | visual | non-wearable | EMG-FES (tibialis anterior muscle) | NM | healthy, NM, PR and NR |
| Hsu et al. (2019) [28] | pneumatic insole (NM) | vertical force | under feet | tv screen (NM) | visual | non-wearable | treadmill (Woodway, WI), cable-driven robotic system (corrective force on pelvis) | NM | body weight, gait cycle, PR |
| Givon et al. (2016) [29] | BS1 and BS3: camera (Microsoft Xbox Kinect, SeeMe VR system); BS2: balance board (Nintendo Wii Fit) | BS1 and BS3: body movement; BS2: centre of pressure | non-wearable | tv screen, speakers (NM) | visual, auditory | non-wearable | NA | NM | NM |
| Nagano et al. (2020) [30] | Optotrak Certus optical motion capture system (Northern Digital, ON) | minimum foot clearance | one marker on the toe of the affected limb | monitor (NM) | visual | non-wearable | treadmill | NM | baseline, gait cycle, PR and NR |
| Byl et al. (2015) [31] | barometric pressure sensors (NM); IMUs (accelerometer, magnetometer, gyroscope; NM) | ground reaction forces (foot pressing indicators); step lengths, stride widths, and toe-out angles | toe, the first and second metatarsophalangeal joint, the fourth and fifth metatarsophalangeal joint, and the heel of feet; feet | iPad screen (Apple, USA) | visual | non-wearable | treadmill | visual | NM |
| K-S Jung et al. (2020) [32] | pressure sensor (NM) | peak vertical force | cane | indicator (NM) | auditory | waist | cane | NM | baseline, NM, PR |
| Shin et al. (2017) [33] | infrared sensors (NM) | step length, step cycle initiation | on the rail and both sides of treadmill | tv screen, speakers (NM) | visual, auditory | non-wearable | treadmill (Motorika Reoambulator) | NM | non-paretic limb, gait cycle, PR and NR |
| Song et al. (2015) [34] | camera (Microsoft Xbox Kinect) | body movement | non-wearable | tv screen, speakers (NM) | visual, auditory | non-wearable | NA | NM | NM |
| Kim et al. (2020) [35] | pressure sensors (F-scan system, Teckscan Inc., USA) | pressure load | feet | computer monitor (NM) | visual | non-wearable | NA | NM | non-paretic limb, NM, PR and NR |
| J Jung et al. (2020) [36] | plantar pressure measurement mat with smart socks made of conductive material (GAITRite, CIR System Inc., USA) | pressure load | feet | beam projector and screen (NM) | visual | non-wearable | NA | NM | non-paretic limb, NM, PR and NR |
| Mottaz et al. (2018) [37] | MRI system (3T Siemens Trio TIM scanner, Siemens Medical Solutions, Germany), EEG system (BioSemi ActiveTwo, BioSemi B. V., Amsterdam, The Netherlands) | realistic head geometry, alpha-band weighted node degree of functional connectivity using absolute imagery part of coherency in the target area (ipsilesional hand motor cortex area, Brodmann area 10) | non-wearable, head | computer screen (NM) | visual | non-wearable | NA | NM | NM, 0.3 s, PR |
| Boehm et al. (2018) [38] | force plates (NM) | vertical force distribution on each foot | KIINCE | screen, programable plates (NM) | visual, haptic | non-wearable | kinetic immersive interface for neuromuscular coordination enhancement (KIINCE) | NM | non-paretic limb, gait cycle, PR |
| Tsaih et al. (2018) [39] | EMG system (NM) | EMG signal | tibialis anterior muscle | monitor, speaker (NM) | visual, auditory | non-wearable | NA | NM | maximum voluntary contraction, NM, PR |
| Schlieβmann et al. (2018) [40] | IMUs (accelerometer, gyroscope, magnetometer; NM) | foot-to-ground angle at heel-strike, stride length, stance duration, swing duration | feet | tablet, speaker or earphones (NM) | visual, auditory | non-wearable, ears | NA | NM, NA | healthy, third stride, PR and NR |
| Choi et al. (2019) [41] | loading sensor (NM) | user’s weight | center of the metatarsal heads under the paralyzed foot | Speaker (NM) | auditory | foot | NA | NM | non-paretic limb, gait cycle, PR |
| Lee et al. (2015) [42] | EEG system (QEEG-8 LXE3208, LAXHA Inc., Daejeon, Korea) | sensorimotor rhythm waves amplitude | head | computer screen (NM) | visual | non-wearable | NA | visual | healthy, NM, PR |
| Hankinson et al. (2022) [43] | IMUs (Mbientlab Inc., San Francisco, CA, USA) | yaw, pitch, roll angles | upper or lower limbs | mobile phone speaker | auditory | non-wearable | NA | NM | baseline, NM, PR and NR |
| Mihara et al. (2021) [44] | dunctional near-infrared spectroscopy (fNIRS) (OMM-3000, Shimadzu Corp., Tokyo, Japan) | supplementary motor area (SMA) activation | head | screen | visual | non-wearable | NA | NM | NM |
| Park et al. (2021) [45] | Qualisys Oqus motion capture system | position of reflective markers during swing phase | ankle | screen | visual | non-wearable | treadmill, ankle-foot orthosis | NM | baseline, gait cycle, PR and NR |
| Skvortsov et al. (2021) [46] | Stadis system (Neurosoft, Ivanovo, Russia) | stance time | ankle | screen | visual | non-wearable | treadmill | NM | baseline, NM, NR |
| Studies | Study Design | Sample Size (EG1, EG2/CG) | Lower Limb Activity | Training Dosing | Evaluation Time Points |
|---|---|---|---|---|---|
| Ma et al. (2018) [16] | uncontrolled design | 8 (8/0) | walking | 7 m-long walkway five times | during procedures |
| Genthe et al. (2018) [17] | uncontrolled design | 9 (9/0) | walking | 6 min three times | pre-, post-control and training procedures, 2, 15, 30 min follow-up |
| Afzal et al. (2019) [18] | uncontrolled design | 8 (8/0) | walking | not mentioned | during procedures |
| Khoo et al. (2017) [19] | non-randomized controlled design | 6 (2,2/2) | walking | 20 min twice a week for 8 weeks | pre-, mid-, and post-training |
| Nan et al. (2019) [20] | uncontrolled design | 2 (2/0) | sitting | 60 min twice a week until 15 sessions | pre-, during, and post-training |
| Arpa et al. (2019) [21] | RCT | 34 (17/17) | sitting | 15 min five times a week for 2 weeks | pre-training, immediate post-training, 1-, 3-month follow-up |
| Tamburella et al. (2019) [22] | randomized cross-over design | 10 (5,5/0) | walking | 40 min three times a week until 12 sessions | pre- and post-training |
| Day et al. (2019) [23] | uncontrolled design | 10 (10/0) | walking | 15 min two sessions at least 3 days apart | during procedures, pre-, and post-training |
| Guzik et al. (2020) [24] | uncontrolled design | 50 (50/0) | walking | 20 min five times a week for 2 weeks | pre- and post-training |
| Surucu et al. (2021) [25] | non-randomized controlled design | 40 (20/20) | sitting | 20 min five times a week for 3 weeks | pre- and post-training |
| Ochi et al. (2015) [26] | RCT | 26 (13/13) | walking | 20 min five times a week for 4 weeks | pre- and post-training |
| Bae et al. (2020) [27] | RCT | 26 (13/13) | sitting | 40 min five times a week for 4 weeks | pre- and post-training |
| Hsu et al. (2019) [28] | uncontrolled design | 15 (15/0) | walking | 5-min | pre-, early-, late-, and post-training |
| Givon et al. (2016) [29] | RCT | 47 (23/24) | standing | 1 h twice a week for 3 months | pre-training, immediate post-training, and 3-month follow-up |
| Nagano et al. (2020) [30] | uncontrolled design | 6 (6/0) | walking | 10 min eight sessions over 4 weeks | pre-training, immediate post-training, and 1-month follow-up |
| Byl et al. (2015) [31] | non-randomized controlled design | 12 (7/4) | walking | 30 min 12 sessions over 6–8 weeks | pre- and post-training |
| K-S Jung et al. (2020) [32] | RCT | 20 (10/10) | walking | 30 min five times a week for 4 weeks | pre- and post-training |
| Shin et al. (2017) [33] | uncontrolled design | 17 (17/0) | walking | 5 min | during procedures, post-training |
| Song et al. (2015) [34] | non-randomized controlled design | 40 (20/20) | standing | 30 min five times a week for 8 weeks | pre- and post-training |
| Kim et al. (2020) [35] | RCT | 24 (12/12) | walking | 30 min three times a week for 4 weeks | pre-training, immediate post-training, and three times per week for 2 weeks follow-up |
| J Jung et al. (2020) [36] | uncontrolled design | 10 (10/0) | walking | not mentioned | during procedures |
| Mottaz et al. (2018) [37] | uncontrolled design | 10 (10/0) | sitting | 50 min twice a week over 2 months | pre-training, immediate post-training, and 1-month follow-up |
| Boehm et al. (2018) [38] | uncontrolled design | 10 (10/0) | walking | 30-s | during procedures |
| Tsaih et al. (2018) [39] | RCT | 33 (13,11/9) | sitting | 40 min 18 sessions over 6 weeks | pre-training, 1-day, 2-week, and 6-week follow-up |
| Schlieβmann et al. (2018) [40] | uncontrolled design | 11 (11/0) | walking | 15 min three consecutive sessions | pre-training, immediate post-training, and 4-week follow-up |
| Choi et al. (2019) [41] | RCT | 24 (12/12) | walking | 20 min three times a week for 6 weeks | pre- and post-training |
| Lee et al. (2015) [42] | RCT | 20 (20/0) | sitting | 30 min three times a week for 8 weeks | pre- and post-training |
| Hankinson et al. (2022) [43] | RCT | 22 (10/12) | upper or lower limb tasks | 20 min three times a week for 6 weeks | pre-, mid-, and post-training |
| Mihara et al. (2021) [44] | RCT | 54 (28/26) | sitting | 6 min three times a week for 2 weeks | pre-training, immediate post-training, and 2-week follow-up |
| Park et al. (2021) [45] | uncontrolled design | 36 | walking | 3 min three times | during procedures |
| Skvortsov et al. (2021) [46] | uncontrolled design | 20 | walking | 18 min eight to eleven times | pre- and post-training |
| Studies | Sensor-Based Outcomes | Clinical Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|
| Spatiotemporal | Kinetic | Kinematic | Physiological | Post-Stroke Stage | Motor | Mental | Sensory | |
| Ma et al. [16] | Walking speed Stance time Stride time | Plantar pressure Foot-floor contact area | Peak joint angle | |||||
| Genthe et al. [17] | Step length Spatial asymmetry | Peak joint moment Peak AGRF AGRF deficit | Peak joint angle Peak joint angle deficit | |||||
| Afzal et al. [18] | Walking speed Time asymmetry | |||||||
| Khoo et al. [19] | Time asymmetry | |||||||
| Nan et al. [20] | IAB amplitude | BBS 10-m walk test TUG | HADS MMSE Brief Aphasia Evaluation | |||||
| Arpa et al. [21] | Peak joint moment | Joint ROM | Muscle activation | 10 m walk test MAS BI | ||||
| Tamburella et al. [22] | Human–robot interaction torque | BBS MAS BI MMT FAC Trunk Control Test | QUEST 2.0 VAS of motivation, mood, satisfaction NASA-TLX QCM CESD | |||||
| Day et al. [23] | Walking speed Foot clearance | Joint ROM One-dimensional summary of performance from joint angles Performance deficit | FM ESS | MoCA | Proprioception test Start Cancellation Test | |||
| Guzik et al. [24] | 10 m walk test TUG FIM BI 2 min walk test | |||||||
| Surucu et al. [25] | Joint ROM | Muscle activation | Brunnstrom neurophysiological assessment | MAS Modified Motor Assessment Scale | ||||
| Ochi et al. [26] | Muscle torque | FM | 10 m walk test FIM FAC | |||||
| Bae et al. [27] | Step length Walking speed Stride length Stance time Swing time Cadence | TUG Tardieu scale WBLT | ||||||
| Hsu et al. [28] | Step length Walking speed Spatial asymmetry | Peak vertical force | Muscle activation | |||||
| Givon et al. [29] | Number of steps walked per day | Grip strength | 10 m walk test FIM ARAT FRT IADL questionnaire | Likert-type satisfaction scale Session attendance GDS | ||||
| Nagano et al. [30] | Step length Foot clearance Step time Step width | Peak joint angle at MFC | ||||||
| Byl et al. [31] | Step length | Joint ROM | FM | 10 m walk test TUG BBS MMT 6 min walk test TGA 5XSST DGI Number of falls experienced before admitted and during training | VAS of pain | |||
| K-S Jung et al. [32] | Peak vertical force | Muscle activation | TUG TIS | |||||
| Shin et al. [33] | Step length Spatial asymmetry Step length deficit | Likert-type satisfaction scale | ||||||
| Song et al. [34] | 10 m walk test TUG LOS test | BDI RCS | ||||||
| Kim et al. [35] | Step length Walking speed Stride length Spatial asymmetry Single support time Double support time | TUG | ||||||
| J Jung et al. [36] | Walking speed Stride length Stance time Swing time Time asymmetry Cadence Single support time Double support time Toe-only time Heel-only time | |||||||
| Mottaz et al. [37] | Grip strength | Alpha-band weighted node degree of functional connectivity | FM | 10 m walk test TUG MAS MAL MRC muscle scale 9HPT BBT | ||||
| Boehm et al. [38] | Force asymmetry | |||||||
| Tsaih et al. [39] | Muscle strength | 10 m walk test TUG LOS test 6 min walk test | ||||||
| Schlieβmann et al. [40] | Stride length Swing time | Peak joint angle at heel-strike | 10 m walk test TUG MMT WISCI II | QUEST 2.0 | PP test | |||
| Choi et al. [41] | Centre of pressure | 10 m walk test TUG FGA | ||||||
| Lee et al. [42] | Stance time Cadence | Plantar pressure | SMR waves amplitude | 10 m walk test Dual task error | ||||
| Hankinson et al. (2022) [43] | FM | |||||||
| Mihara et al. (2021) [44] | Walking speed | FM | TUG FIM BBS | |||||
| Park et al. (2021) [45] | Step length asymmetry | Whole-body angular momentum | ||||||
| Skvortsov et al. (2021) [46] | Walking speed Foot clearance Stance time Single support time Double support time | Joint angles | Muscle activation | TUG BBS Hauser Ambulance Index (HAI) Standing Balance Test (SBT) | ||||
| Studies | Component Ratings | Global Rating | |||||
|---|---|---|---|---|---|---|---|
| Selection Bias | Study Design | Confounders | Blinding | Data Collection Methods | Withdrawals and Drop-outs | ||
| Ma et al. [16] | moderate | moderate | strong | strong | strong | weak | moderate |
| Genthe et al. [17] | weak | moderate | strong | strong | strong | weak | weak |
| Afzal et al. [18] | weak | moderate | strong | strong | strong | weak | weak |
| Khoo et al. [19] | moderate | strong | weak | weak | strong | weak | weak |
| Nan et al. [20] | weak | moderate | strong | strong | strong | weak | weak |
| Arpa et al. [21] | weak | strong | strong | strong | strong | strong | moderate |
| Tamburella et al. [22] | moderate | strong | strong | strong | strong | strong | strong |
| Day et al. [23] | weak | moderate | strong | strong | strong | weak | weak |
| Guzik et al. [24] | moderate | moderate | strong | strong | strong | weak | moderate |
| Surucu et al. [25] | moderate | strong | strong | weak | strong | strong | moderate |
| Ochi et al. [26] | strong | strong | strong | strong | strong | strong | strong |
| Bae et al. [27] | moderate | strong | strong | weak | strong | strong | moderate |
| Hsu et al. [28] | weak | moderate | strong | strong | strong | weak | weak |
| Givon et al. [29] | weak | strong | strong | strong | strong | strong | moderate |
| Nagano et al. [30] | weak | moderate | strong | strong | strong | weak | weak |
| Byl et al. [31] | moderate | strong | strong | weak | strong | weak | weak |
| K-S Jung et al. [32] | weak | strong | strong | strong | strong | weak | weak |
| Shin et al. [33] | moderate | moderate | strong | strong | strong | weak | moderate |
| Song et al. [34] | moderate | strong | strong | weak | strong | weak | weak |
| Kim et al. [35] | weak | strong | strong | strong | strong | strong | moderate |
| J Jung et al. [36] | moderate | moderate | strong | strong | strong | weak | moderate |
| Mottaz et al. [37] | weak | moderate | strong | strong | strong | weak | weak |
| Boehm et al. [38] | weak | moderate | strong | strong | strong | weak | weak |
| Tsaih et al. [39] | moderate | strong | strong | strong | strong | strong | strong |
| Schlieβmann et al. [40] | weak | moderate | strong | strong | strong | strong | moderate |
| Choi et al. [41] | moderate | strong | strong | strong | strong | weak | moderate |
| Lee et al. [42] | moderate | strong | strong | strong | strong | strong | strong |
| Hankinson et al. (2022) [43] | strong | strong | weak | strong | strong | strong | moderate |
| Mihara et al. (2021) [44] | strong | strong | strong | strong | strong | strong | strong |
| Park et al. (2021) [45] | weak | moderate | strong | weak | strong | strong | weak |
| Skvortsov et al. (2021) [46] | weak | moderate | strong | weak | strong | strong | weak |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, C.; Figueiredo, J.; Cerqueira, J.; Santos, C.P. Robotic Biofeedback for Post-Stroke Gait Rehabilitation: A Scoping Review. Sensors 2022, 22, 7197. https://doi.org/10.3390/s22197197
Pinheiro C, Figueiredo J, Cerqueira J, Santos CP. Robotic Biofeedback for Post-Stroke Gait Rehabilitation: A Scoping Review. Sensors. 2022; 22(19):7197. https://doi.org/10.3390/s22197197
Chicago/Turabian StylePinheiro, Cristiana, Joana Figueiredo, João Cerqueira, and Cristina P. Santos. 2022. "Robotic Biofeedback for Post-Stroke Gait Rehabilitation: A Scoping Review" Sensors 22, no. 19: 7197. https://doi.org/10.3390/s22197197
APA StylePinheiro, C., Figueiredo, J., Cerqueira, J., & Santos, C. P. (2022). Robotic Biofeedback for Post-Stroke Gait Rehabilitation: A Scoping Review. Sensors, 22(19), 7197. https://doi.org/10.3390/s22197197









