Measuring Respiratory Motion for Supporting the Minimally Invasive Destruction of Liver Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Minimally Invasive Methods of Measuring Respiratory Movements of the Liver
Laparoscopic Stereoscopic Reconstruction of Point Displacements on the Surface of the Liver
- the relevance of the images in the video sequences of the left and right cameras—in the time domain—is described below,
- the correspondence of points in the images of the left and right cameras—in the spatial domain—was determined manually on the basis of finding local maxima in the image content.
2.2. Non-Invasive Methods of Measuring Respiratory Movements on the Patient’s Surface
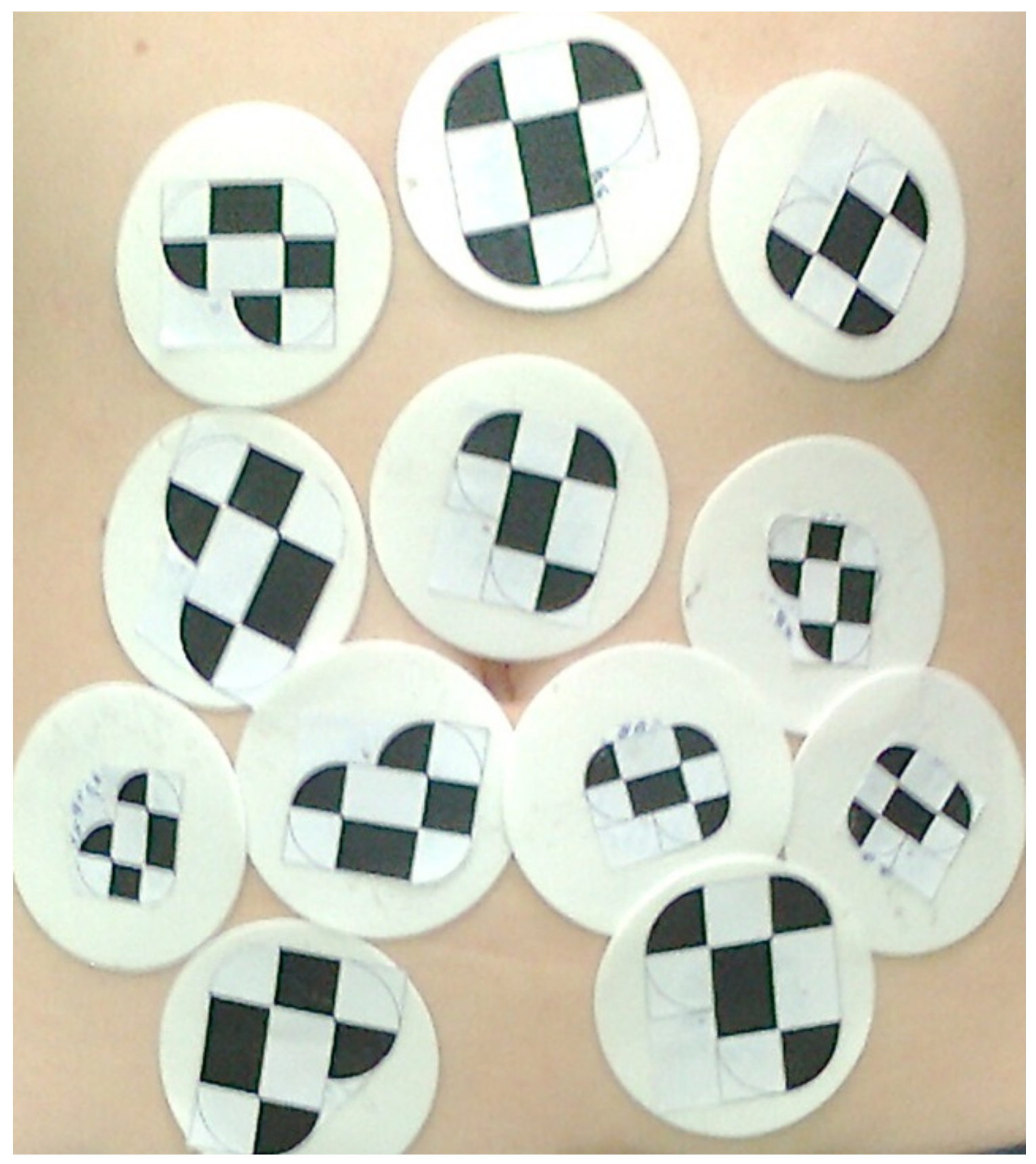
2.2.1. Observation of Breathing Based on External Markers Placed on the Surface of the Abdominal Cavity
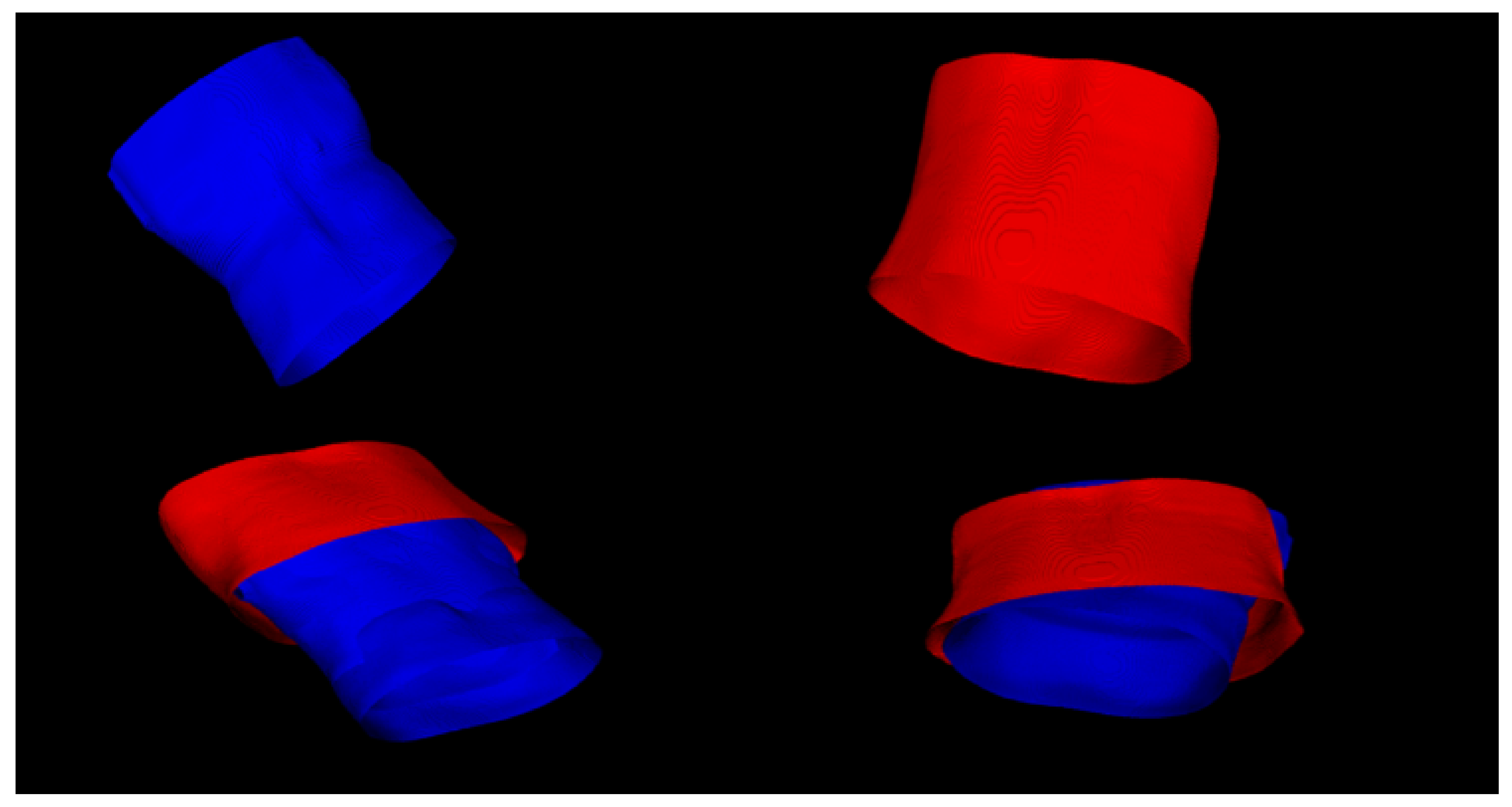
2.2.2. Registration of the Surface of the Abdominal Cavity during Breathing
Proposed Changes in the Method for Searching for Equivalents
- Representation of the point cloud by means of a flat table was abandoned in favor of a tree structure.
- The Euclidean distance condition was replaced by searching for an equivalent along the normal vector of the destination point.
- The search for the best match was preceded by initially selecting a starting set with the condition of the maximum difference of angles between the normal vectors of potential equivalents.
Proposed Changes in the Method for Finding the Result Transform
- Ed (X) is a measure of the distance between the points of the target set and the transposed points of the source set, with each point of the target set having its own transformation.
- Es (X) is a measure of point cloud stiffness that uses the cloud topology matrix and is created on the basis of neighborhood of points, which prevents the cloud shape from changing during algorithm iteration. The alpha coefficient represents the stiffness coefficient, which is changed in the course of the algorithm’s operation at moments when the difference of transforms in subsequent iterations is smaller than the given threshold, and this represents the situation when the source set stops approaching the target set.
- El(X) is the term that introduces correct pairs of equivalents on the basis of known marker positions in the input and target set, whilst the beta ratio represents a measure of the quality of matching.
- α is a stiffness vector influencing the flexibility of the point cloud. The implementation of the process of finding a new transform of the non-rigid version of the ICP algorithm consists of two loops. In the outer loop, the stiffness coefficient is reduced to allow for ever greater local deformations of the initial shape in subsequent iterations. Then, for a given value of the stiffness coefficient, new values of the coefficients of the resultant transformation in the inner loop are calculated.
- β is a weighting factor that enables the gradation of the influence of individual reference points.
3. Results
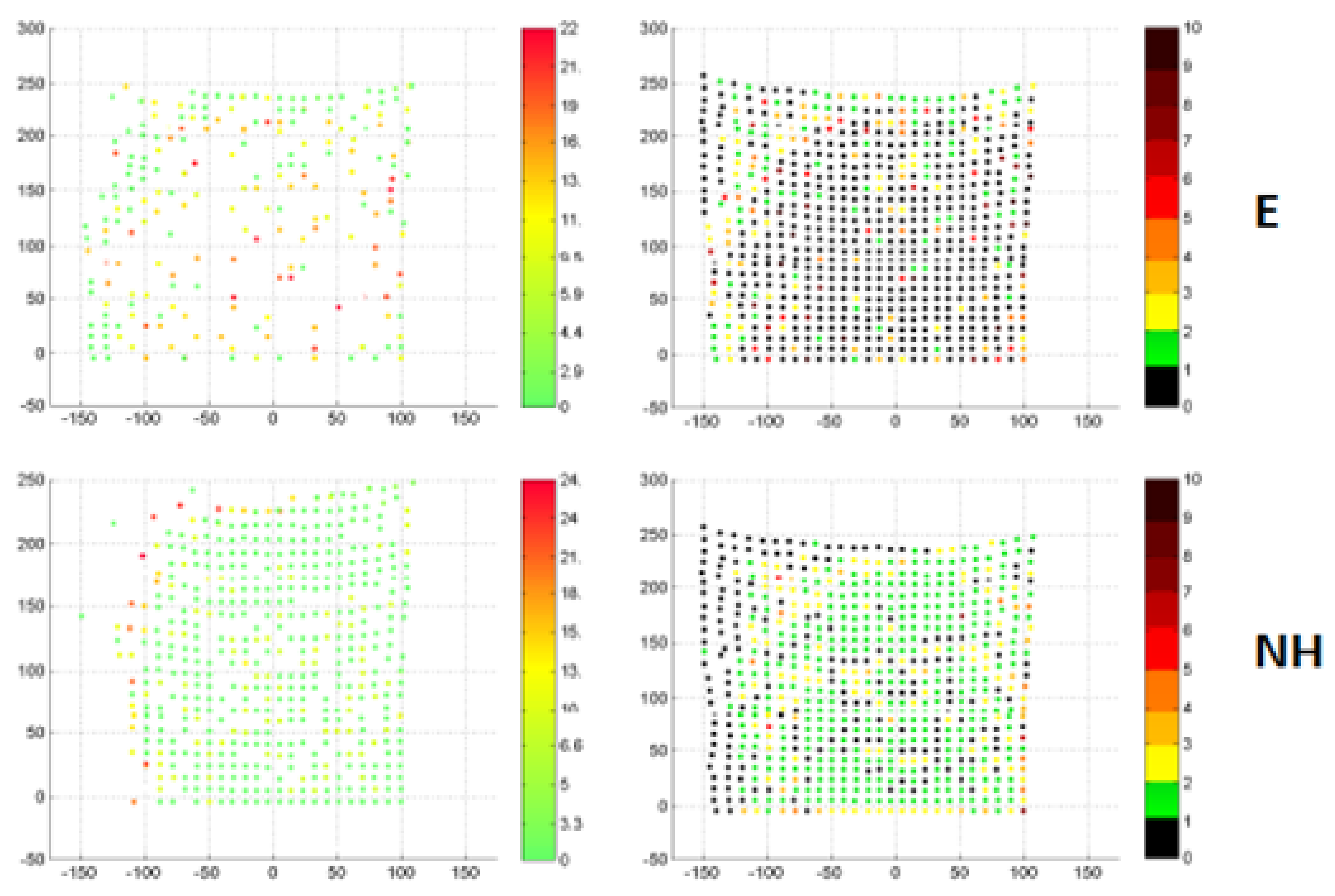
3.1. Stereoscopic Reconstruction of Point Displacements on the Surface of the Liver
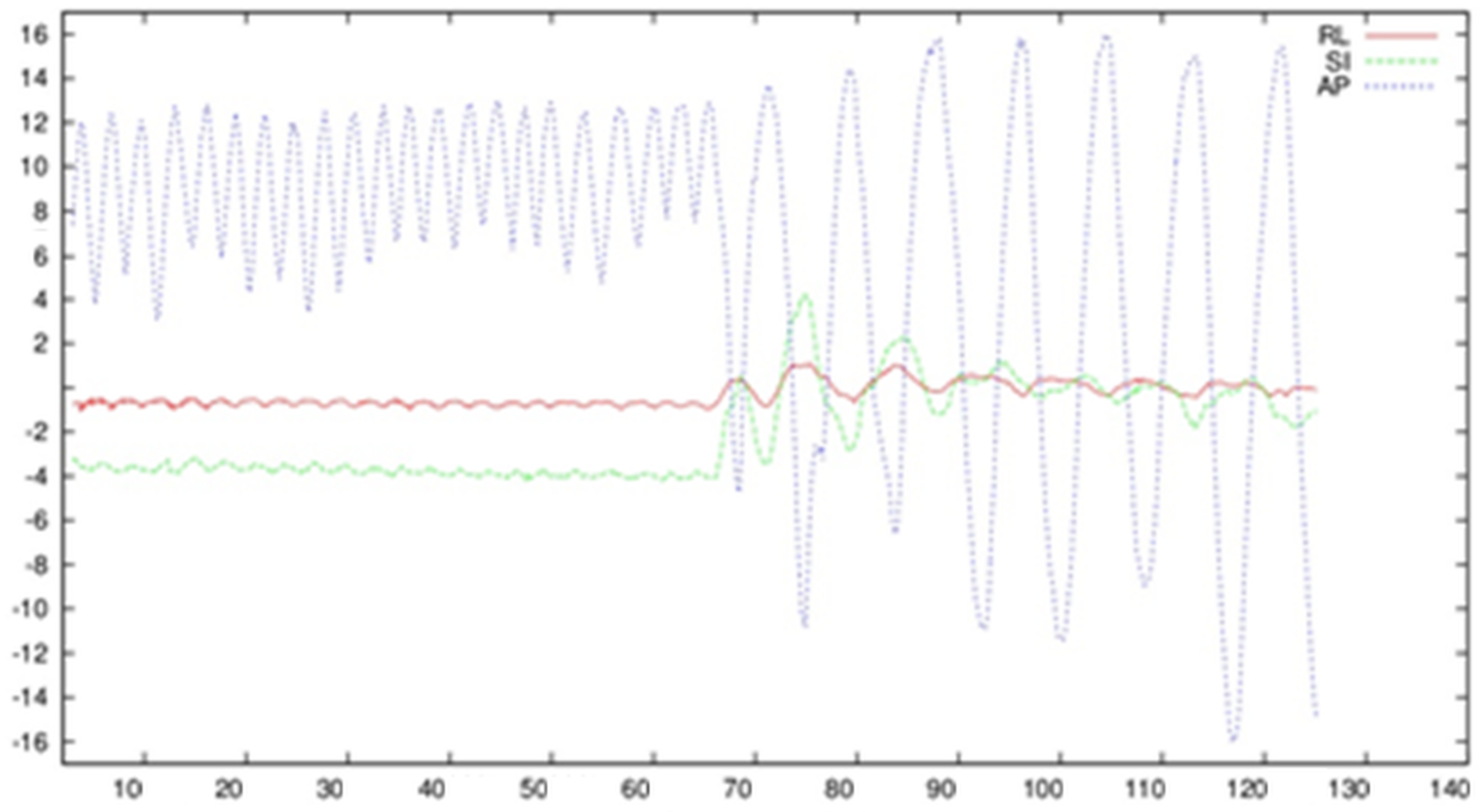
3.2. Observation of Breathing Based on External Markers Placed on the Surface of the Abdominal Cavity
3.3. Recording of the Surface of the Abdominal Cavity during Breathing
4. Discussion
- Percutaneous ablation under the control of CT/external ultrasound probe.
- Ablation using the laparoscopic technique, which uses a laparoscopic trocar under the control of an image from a laparoscopic camera/ultrasound probe.
- Open surgery under the control of an intraoperative probe.
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abou-Alfa, G.K.; Jarnagin, W.; Lowery, M.; D'Angelica, M.; Brown, K.; Ludwig, E.; Covey, A.; Kemeny, N.; Goodman, K.A.; Shia, J.; et al. Liver and Bile Duct Cancer. Abeloff’s Clin. Oncol. Fifth Ed. 2014, 1373–1396.e8. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L.; Atienza, F.; Eidelman, G.; Ávila, P.; Pelliza, M.; Castellanos, E.; Loughlin, G.; Datino, T.; Torrecilla, E.G.; Almendral, J.; et al. Safety and efficacy of cryoablation vs. radiofrequency ablation of septal accessory pathways: Systematic review of the literature and meta-analyses. EP Eur. 2018, 20, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, A.D.; Onur, M.R.; Ozmen, M.N.; Akata, D.; Karcaaltincaba, M. Magnetic Resonance Imaging of Liver Metastasis. Semin. Ultrasound CT MRI 2016, 37, 533–548. [Google Scholar] [CrossRef]
- Seminars in Ultrasound, CT and MRI|Liver Imaging|ScienceDirect.com by Elsevier. Available online: https://www.sciencedirect.com/journal/seminars-in-ultrasound-ct-and-mri/vol/37/issue/6 (accessed on 12 August 2022).
- Banz, V.M.; Müller, P.C.; Tinguely, P.; Inderbitzin, D.; Ribes, D.; Peterhans, M.; Candinas, D.; Weber, S. Intraoperative image-guided navigation system: Development and applicability in 65 patients undergoing liver surgery. Langenbeck’s Arch. Surg. 2016, 401, 495–502. [Google Scholar] [CrossRef]
- Spinczyk, D.; Stronczek, M.; Badura, A.; Sperka, P.; Krywalska, D.; Wolinska, A.; Krasoń, A.; Fabian, S.; Bas, M.; Woloshuk, A.; et al. Initial clinical evaluation of image fusion based on rigid registration and supporting percutaneous liver tumor ablation. Biocybern. Biomed. Eng. 2020, 40, 1378–1390. [Google Scholar] [CrossRef]
- Hartley, R.; Zisserman, A. Multiple View Geometry in Computer Vision, 2nd ed.; Cambridge University Press: Cambridge, UK, 2003; pp. 262–278. [Google Scholar] [CrossRef]
- Spinczyk, D.; Karwan, A.; Rudnicki, J.; Wróblewski, T. Stereoscopic liver surface reconstruction. Wideochirurgia I Inne Technol. Maloinwazyjne 2012, 3, 181–187. [Google Scholar] [CrossRef]
- Tsai, R.Y. A Versatile Camera Calibration Technique for High-Accuracy 3D Machine Vision Metrology Using Off-the-Shelf TV Cameras and Lenses. IEEE J. Robot. Autom. 1987, 3, 323–344. [Google Scholar] [CrossRef]
- Hast, A.; Nysjö, J.; Marchetti, A. Optimal RANSAC-Towards a Repeatable Algorithm for Finding the Optimal Set. J. WSCG 2013, 21, 21–30. [Google Scholar]
- Lin, C.-H.; Zhu, R.; Lucey, S. The conditional lucas & kanade algorithm. Lect. Notes Comput. Sci. 2016, 9909, 793–808. [Google Scholar] [CrossRef]
- Schaerer, J.; Fassi, A.; Riboldi, M.; Cerveri, P.; Baroni, G.; Sarrut, D. Multi-dimensional respiratory motion tracking from markerless optical surface imaging based on deformable mesh registration. Phys. Med. Biol. 2012, 57, 357–373. [Google Scholar] [CrossRef]
- Claron Nav Hx40—Specification. Available online: https://www.claronav.com/microntracker/microntracker-specifications/ (accessed on 21 July 2022).
- Holz, D.; Ichim, A.E.; Tombari, F.; Rusu, R.B.; Behnke, S. Registration with the point cloud library: A modular framework for aligning in 3-D. IEEE Robot. Autom. Mag. 2015, 22, 110–124. [Google Scholar] [CrossRef]
- Otsu, N. Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Amberg, B.; Romdhani, S.; Vetter, T. Optimal Step Nonrigid ICP Algorithms for Surface Registration. In Proceedings of the Computer Vision and Pattern Recognition Conference, CVPR’07, Minneapolis, MN, USA, 17–22 June 2007; pp. 1–8. [Google Scholar] [CrossRef]
- Schaller, C. Time-of-Flight-A New Modality for Radiotherapy. Ph.D. Thesis, University of Erlangen-Nuremberg, Erlangen, Germany, 2011. [Google Scholar]
- Horn, B.K.P.; Hilden, H.M.; Negahdaripour, S. Closed-form solution of absolute orientation using orthonormal matrices. J. Opt. Soc. Am. A 1988, 5, 1127–1135. [Google Scholar] [CrossRef]
- Ivashchenko, O.V.; Kuhlmann, K.F.; van Veen, R.; Pouw, B.; Kok, N.F.M.; Hoetjes, N.J.; Smit, J.N.; Klompenhouwer, E.G.; Nijkamp, J.; Ruers, T.J.M. CBCT-based navigation system for open liver surgery: Accurate guidance toward mobile and deformable targets with a semi-rigid organ approximation and electromagnetic tracking of the liver. Med Phys. 2021, 48, 2145–2159. [Google Scholar] [CrossRef]
- Bernhardt, S.; Nicolau, S.A.; Soler, L.; Doignon, C. The status of augmented reality in laparoscopic surgery as of 2016. Med. Image Anal. 2017, 37, 66–90. [Google Scholar] [CrossRef]
- Liu, X.; Plishker, W.; Zaki, G.F.; Kang, S.; Kane, T.D.; Shekhar, R. On-demand calibration and evaluation for electromagnetically tracked laparoscope in augmented reality visualization. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 1163–1171. [Google Scholar] [CrossRef]
- Xiao, G.; Bonmati, E.; Thompson, S.; Evans, J.; Hipwell, J.; Nikitichev, D.; Gurusamy, K.; Ourselin, S.; Hawkes, D.J.; Davidson, B.; et al. Electromagnetic tracking in image-guided laparoscopic surgery: Comparison with optical tracking and feasibility study of a combined laparoscope and laparoscopic ultrasound system. Med. Phys. 2018, 45, 5094–5104. [Google Scholar] [CrossRef]
- Heiselman, J.S.; Clements, L.W.; Collins, J.A.; Weis, J.; Simpson, A.L.; Geevarghese, S.K. Characterization and correction of intraoperative soft tissue deformation in image-guided laparoscopic liver surgery. J. Med Imaging 2018, 5, 021203. [Google Scholar] [CrossRef]
- Galmén, K.; Jakobsson, J.G.; Freedman, J.; Harbut, P. High frequency jet ventilation during stereotactic ablation of liver tumours: An observational study on blood gas analysis as a measure of lung function during general anaesthesia. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Maier-Hein, L.; Franz, A.; Meinzer, H.-P.; Wolf, I. Comparative assessment of optical tracking systems for soft tissue navigation with fiducial needles. Med. Imaging 2008, 6918, 69181Z. [Google Scholar] [CrossRef]
- Spinczyk, D.; Bas, M. Anisotropic non-rigid Iterative Closest Point Algorithm for respiratory motion abdominal surface matching. Biomed. Eng. Online 2019, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Aliverti, A.; Lacca, D.; LoMauro, A. Quantitative Analysis by 3D Graphics of Thoraco-Abdominal Surface Shape and Breathing Motion. Front. Bioeng. Biotechnol. 2022, 10, 910499. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Chen, R.E.; Kim, E.J.; Akinduro, O.O.; Kerezoudis, P.; Han, P.K.; Si, P.; Freeman, W.D.; Diaz, R.J.; Komotar, R.J.; et al. Augmented reality for the surgeon: Systematic review. Int. J. Med. Robot. 2018, 14, e1914. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Hunt, S.J.; Martin, C.; Nadolski, G.J.; Wood, B.J.; Gade, T.P. Augmented and Mixed Reality: Technologies for Enhancing the Future of IR. J. Vasc. Interv. Radiol. 2020, 31, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Bosch, V.V.D.; Salim, H.S.; Chen, N.-Z.; Stroosma, O.; Bruners, P.; Kuhl, C.K.; Pedersoli, F.; Isfort, P. Augmented Reality-Assisted CT-Guided Puncture: A Phantom Study. Cardiovasc. Interv. Radiol. 2022, 45, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Gadodia, G.; Yanof, J.; Hanlon, A.; Bustos, S.; Weunski, C.; West, K.; Martin, C. Early Clinical Feasibility Evaluation of an Augmented Reality Platform for Guidance and Navigation during Percutaneous Tumor Ablation. J. Vasc. Interv. Radiol. 2022, 33, 333–338. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinczyk, D. Measuring Respiratory Motion for Supporting the Minimally Invasive Destruction of Liver Tumors. Sensors 2022, 22, 6446. https://doi.org/10.3390/s22176446
Spinczyk D. Measuring Respiratory Motion for Supporting the Minimally Invasive Destruction of Liver Tumors. Sensors. 2022; 22(17):6446. https://doi.org/10.3390/s22176446
Chicago/Turabian StyleSpinczyk, Dominik. 2022. "Measuring Respiratory Motion for Supporting the Minimally Invasive Destruction of Liver Tumors" Sensors 22, no. 17: 6446. https://doi.org/10.3390/s22176446
APA StyleSpinczyk, D. (2022). Measuring Respiratory Motion for Supporting the Minimally Invasive Destruction of Liver Tumors. Sensors, 22(17), 6446. https://doi.org/10.3390/s22176446





