A Platinized Carbon Fiber Microelectrode-Based Oxidase Biosensor for Amperometric Monitoring of Lactate in Brain Slices
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Electrochemical Instrumentation
2.3. Lactate Microbiosensors Fabrication
2.4. Scanning Electron Microscopy
2.5. In Vitro Evaluation of the Lactate Microbiosensor
2.6. Preparation of Hippocampal Slices
2.7. Experiments in Hippocampal Slices
2.8. Evaluation of Lactate Microbiosensor Biofouling upon Successive Slice Recordings
2.9. Data Analysis
2.10. Statistical Analysis
3. Results
3.1. Analytical Properties of CFM/Pt-Based Lactate Microbiosensors
3.1.1. Kinetic Parameters
3.1.2. Temperature and pH Dependency
3.1.3. Selectivity
3.2. Evaluation of the Operational Stability of the Lactate Microbiosensor for Multiple Recordings
3.3. Measurement of Extracellular Lactate in Hippocampal Slices
3.4. High Potassium-Evoked Changes in Extracellular Lactate in the CA1 Subregion of Hippocampal Slices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brooks, G.A. Lactate as a Fulcrum of Metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Sawa, T.; Okuda, C.; Matsuda, T.; Tanaka, Y. Effects of Glucose Load on Brain Extracellular Lactate Concentration in Conscious Rats Using a Microdialysis Technique. Horm. Metab. Res. 1993, 25, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Abi-Saab, W.M.; Maggs, D.G.; Jones, T.; Jacob, R.; Srihari, V.; Thompson, J.; Kerr, D.; Leone, P.; Krystal, J.H.; Spencer, D.D.; et al. Striking Differences in Glucose and Lactate Levels between Brain Extracellular Fluid and Plasma in Conscious Human Subjects: Effects of Hyperglycemia and Hypoglycemia. J. Cereb. Blood Flow Metab. 2002, 22, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Zilberter, Y.; Zilberter, T.; Bregestovski, P. Neuronal Activity in Vitro and the in Vivo Reality: The Role of Energy Homeostasis. Trends Pharmacol. Sci. 2010, 31, 394–401. [Google Scholar] [CrossRef]
- Yager, J.Y.; Armstrong, E.A.; Miyashita, H.; Wirrell, E.C. Prolonged Neonatal Seizures Exacerbate Hypoxic-Ischemic Brain Damage: Correlation with Cerebral Energy Metabolism and Excitatory Amino Acid Release. Dev. Neurosci. 2002, 24, 367–381. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, L.; Shangguan, D.; Yu, X.; Zhao, R.; Han, H.; Liu, G. Analysis of Glucose and Lactate in Hippocampal Dialysates of Rats during the Operant Conditioned Reflex Using Microdialysis. Neurochem. Int. 2003, 43, 67–72. [Google Scholar] [CrossRef]
- Zuend, M.; Saab, A.S.; Wyss, M.T.; Ferrari, K.D.; Hösli, L.; Looser, Z.J.; Stobart, J.L.; Duran, J.; Guinovart, J.J.; Barros, L.F.; et al. Arousal-Induced Cortical Activity Triggers Lactate Release from Astrocytes. Nat. Metab. 2020, 2, 179–191. [Google Scholar] [CrossRef]
- Forderhase, A.G.; Styers, H.C.; Lee, C.A.; Sombers, L.A. Simultaneous Voltammetric Detection of Glucose and Lactate Fluctuations in Rat Striatum Evoked by Electrical Stimulation of the Midbrain. Anal. Bioanal. Chem. 2020, 412, 6611–6624. [Google Scholar] [CrossRef]
- Bingul, D.; Kalra, K.; Murata, E.M.; Belser, A.; Dash, M.B. Persistent Changes in Extracellular Lactate Dynamics Following Synaptic Potentiation. Neurobiol. Learn. Mem. 2020, 175, 107314. [Google Scholar] [CrossRef]
- Hu, Y.; Wilson, G.S. A Temporary Local Energy Pool Coupled to Neuronal Activity: Fluctuations of Extracellular Lactate Levels in Rat Brain Monitored with Rapid-Response Enzyme-Based Sensor. J. Neurochem. 1997, 69, 1484–1490. [Google Scholar] [CrossRef]
- Fernandes, E.; Ledo, A.; Barbosa, R.M. Design and Evaluation of a Lactate Microbiosensor: Toward Multianalyte Monitoring of Neurometabolic Markers In Vivo in the Brain. Molecules 2022, 27, 514. [Google Scholar] [CrossRef]
- Monroe, G.R.; van Eerde, A.M.; Tessadori, F.; Duran, K.J.; Savelberg, S.M.C.; van Alfen, J.C.; Terhal, P.A.; van der Crabben, S.N.; Lichtenbelt, K.D.; Fuchs, S.A.; et al. Identification of Human D Lactate Dehydrogenase Deficiency. Nat. Commun. 2019, 10, 1477. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.F. Gold Nanoparticles Assisted Laser Desorption/Ionization Mass Spectrometry and Applications: From Simple Molecules to Intact Cells. Anal. Bioanal. Chem. 2016, 408, 4485–4502. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, S.; Li, T.; Yang, H.; Zhang, Y.; Zhao, Z. Co-Incorporated Mesoporous Carbon Material-Assisted Laser Desorption/Ionization Ion Source as an Online Interface of in Vivo Microdialysis Coupled with Mass Spectrometry. Anal. Chem. 2020, 92, 5482–5491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, C.; Jiang, K.; Ying, M.; Hu, X. Quantification of Lactate from Various Metabolic Pathways and Quantification Issues of Lactate Isotopologues and Isotopmers. Sci. Rep. 2017, 7, 8489. [Google Scholar] [CrossRef]
- Barros, L.F.; Bolaños, J.P.; Bonvento, G.; Bouzier-Sore, A.K.; Brown, A.; Hirrlinger, J.; Kasparov, S.; Kirchhoff, F.; Murphy, A.N.; Pellerin, L.; et al. Current Technical Approaches to Brain Energy Metabolism. Glia 2018, 66, 1138–1159. [Google Scholar] [CrossRef] [PubMed]
- Weltin, A.; Kieninger, J.; Urban, G.A. Microfabricated, Amperometric, Enzyme-Based Biosensors for in Vivo Applications. Anal. Bioanal. Chem. 2016, 408, 4503–4521. [Google Scholar] [CrossRef] [PubMed]
- Katsounaros, I.; Schneider, W.B.; Meier, J.C.; Benedikt, U.; Biedermann, P.U.; Auer, A.A.; Mayrhofer, K.J.J. Hydrogen Peroxide Electrochemistry on Platinum: Towards Understanding the Oxygen Reduction Reaction Mechanism. Phys. Chem. Chem. Phys. 2012, 14, 7384. [Google Scholar] [CrossRef]
- Meyerhoff, J.B.; Ewing, M.A.; Ewing, A.G. Ultrasmall Enzyme Electrodes with Response Time Less than 100 Milliseconds. Electroanalysis 1999, 11, 308–312. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Caetano, M.; Ledo, A.; Barbosa, R.M. Platinized Carbon Fiber-Based Glucose Microbiosensor Designed for Metabolic Studies in Brain Slices. Bioelectrochemistry 2019, 130, 107325. [Google Scholar] [CrossRef]
- Hadjihambi, A.; Karagiannis, A.; Theparambil, S.M.; Ackland, G.L.; Gourine, A.v. The Effect of General Anaesthetics on Brain Lactate Release. Eur. J. Pharmacol. 2020, 881, 173188. [Google Scholar] [CrossRef] [PubMed]
- Lein, P.J.; Barnhart, C.D.; Pessah, I.N. Acute Hippocampal Slice Preparation and Hippocampal Slice Cultures. Methods Mol. Biol. 2011, 758, 115–134. [Google Scholar] [CrossRef]
- Hertz, L. Metabolic Studies in Brain Slices—Past, Present, and Future. Front. Pharmacol. 2012, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kass, I.S. Preparation of Brain Slices BT—Neurotransmitter Methods; Rayne, R.C., Ed.; Springer: Totowa, NJ, USA, 1997; pp. 1–14. ISBN 978-1-59259-558-7. [Google Scholar]
- Okada, Y.; Lipton, P. 1.2 Glucose, Oxidative Energy Metabolism, and Neural Function in Brain Slices—Glycolysis Plays a Key Role in Neural Activity BT—Handbook of Neurochemistry and Molecular Neurobiology: Brain Energetics. Integration of Molecular and Cellular Processes; Lajtha, A., Gibson, G.E., Dienel, G.A., Eds.; Springer: Boston, MA, USA, 2007; pp. 17–39. ISBN 978-0-387-30411-3. [Google Scholar]
- Santos, R.M.; Lourenço, C.F.; Piedade, A.P.; Andrews, R.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Laranjinha, J.; Barbosa, R.M. A Comparative Study of Carbon Fiber-Based Microelectrodes for the Measurement of Nitric Oxide in Brain Tissue. Biosens. Bioelectron. 2008, 24, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Ledo, A.; Barbosa, R.M.; Gerhardt, G.A.; Cadenas, E.; Laranjinha, J. Concentration Dynamics of Nitric Oxide in Rat Hippocampal Subregions Evoked by Stimulation of the NMDA Glutamate Receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 17483–17488. [Google Scholar] [CrossRef] [PubMed]
- Vaddiraju, S.; Legassey, A.; Wang, Y.; Qiang, L.; Burgess, D.J.; Jain, F.; Papadimitrakopoulos, F. Design and Fabrication of a High-Performance Electrochemical Glucose Sensor. J. Diabetes Sci. Technol. 2011, 5, 1044–1051. [Google Scholar] [CrossRef]
- Panuccio, G.; Colombi, I.; Chiappalone, M. Recording and Modulation of Epileptiform Activity in Rodent Brain Slices Coupled to Microelectrode Arrays. JoVE 2018, 135, e57548. [Google Scholar] [CrossRef]
- Manz, K.M.; Siemann, J.K.; McMahon, D.G.; Grueter, B.A. Patch-Clamp and Multi-Electrode Array Electrophysiological Analysis in Acute Mouse Brain Slices. STAR Protoc. 2021, 2, 100442. [Google Scholar] [CrossRef]
- Teyler, T.J. Brain Slice Preparation: Hippocampus. Brain Res. Bull. 1980, 5, 391–403. [Google Scholar] [CrossRef]
- Reid, K.H.; Edmonds, H.L.; Schurr, A.; Tseng, M.T.; West, C.A. Pitfalls in the Use of Brain Slices. Prog. Neurobiol. 1988, 31, 1–18. [Google Scholar] [CrossRef]
- Rice, M.E. Ascorbate Regulation and Its Neuroprotective Role in the Brain. Trends Neurosci. 2000, 23, 209–216. [Google Scholar] [CrossRef]
- Ferreira, N.R.; Ledo, A.; Laranjinha, J.; Gerhardt, G.A.; Barbosa, R.M. Simultaneous Measurements of Ascorbate and Glutamate in Vivo in the Rat Brain Using Carbon Fiber Nanocomposite Sensors and Microbiosensor Arrays. Bioelectrochemistry 2018, 121, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.R.; Santos, R.M.; Laranjinha, J.; Barbosa, R.M. Real Time In Vivo Measurement of Ascorbate in the Brain Using Carbon Nanotube-Modified Microelectrodes. Electroanalysis 2013, 25, 1757–1763. [Google Scholar] [CrossRef]
- Bucur, B.; Purcarea, C.; Andreescu, S.; Vasilescu, A. Addressing the Selectivity of Enzyme Biosensors: Solutions and Perspectives. Sensors 2021, 21, 3038. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Pomerleau, F.; Huettl, P.; Gash, C.R.; Werner, C.E.; Bruno, J.P.; Gerhardt, G.A. Ceramic-Based Multisite Microelectrode Arrays for Simultaneous Measures of Choline and Acetylcholine in CNS. Biosens. Bioelectron. 2008, 23, 1382–1389. [Google Scholar] [CrossRef]
- Singh, Y.S.; Sawarynski, L.E.; Dabiri, P.D.; Choi, W.R.; Andrews, A.M. Head-to-Head Comparisons of Carbon Fiber Microelectrode Coatings for Sensitive and Selective Neurotransmitter Detection by Voltammetry. Anal. Chem. 2011, 83, 6658–6666. [Google Scholar] [CrossRef]
- Wisniewski, N.; Moussy, F.; Reichert, W.M. Characterization of Implantable Biosensor Membrane Biofouling. Fresenius J. Anal. Chem. 2000, 366, 611–621. [Google Scholar] [CrossRef]
- Barros, L.F.; San Martín, A.; Ruminot, I.; Sandoval, P.Y.; Baeza-Lehnert, F.; Arce-Molina, R.; Rauseo, D.; Contreras-Baeza, Y.; Galaz, A.; Valdivia, S. Fluid Brain Glycolysis: Limits, Speed, Location, Moonlighting, and the Fates of Glycogen and Lactate. Neurochem. Res. 2020, 45, 1328–1334. [Google Scholar] [CrossRef]
- Barros, L.F.; Ruminot, I.; San Martín, A.; Lerchundi, R.; Fernández-Moncada, I.; Baeza-Lehnert, F. Aerobic Glycolysis in the Brain: Warburg and Crabtree Contra Pasteur. Neurochem. Res. 2021, 46, 15–22. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the Brain: From Metabolic End-Product to Signalling Molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Honegger, P.; Pardo, B. Separate Neuronal and Glial Na+, K+ -ATPase Isoforms Regulate Glucose Utilization in Response to Membrane Depolarization and Elevated Extracellular Potassium. J. Cereb. Blood Flow Metab. 1999, 19, 1051–1059. [Google Scholar] [CrossRef]
- Hertz, L.; Xu, J.; Song, D.; Yan, E.; Gu, L.; Peng, L. Astrocytic and Neuronal Accumulation of Elevated Extracellular K+ with a 2/3 K+/Na+ Flux Ratio—Consequences for Energy Metabolism, Osmolarity and Higher Brain Function. Front. Comput. Neurosci. 2013, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- van Hall, G.; Strømstad, M.; Rasmussen, P.; Jans, O.; Zaar, M.; Gam, C.; Quistorff, B.; Secher, N.H.; Nielsen, H.B. Blood Lactate Is an Important Energy Source for the Human Brain. J. Cereb. Blood Flow Metab. 2009, 29, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Chatard, C.; Sabac, A.; Moreno-Velasquez, L.; Meiller, A.; Marinesco, S. Minimally Invasive Microelectrode Biosensors Based on Platinized Carbon Fibers for in Vivo Brain Monitoring. ACS Cent. Sci. 2018, 4, 1751–1760. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; Roche, R.; González-Mora, J.L.; O’Neill, R.D. Microbiosensors for Glucose Based on Prussian Blue Modified Carbon Fiber Electrodes for in Vivo Monitoring in the Central Nervous System. Biosens. Bioelectron. 2010, 26, 748–753. [Google Scholar] [CrossRef]
- Smith, S.K.; Gosrani, S.P.; Lee, C.A.; McCarty, G.S.; Sombers, L.A. Carbon-Fiber Microbiosensor for Monitoring Rapid Lactate Fluctuations in Brain Tissue Using Fast-Scan Cyclic Voltammetry. Anal. Chem. 2018, 90, 12994–12999. [Google Scholar] [CrossRef] [PubMed]
- Schuvailo, O.M.; Soldatkin, O.O.; Lefebvre, A.; Cespuglio, R.; Soldatkin, A.P. Highly Selective Microbiosensors for in Vivo Measurement of Glucose, Lactate and Glutamate. Anal. Chim. Acta 2006, 573–574, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Ledo, A.; Barbosa, R.; Cadenas, E.; Laranjinha, J. Dynamic and Interacting Profiles of *NO and O2 in Rat Hippocampal Slices. Free Radic. Biol. Med. 2010, 48, 1044–1050. [Google Scholar] [CrossRef][Green Version]
- Dias, C.; Lourenço, C.F.; Barbosa, R.M.; Laranjinha, J.; Ledo, A. Analysis of Respiratory Capacity in Brain Tissue Preparations: High-Resolution Respirometry for Intact Hippocampal Slices. Anal. Biochem. 2018, 551, 43–50. [Google Scholar] [CrossRef]
- Taurino, I.; Reiss, R.; Richter, M.; Fairhead, M.; Thöny-Meyer, L.; de Micheli, G.; Carrara, S. Comparative Study of Three Lactate Oxidases from Aerococcus Viridans for Biosensing Applications. Electrochim. Acta 2013, 93, 72–79. [Google Scholar] [CrossRef][Green Version]
- Yorita, K.; Matsuoka, T.; Misaki, H.; Massey, V. Interaction of Two Arginine Residues in Lactate Oxidase with the Enzyme Flavin: Conversion of FMN to 8-Formyl-FMN. Proc. Natl. Acad. Sci. USA 2000, 97, 13039–13044. [Google Scholar] [CrossRef] [PubMed]
- Spehar-Délèze, A.-M.; Anastasova, S.; Vadgama, P. Monitoring of Lactate in Interstitial Fluid, Saliva and Sweat by Electrochemical Biosensor: The Uncertainties of Biological Interpretation. Chemosensors 2021, 9, 195. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Palmer, M.; Gerhardt, G.A. L-Lactate Measures in Brain Tissue with Ceramic-Based Multisite Microelectrodes. Biosens. Bioelectron. 2005, 20, 1772–1779. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Ledo, A.; Gerhardt, G.A.; Laranjinha, J.; Barbosa, R.M. Neurometabolic and Electrophysiological Changes during Cortical Spreading Depolarization: Multimodal Approach Based on a Lactate-Glucose Dual Microbiosensor Arrays. Sci. Rep. 2017, 7, 6764. [Google Scholar] [CrossRef] [PubMed]
- Scopes, R.K. The Effect of Temperature on Enzymes Used in Diagnostics. Clin. Chim. Acta 1995, 237, 17–23. [Google Scholar] [CrossRef]
- Heruye, S.H.; Warren, T.J.; Kostansek, J.A.; Draves, S.B.; Matthews, S.A.; West, P.J.; Simeone, K.A.; Simeone, T.A. Ascorbic Acid Reduces Neurotransmission, Synaptic Plasticity, and Spontaneous Hippocampal Rhythms in In Vitro Slices. Nutrients 2022, 14, 613. [Google Scholar] [CrossRef]
- Moretti, M.; Rodrigues, A.L.S. Functional Role of Ascorbic Acid in the Central Nervous System: A Focus on Neurogenic and Synaptogenic Processes. Nutr. Neurosci. 2021, 1–11. [Google Scholar] [CrossRef]
- Vizi, E.S.; Kiss, J.P. Neurochemistry and Pharmacology of the Major Hippocampal Transmitter Systems: Synaptic and Nonsynaptic Interactions. Hippocampus 1998, 8, 566–607. [Google Scholar] [CrossRef]
- Patel, B.A. Chapter 12—Electrochemical Biosensors; Patel, B.B.T.-E.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 267–284. ISBN 978-0-12-821203-5. [Google Scholar]
- Shram, N.F.; Netchiporouk, L.I.; Martelet, C.; Jaffrezic-Renault, N.; Bonnet, C.; Cespuglio, R. In Vivo Voltammetric Detection of Rat Brain Lactate with Carbon Fiber Microelectrodes Coated with Lactate Oxidase. Anal. Chem. 1998, 70, 2618–2622. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; O’Neill, R.D.; Roche, R.; González-Mora, J.L. Biosensors Based on Prussian Blue Modified Carbon Fibers Electrodes for Monitoring Lactate in the Extracellular Space of Brain Tissue. Int. J. Electrochem. Sci. 2012, 7, 5910–5926. [Google Scholar]
- Salazar, P.; Martín, M.; O’Neill, R.D.; Roche, R.; González-Mora, J.L. Surfactant-Promoted Prussian Blue-Modified Carbon Electrodes: Enhancement of Electro-Deposition Step, Stabilization, Electrochemical Properties and Application to Lactate Microbiosensors for the Neurosciences. Colloids Surf B Biointerfaces 2012, 92, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Karyakin, A.A. Advances of Prussian Blue and Its Analogues in (Bio)Sensors. Curr. Opin. Electrochem. 2017, 5, 92–98. [Google Scholar] [CrossRef]
- Itaya, K.; Shoji, N.; Uchida, I. Catalysis of the Reduction of Molecular Oxygen to Water at Prussian Blue Modified Electrodes. J. Am. Chem. Soc. 1984, 106, 3423–3429. [Google Scholar] [CrossRef]
- Ricci, F.; Palleschi, G. Sensor and Biosensor Preparation, Optimisation and Applications of Prussian Blue Modified Electrodes. Biosens/ Bioelectron. 2005, 21, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Feldman, B.J.; Murray, R.W. Electron Diffusion in Wet and Dry Prussian Blue Films on Interdigitated Array Electrodes. Inorg. Chem. 1987, 26, 1702–1708. [Google Scholar] [CrossRef]
- Ledo, A.; Fernandes, E.; Brett, C.M.A.; Barbosa, R.M. Enhanced Selectivity and Stability of Ruthenium Purple-Modified Carbon Fiber Microelectrodes for Detection of Hydrogen Peroxide in Brain Tissue. Sens. Actuators B Chem. 2020, 311, 127899. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Gaetani, G.F. Mammalian Catalase: A Venerable Enzyme with New Mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef]
- Wahono, N.; Qin, S.; Oomen, P.; Cremers, T.I.F.; de Vries, M.G.; Westerink, B.H.C. Evaluation of Permselective Membranes for Optimization of Intracerebral Amperometric Glutamate Biosensors. Biosens. Bioelectron. 2012, 33, 260–266. [Google Scholar] [CrossRef]
- Fang, L.; Liang, B.; Yang, G.; Hu, Y.; Zhu, Q.; Ye, X. A Needle-Type Glucose Biosensor Based on PANI Nanofibers and PU/E-PU Membrane for Long-Term Invasive Continuous Monitoring. Biosens. Bioelectron. 2017, 97, 196–202. [Google Scholar] [CrossRef]
- Sotelo-Hitschfeld, T.; Niemeyer, M.I.; Machler, P.; Ruminot, I.; Lerchundi, R.; Wyss, M.T.; Stobart, J.; Fernandez-Moncada, I.; Valdebenito, R.; Garrido-Gerter, P.; et al. Channel-Mediated Lactate Release by K+-Stimulated Astrocytes. J. Neurosci. 2015, 35, 4168–4178. [Google Scholar] [CrossRef]
- Ruminot, I.; Schmälzle, J.; Leyton, B.; Barros, L.F.; Deitmer, J.W. Tight Coupling of Astrocyte Energy Metabolism to Synaptic Activity Revealed by Genetically Encoded FRET Nanosensors in Hippocampal Tissue. J. Cereb. Blood Flow Metab. 2019, 39, 513–523. [Google Scholar] [CrossRef] [PubMed]

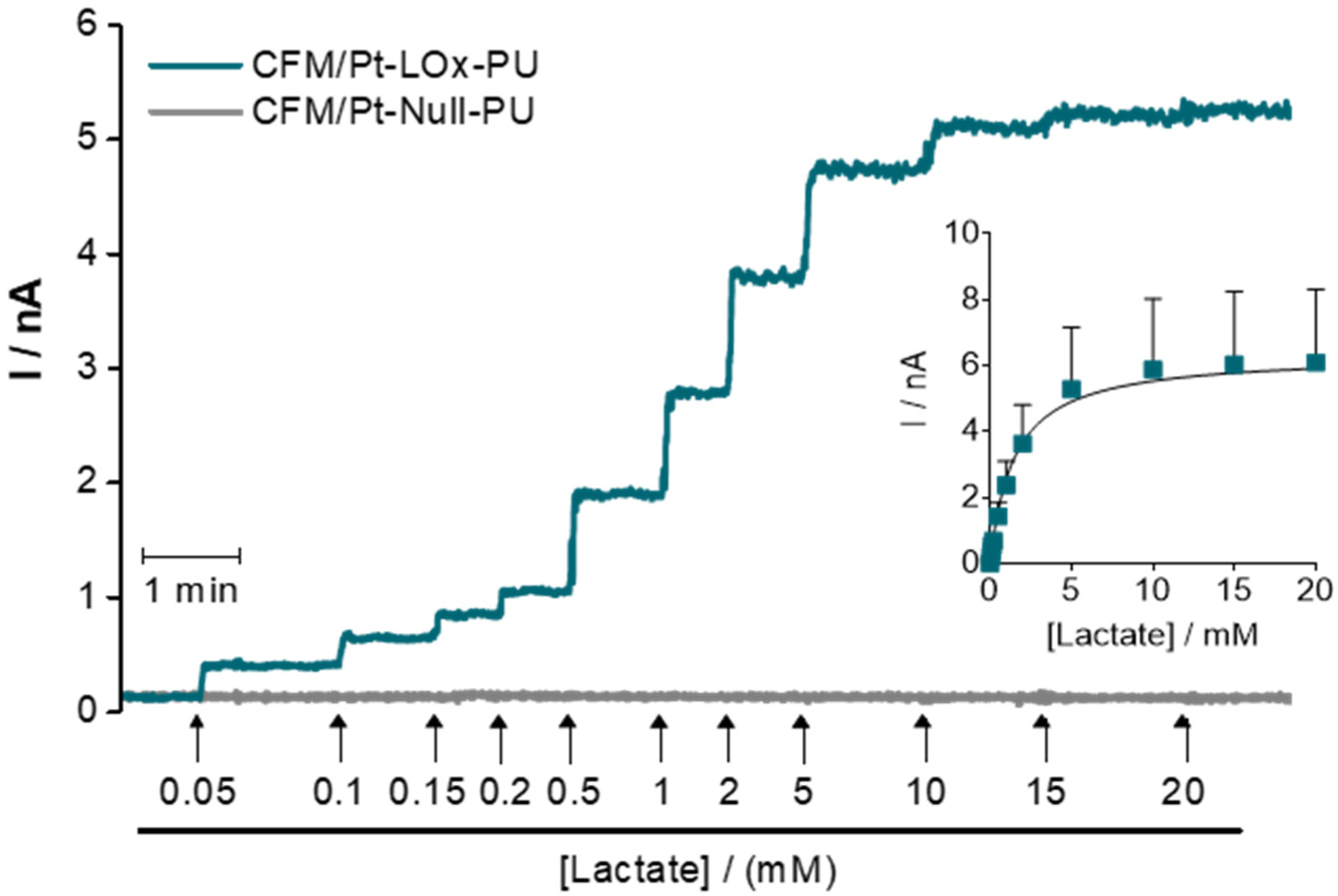

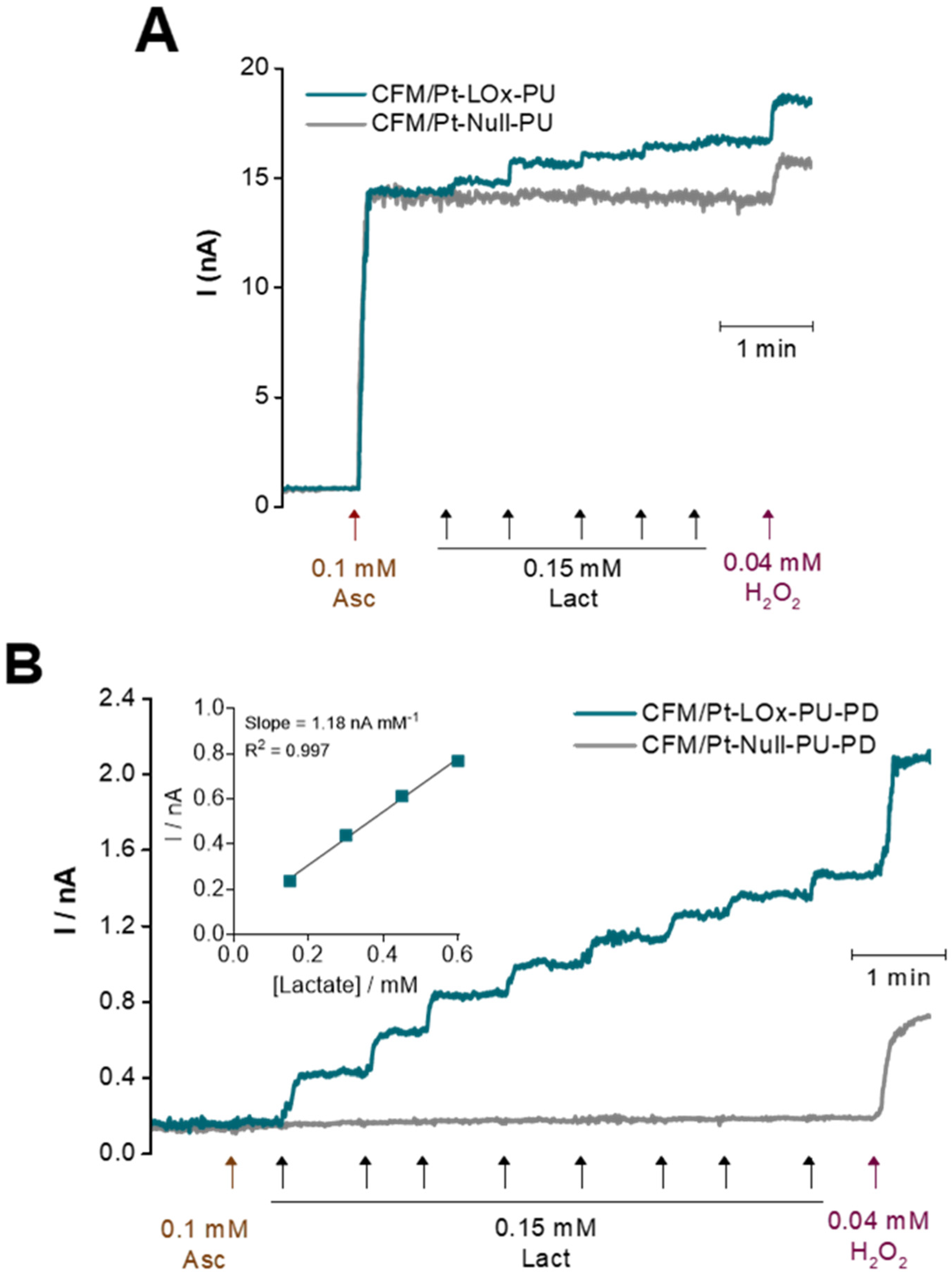
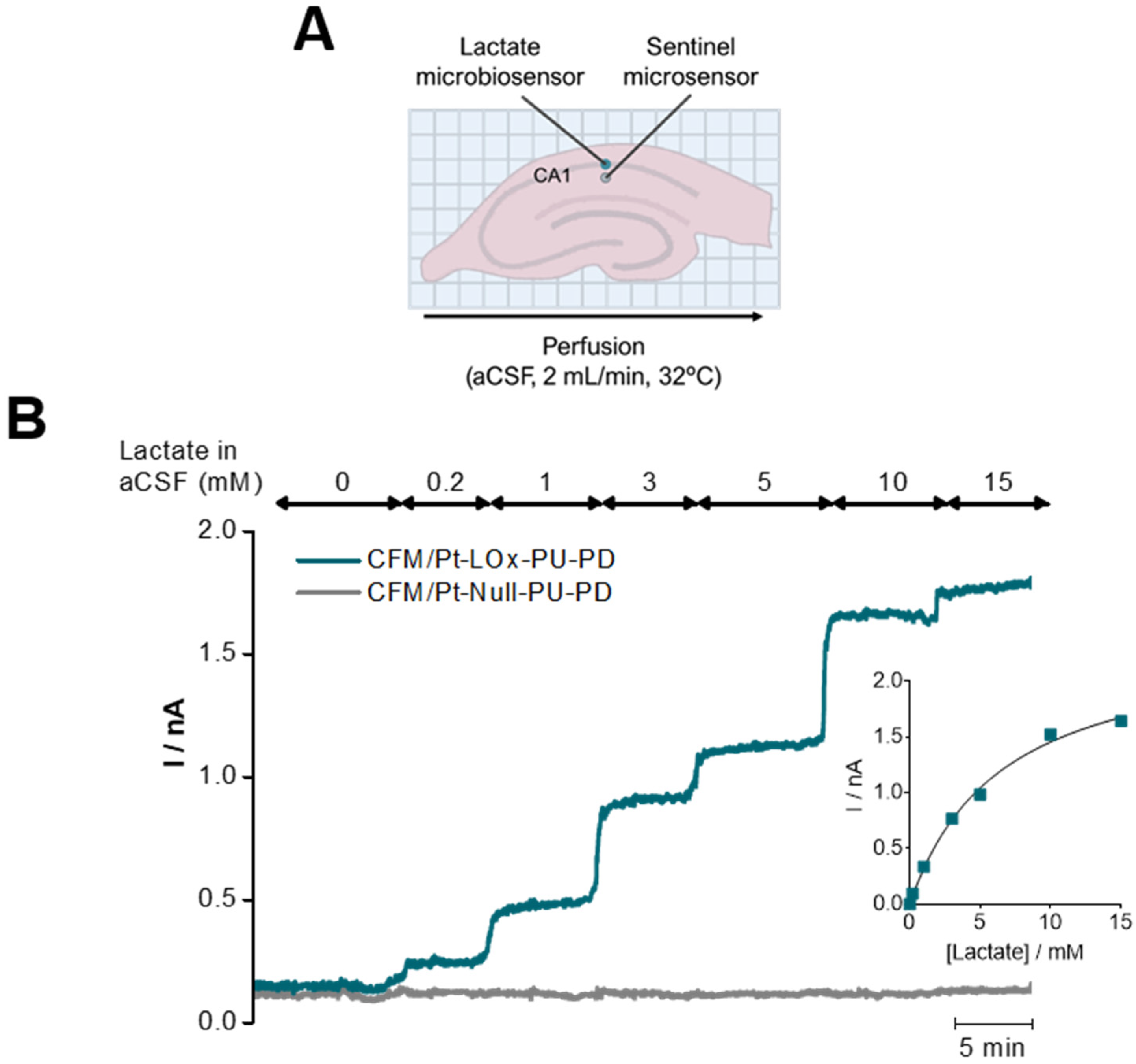
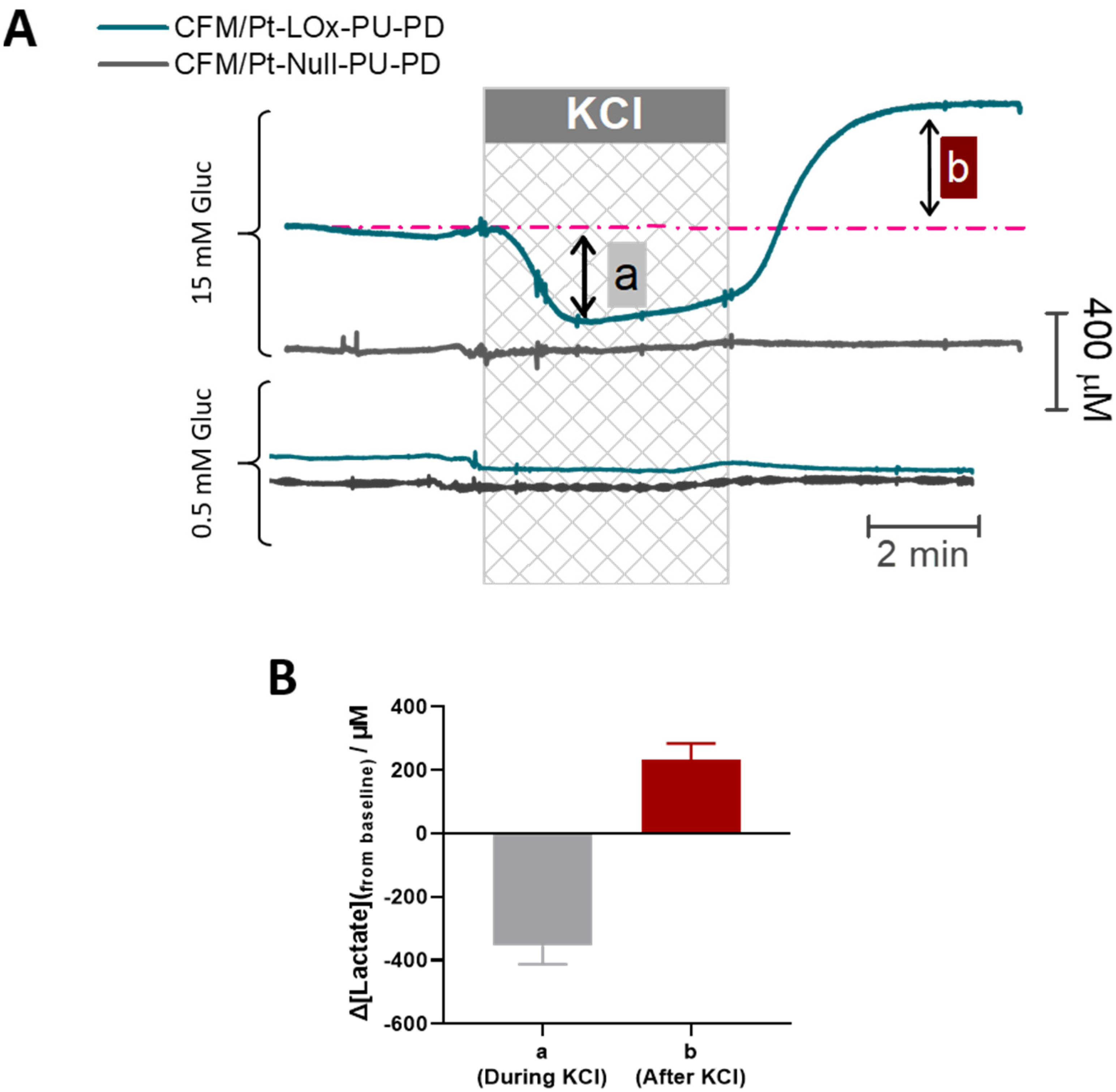
| Microbiosensor (n = 8) | KM,app (mM) | Imax (nA) | Lactate | H2O2 Sensitivity (nA mM−1) | Selectivity (Lactate/AA) | ||
|---|---|---|---|---|---|---|---|
| Sensitivity (nA mM−1) | Linearity (R2) | LOD (mM) | |||||
| CFM/Pt-LOx-PU | 1.4 ± 0.2 | 6.8 ± 2.6 | 2.2 ± 0.7 | 0.994 ± 0.002 | 0.04 ± 0.01 | 109 ± 22 | 0.15 ± 0.06 |
| CFM/Pt-LOx-PU-PD | - | - | 0.6 ± 0.2 | 0.993 ± 0.002 | 0.07 ± 0.01 | 33 ± 11 | 5.31 ± 1.37 * |
| Condition (n) | Sensitivity | ||
|---|---|---|---|
| Lactate (% of Pre-Cal) | H2O2 (% of Pre-Cal) | Ascorbate (nA mM−1) | |
| Pre-calibration (14) | 100 | 100 | 0.20 ± 0.07 |
| After 1st recording (6) | 90.0 ± 9.4 | 92.8 ± 4.0 | 0.08 ± 0.08 |
| After 2nd recording (4) | 91.0 ± 16.1 | 74.8 ± 7.4 * | 0.12 ± 0.12 |
| After 3rd recording (8) | 90.8 ± 5.6 | 71.4 ± 10.8 * | 0.58 ± 0.15 * |
| After 4th recording (4) | 74.0 ± 17.2 | 61.8 ± 7.5 * | 0.69 ± 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, C.; Fernandes, E.; Barbosa, R.M.; Ledo, A. A Platinized Carbon Fiber Microelectrode-Based Oxidase Biosensor for Amperometric Monitoring of Lactate in Brain Slices. Sensors 2022, 22, 7011. https://doi.org/10.3390/s22187011
Dias C, Fernandes E, Barbosa RM, Ledo A. A Platinized Carbon Fiber Microelectrode-Based Oxidase Biosensor for Amperometric Monitoring of Lactate in Brain Slices. Sensors. 2022; 22(18):7011. https://doi.org/10.3390/s22187011
Chicago/Turabian StyleDias, Cândida, Eliana Fernandes, Rui M. Barbosa, and Ana Ledo. 2022. "A Platinized Carbon Fiber Microelectrode-Based Oxidase Biosensor for Amperometric Monitoring of Lactate in Brain Slices" Sensors 22, no. 18: 7011. https://doi.org/10.3390/s22187011
APA StyleDias, C., Fernandes, E., Barbosa, R. M., & Ledo, A. (2022). A Platinized Carbon Fiber Microelectrode-Based Oxidase Biosensor for Amperometric Monitoring of Lactate in Brain Slices. Sensors, 22(18), 7011. https://doi.org/10.3390/s22187011







