Evaluating Different TiO2 Nanoflower-Based Composites for Humidity Detection
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Properties
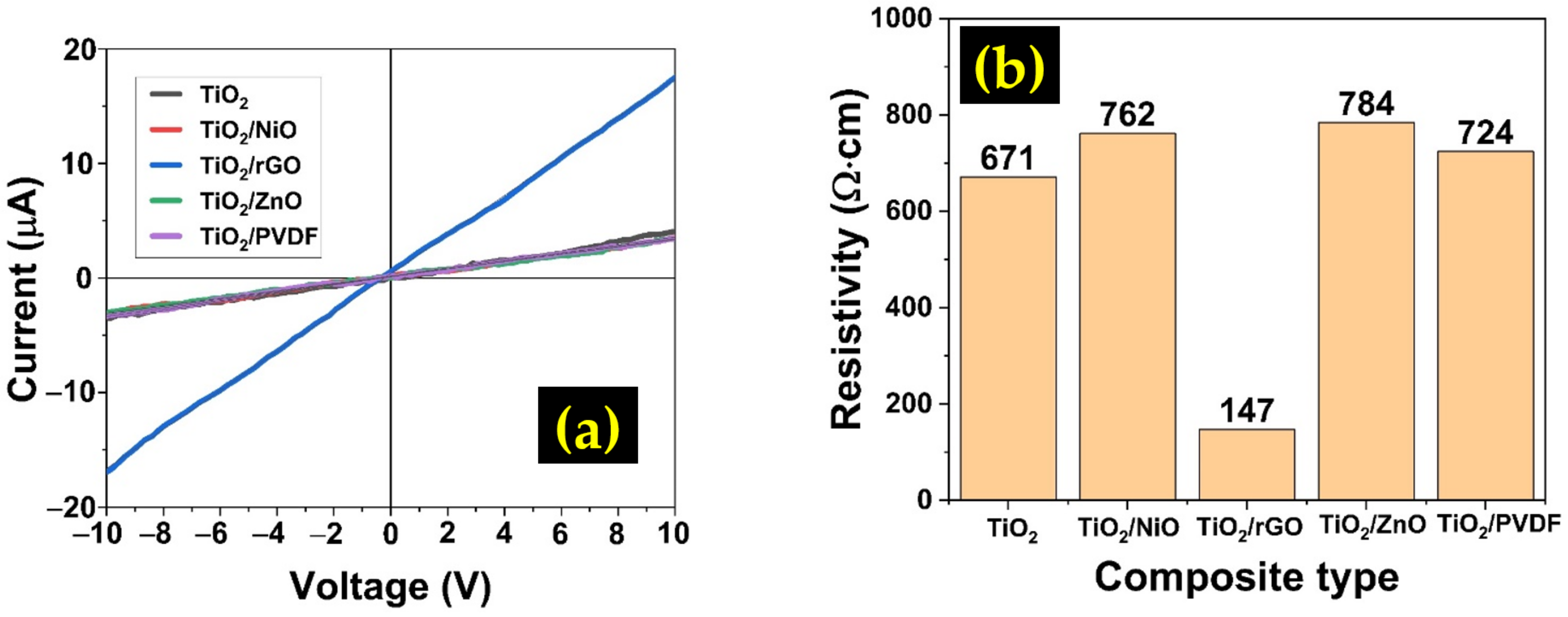
3.2. Electrical Properties
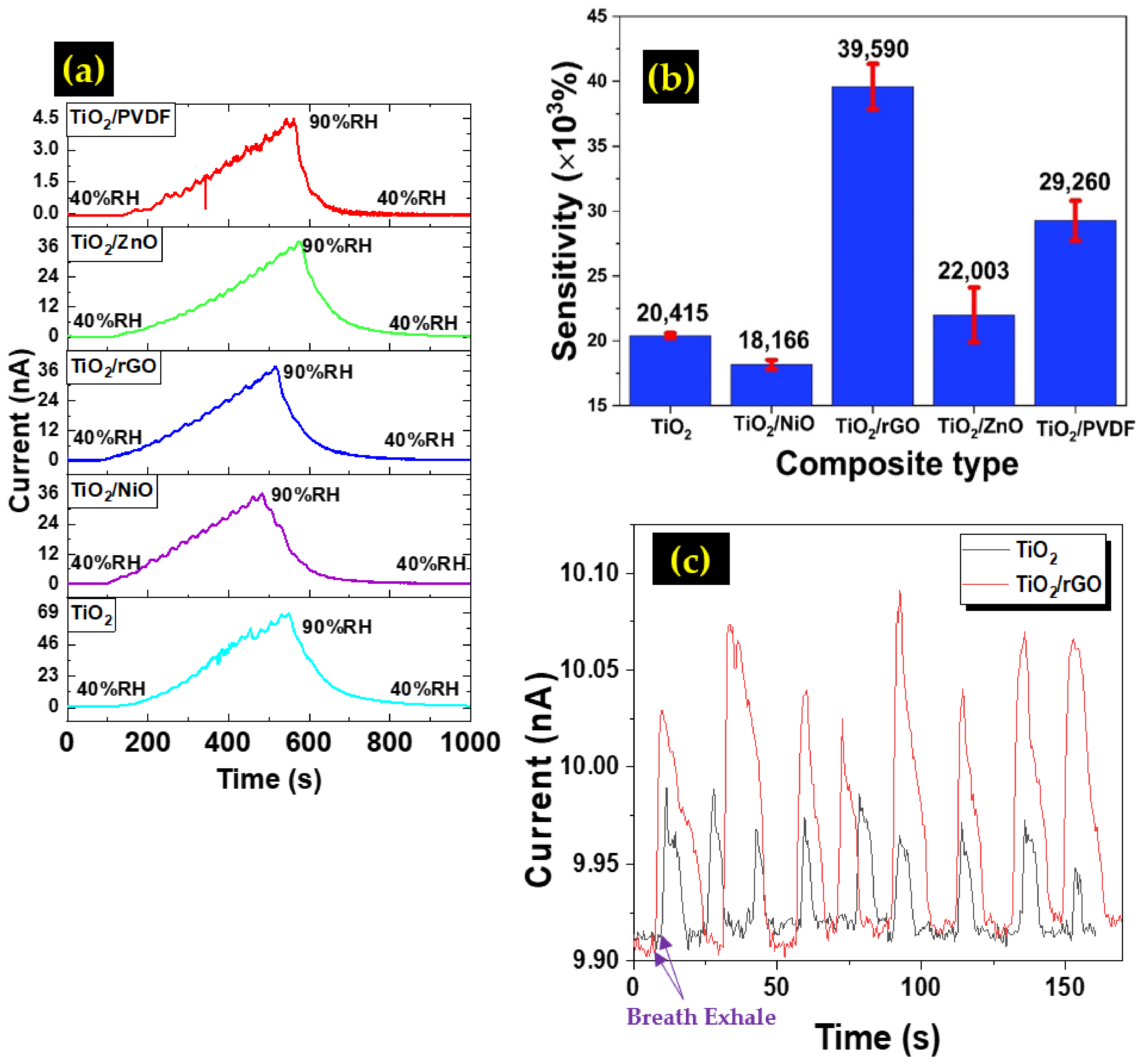
3.3. Humidity Sensor Performance
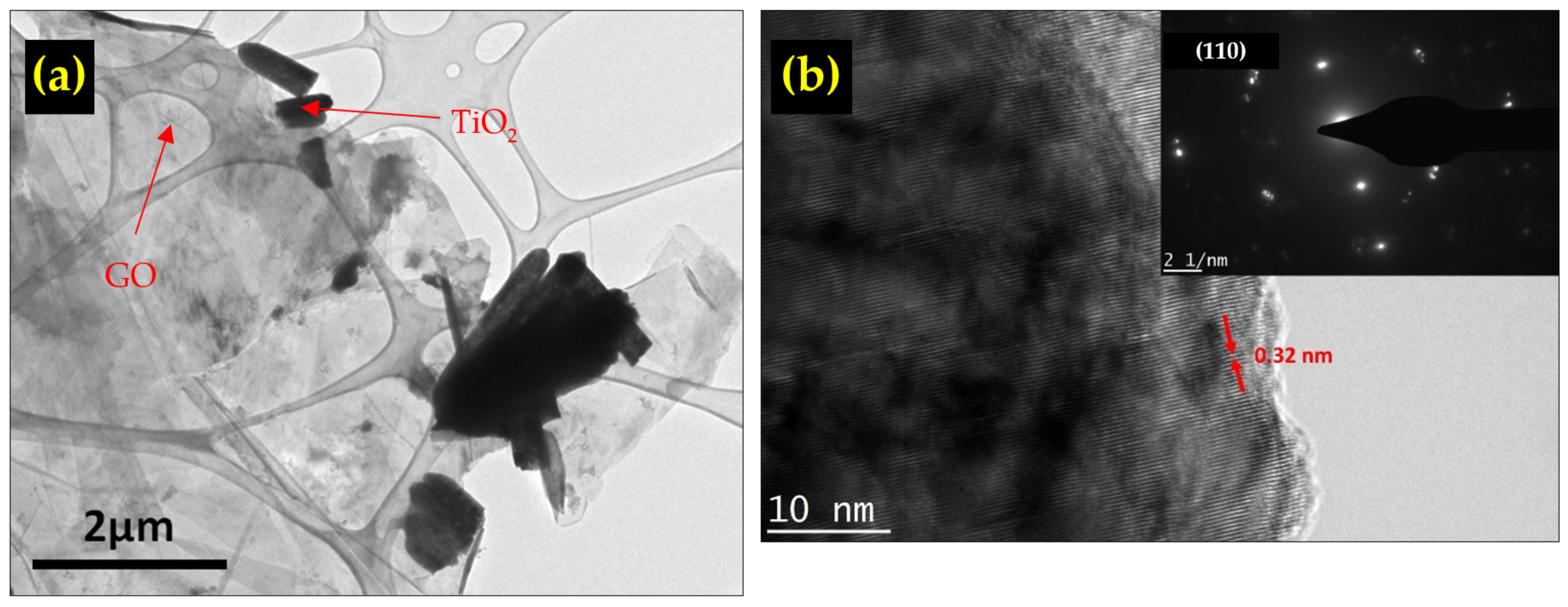
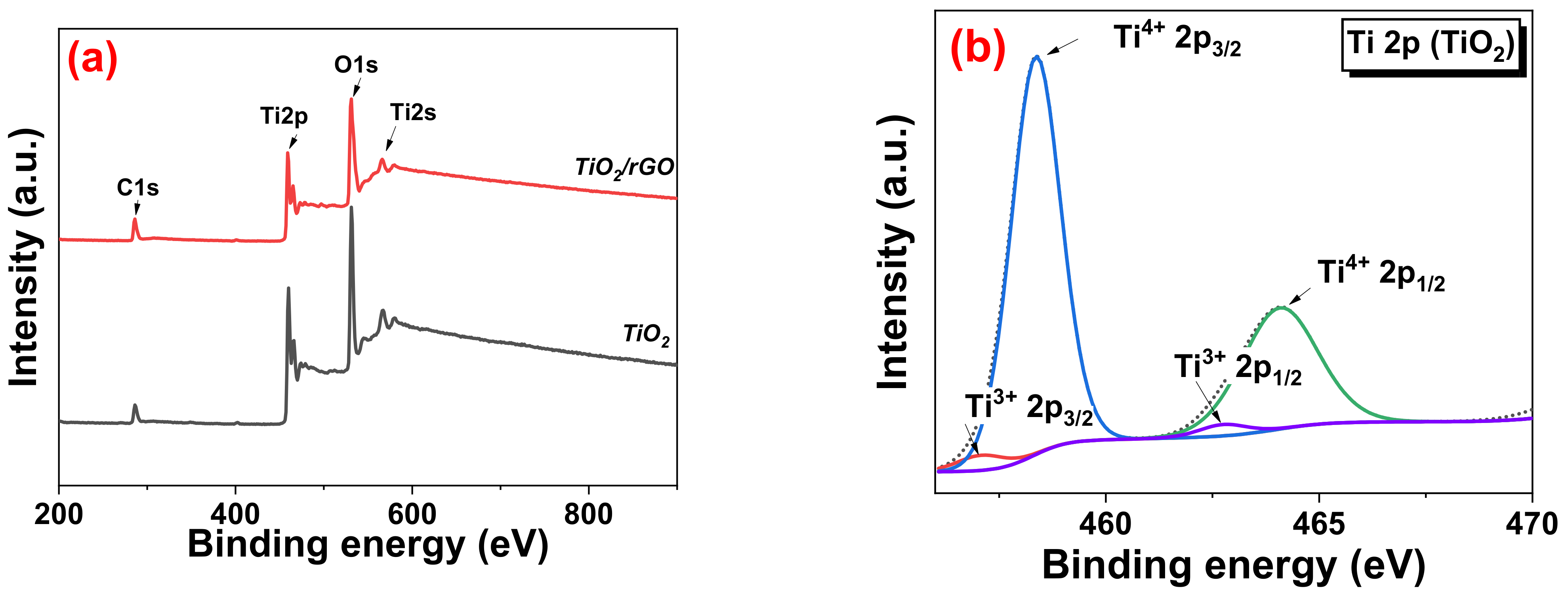
3.4. HRTEM and XPS Analysis of TiO2/rGO
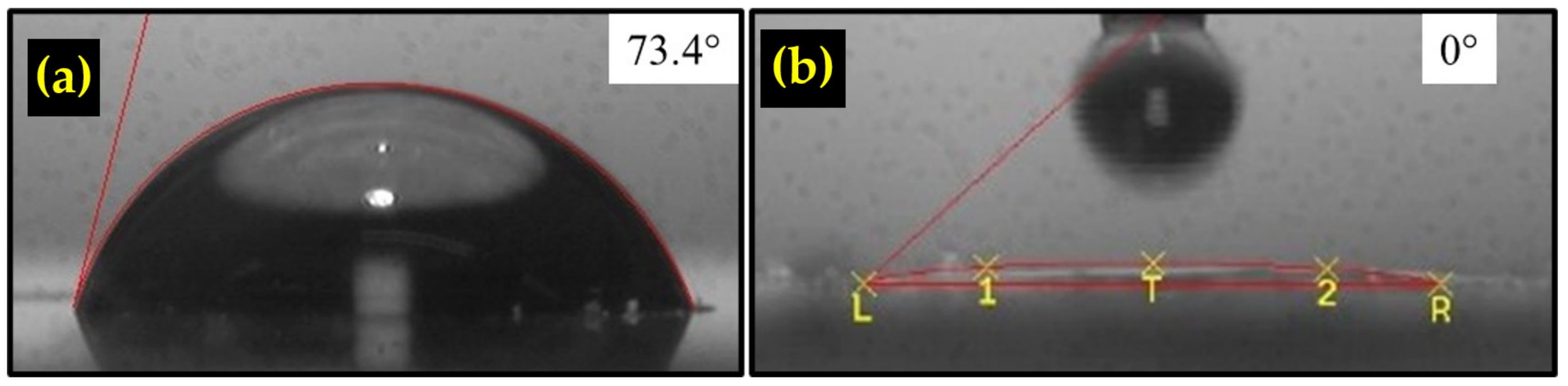
3.5. Hydrophilicity of TiO2/rGO
3.6. Humidity Detection Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cappelli, I.; Fort, A.; Lo Grasso, A.; Panzardi, E.; Mugnaini, M.; Vignoli, V. RH Sensing by Means of TiO2 Nanoparticles: A Comparison among Different Sensing Techniques Based on Modeling and Chemical/Physical Interpretation. Chemosensors 2020, 8, 89. [Google Scholar] [CrossRef]
- Farahani, E.; Mohammadpour, R. Fabrication of flexible self-powered humidity sensor based on super-hydrophilic titanium oxide nanotube arrays. Sci. Rep. 2020, 10, 13032. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liang, K.; Xu, C.; Wang, X.; Ouyang, H.; Huang, J.; Feng, L. Humidity sensor based on heterogeneous CoTiO3/TiO2 film with vertically aligned nanocrystalline structure. Vacuum 2019, 163, 292–300. [Google Scholar] [CrossRef]
- Hou, X.; Lund, P.D.; Li, Y. Controlling anodization time to monitor film thickness, phase composition and crystal orientation during anodic growth of TiO2 nanotubes. Electrochem. Commun. 2022, 134, 107168. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Mamat, M.H.; Malek, M.F.; Suriani, A.B.; Mohamed, A.; Ahmad, M.K.; Alrokayan, S.A.H.; Khan, H.A.; Rusop, M. Growth of titanium dioxide nanorod arrays through the aqueous chemical route under a novel and facile low-cost method. Mater. Lett. 2016, 164, 294–298. [Google Scholar] [CrossRef]
- Ahmad, M.K.; Mokhtar, S.M.; Soon, C.F.; Nafarizal, N.; Suriani, A.B.; Mohamed, A.; Mamat, M.H.; Malek, M.F.; Shimomura, M.; Murakami, K. Raman investigation of rutile-phased TiO2 nanorods/nanoflowers with various reaction times using one step hydrothermal method. J. Mater. Sci. Mater. Electron. 2016, 27, 7920–7926. [Google Scholar] [CrossRef]
- Li, L.; Feng, K.; Huang, D.; Wang, K.; Li, Y.; Guo, Z.; Ng, Y.H.; Jiang, F. Surface plasmon resonance effect of a Pt-nano-particles-modified TiO2 nanoball overlayer enables a significant enhancement in efficiency to 3.5% for a Cu2ZnSnS4-based thin film photocathode used for solar water splitting. Chem. Eng. J. 2020, 396, 125264. [Google Scholar] [CrossRef]
- Jyothilal, H.; Shukla, G.; Walia, S.; Kundu, S.; Angappane, S. Humidity sensing and breath analyzing applications of TiO2 slanted nanorod arrays. Sens. Actuators A Phys. 2020, 301, 111758. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Y.; Zhou, C.; Xiao, Y.; Meng, B.; Wang, Z.; Huang, D.; Xing, C.; Peng, Z. High-Performance Humidity Sensor Based on Urchin-Like Composite of Ti3C2 MXene-Derived TiO2 Nanowires. ACS Appl. Mater. Interfaces 2019, 11, 38116–38125. [Google Scholar] [CrossRef]
- Poonia, E.; Mishra, P.K.; Kiran, V.; Sangwan, J.; Kumar, R.; Rai, P.K.; Tomer, V.K. Aero-gel assisted synthesis of anatase TiO2 nanoparticles for humidity sensing application. Dalton Trans. 2018, 47, 6293–6298. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Y.; Cui, X.; Cheng, P.; Wang, B.; Gao, Y.; Li, X.; Yang, T.; Zhang, T.; Lu, G. Humidity-Sensing Properties of Urchinlike CuO Nanostructures Modified by Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2014, 6, 3888–3895. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, H.; Li, P.; Wang, D.; Yang, Z. Humidity Sensing Properties of Metal Organic Framework-Derived Hollow Ball-Like TiO2 Coated QCM Sensor. IEEE Sens. J. 2019, 19, 2909–2915. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Guo, Y.; Lv, X.; Dou, X. 2D TiO2 nanosheets for ultrasensitive humidity sensing application benefited by abundant surface oxygen vacancy defects. Sens. Actuators B Chem. 2018, 262, 350–358. [Google Scholar] [CrossRef]
- Farzaneh, A.; Esrafili, M.D.; Mermer, Ö. Development of TiO2 nanofibers based semiconducting humidity sensor: Adsorption kinetics and DFT computations. Mater. Chem. Phys. 2020, 239, 121981. [Google Scholar] [CrossRef]
- Haq, Y.-U.; Ullah, R.; Mazhar, S.; Khattak, R.; Qarni, A.A.; Haq, Z.-U.; Amin, S. Synthesis and characterization of 2D MXene: Device fabrication for humidity sensing. J. Sci. Adv. Mater. Devices 2022, 7, 100390. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, Y.Z.; Huang, S.S.; Ren, S.T.; Li, P.; Li, J.J. Resistive and capacitive response of nitrogen-doped TiO2 nanotubes film humidity sensor. Nanotechnology 2011, 22, 025501. [Google Scholar] [CrossRef]
- Si, R.; Xie, X.; Li, T.; Zheng, J.; Cheng, C.; Wang, C. TiO2/NaNbO3 heterojunction for boosted humidity sensing ability. Sens. Actuators B Chem. 2020, 309, 127803. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, J.E.; Kim, Y.H.; Kim, J.J.; Shim, Y.-S.; Kim, S.Y.; Lee, M.H.; Jang, H.W. Room temperature humidity sensors based on rGO/MoS2 hybrid composites synthesized by hydrothermal method. Sens. Actuators B Chem. 2018, 258, 775–782. [Google Scholar] [CrossRef]
- Sasikumar, M.; Subiramaniyam, N.P. Microstructure, electrical and humidity sensing properties of TiO2/polyaniline nanocomposite films prepared by sol–gel spin coating technique. J. Mater. Sci. Mater. Electron. 2018, 29, 7099–7106. [Google Scholar] [CrossRef]
- Araújo, E.S.; Libardi, J.; Faia, P.M.; de Oliveira, H.P. Humidity-sensing properties of hierarchical TiO2:ZnO composite grown on electrospun fibers. J. Mater. Sci. Mater. Electron. 2017, 28, 16575–16583. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Zhang, J. Synthesis of high response gold/titanium dioxide humidity sensor and its application in human respiration. Ceram. Int. 2021, 47, 30880–30887. [Google Scholar] [CrossRef]
- Musa, M.Z.; Mamat, M.H.; Vasimalai, N.; Shameem Banu, I.B.; Malek, M.F.; Ahmad, M.K.; Suriani, A.B.; Mohamed, A.; Rusop, M. Fabrication and structural properties of flower-like TiO2 nanorod array films grown on glass substrate without FTO layer. Mater. Lett. 2020, 273, 127902. [Google Scholar] [CrossRef]
- Parimon, N.; Mamat, M.H.; Shameem Banu, I.B.; Vasimalai, N.; Ahmad, M.K.; Suriani, A.B.; Mohamed, A.; Rusop, M. Fabrication, structural, optical, electrical, and humidity sensing characteristics of hierarchical NiO nanosheet/nanoball-flower-like structure films. J. Mater. Sci. Mater. Electron. 2020, 31, 11673–11687. [Google Scholar] [CrossRef]
- Ismail, A.S.; Mamat, M.H.; Sin, N.D.; Malek, M.F.; Zoolfakar, A.S.; Suriani, A.B.; Mohamed, A.; Ahmad, M.K.; Rusop, M. Fabrication of hierarchical Sn-doped ZnO nanorod arrays through sonicated sol–gel immersion for room temperature, resistive-type humidity sensor applications. Ceram. Int. 2016, 42, 9785–9795. [Google Scholar] [CrossRef]
- Mallick, S.; Ahmad, Z.; Touati, F.; Shakoor, R.A. Improvement of humidity sensing properties of PVDF-TiO2 nanocomposite films using acetone etching. Sens. Actuators B Chem. 2019, 288, 408–413. [Google Scholar] [CrossRef]
- Shuangyu, L.; Ping, L.; Qiutao, Z.; Juan, H. Preparation of (B,N)-codoped TiO2 micro-nano composite powder using TiBN powder as a precursor. Ceram. Int. 2022, 48, 10079–10085. [Google Scholar]
- Khezami, L.; Elamin, N.; Modwi, A.; Taha, K.K.; Amer, M.S.; Bououdina, M. Mesoporous Sn@TiO2 nanostructures as excellent adsorbent for Ba ions in aqueous solution. Ceram. Int. 2022, 48, 5805–5813. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Li, S.; Xi, Y.; Cai, C.; Liu, W.; Golosov, D.; Zavadski, S.; Melnikov, S. Preparation of Fe3+ Doped High-Ordered TiO2 Nanotubes Arrays with Visible Photocatalytic Activities. Nanomaterials 2020, 10, 2107. [Google Scholar] [CrossRef]
- Kibasomba, P.M.; Dhlamini, S.; Maaza, M.; Liu, C.-P.; Rashad, M.M.; Rayan, D.A.; Mwakikunga, B.W. Strain and grain size of TiO2 nanoparticles from TEM, Raman spectroscopy and XRD: The revisiting of the Williamson-Hall plot method. Results Phys. 2018, 9, 628–635. [Google Scholar] [CrossRef]
- Uysal, M.; Algül, H.; Duru, E.; Kahraman, Y.; Alp, A.; Akbulut, H. Tribological properties of Ni–W–TiO2–GO composites produced by ultrasonically–assisted pulse electro co–deposition. Surf. Coat. Technol. 2021, 410, 126942. [Google Scholar]
- Luo, L.; Yuan, M.; Sun, H.; Peng, T.; Xie, T.; Chen, Q.; Chen, J. Effect of calcination temperature on the humidity sensitivity of TiO2/graphene oxide nanocomposites. Mater. Sci. Semicond. Process. 2019, 89, 186–193. [Google Scholar] [CrossRef]
- Megat Hasnan, M.M.I.; Nayan, N.; Hamed, N.K.A.; Azman, Z.; Ahmad, M.K.; Mohamed Ali, M.S.; Mohd Yusop, M.Z.; Noor, I.M. Improvement of facile hydrothermal TiO2 rutile nanorod-flower using second HiPIMS deposition for DSSC performance enhancement. Opt. Mater. 2021, 117, 111149. [Google Scholar]
- Debnath, K.; Majumder, T.; Mondal, S.P. Photoelectrochemical study of hydrothermally grown vertically aligned rutile TiO2 nanorods. Chem. Phys. 2022, 561, 111609. [Google Scholar] [CrossRef]
- Qu, G.; Fan, G.; Zhou, M.; Rong, X.; Li, T.; Zhang, R.; Sun, J.; Chen, D. Graphene-Modified ZnO Nanostructures for Low-Temperature NO2 Sensing. ACS Omega 2019, 4, 4221–4232. [Google Scholar] [CrossRef] [Green Version]
- Radoń, A.; Włodarczyk, P.; Łukowiec, D. Structure, temperature and frequency dependent electrical conductivity of oxidized and reduced electrochemically exfoliated graphite. Phys. E Low Dimens. Syst. Nanostruct. 2018, 99, 82–90. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Selective Removal of Hydroxyl Groups from Graphene Oxide. Chem. Eur. J. 2013, 19, 2005–2011. [Google Scholar] [CrossRef]
- Gupta, S.; Joshi, P.; Narayan, J. Electron mobility modulation in graphene oxide by controlling carbon melt lifetime. Carbon 2020, 170, 327–337. [Google Scholar] [CrossRef]
- Yang, C.R.; Tseng, S.F.; Chen, Y.T. Characteristics of Graphene Oxide Films Reduced by Using an Atmospheric Plasma System. Nanomaterials 2018, 8, 802. [Google Scholar]
- Tan, S.M.; Ambrosi, A.; Chua, C.K.; Pumera, M. Electron transfer properties of chemically reduced graphene materials with different oxygen contents. J. Mater. Chem. A 2014, 2, 10668–10675. [Google Scholar]
- Yang, J.; Hu, Y.; Jin, C.; Zhuge, L.; Wu, X. Preparation of TiO2/single layer graphene composite films via a novel interface-facilitated route. Appl. Surf. Sci. 2020, 503, 144334. [Google Scholar] [CrossRef]
- Ding, H.; Wei, Y.; Wu, Z.; Tao, K.; Ding, M.; Xie, X.; Wu, J. Recent Advances in Gas and Humidity Sensors Based on 3D Structured and Porous Graphene and Its Derivatives. ACS Mater. Lett. 2020, 2, 1381–1411. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Yang, S.-H. Carrier transport and photoresponse for heterojunction diodes based on the reduced graphene oxide-based TiO2 composite and p-type Si. Appl. Phys. A 2014, 116, 91–95. [Google Scholar] [CrossRef]
- Ismail, A.S.; Mamat, M.H.; Malek, M.F.; Yusoff, M.M.; Mohamed, R.; Sin, N.D.M.; Suriani, A.B.; Rusop, M. Heterogeneous SnO2/ZnO nanoparticulate film: Facile synthesis and humidity sensing capability. Mater. Sci. Semicond. Process. 2018, 81, 127–138. [Google Scholar] [CrossRef]
- Mannaa, M.A.; Qasim, K.F.; Alshorifi, F.T.; El-Bahy, S.M.; Salama, R.S. Role of NiO Nanoparticles in Enhancing Structure Properties of TiO2 and Its Applications in Photodegradation and Hydrogen Evolution. ACS Omega 2021, 6, 30386–30400. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhao, H.; Wang, S.; Hu, J.; Chen, Z. NiO-TiO2 p-n Heterojunction for Solar Hydrogen Generation. Catalysts 2021, 11, 1427. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Mamat, M.H.; Abdullah, M.A.R.; Ismail, A.S.; Malek, M.F.; Zoolfakar, A.S.; Al Junid, S.A.M.; Suriani, A.B.; Mohamed, A.; Ahmad, M.K.; et al. Coupling heterostructure of thickness-controlled nickel oxide nanosheets layer and titanium dioxide nanorod arrays via immersion route for self-powered solid-state ultraviolet photosensor applications. Measurement 2020, 149, 106982. [Google Scholar] [CrossRef]
- Yadav, B.C.; Srivastava, R.; Dwivedi, C.D. Synthesis and characterization of ZnO–TiO2 nanocomposite and its application as a humidity sensor. Philos. Mag. 2008, 88, 1113–1124. [Google Scholar] [CrossRef]
- Lee, J.; Cho, D.; Jeong, Y. A resistive-type sensor based on flexible multi-walled carbon nanotubes and polyacrylic acid composite films. Solid State Electron. 2013, 87, 80–84. [Google Scholar] [CrossRef]
- Khalifa, M.; Wuzella, G.; Lammer, H.; Mahendran, A.R. Smart paper from graphene coated cellulose for high-performance humidity and piezoresistive force sensor. Synth. Met. 2020, 266, 116420. [Google Scholar] [CrossRef]
- Mamat, M.H.; Ismail, A.S.; Parimon, N.; Vasimalai, N.; Abdullah, M.H.; Malek, M.F.; Yaakob, M.K.; Ahmad, M.K.; Nafarizal, N.; Suriani, A.B.; et al. Heterojunction of SnO2 nanosheet/arrayed ZnO nanorods for humidity sensing. Mater. Chem. Phys. 2022, 288, 126436. [Google Scholar]
- Parimon, N.; Mamat, M.; Banu, I.S.; Vasimalai, N.; Ahmad, M.; Suriani, A.; Mohamed, A.; Rusop, M. Annealing temperature dependency of structural, optical and electrical characteristics of manganese-doped nickel oxide nanosheet array films for humidity sensing applications. Nanomater. Nanotechnol. 2021, 11, 1847980420982788. [Google Scholar] [CrossRef]
- Turkani, V.S.; Maddipatla, D.; Narakathu, B.B.; Saeed, T.S.; Obare, S.O.; Bazuin, B.J.; Atashbar, M.Z. A highly sensitive printed humidity sensor based on a functionalized MWCNT/HEC composite for flexible electronics application. Nano. Adv. 2019, 1, 2311–2322. [Google Scholar] [CrossRef] [Green Version]
- Kano, S.; Fujii, M. All-Painting Process To Produce Respiration Sensor Using Humidity-Sensitive Nanoparticle Film and Graphite Trace. ACS Sustain. Chem. Eng. 2018, 6, 12217–12223. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Hassan, A.; Hassan, G.; Bae, J.; Lee, C.H. All-printed humidity sensor based on graphene/methyl-red composite with high sensitivity. Carbon 2016, 105, 23–32. [Google Scholar] [CrossRef]
- Yoshida, A.; Wang, Y.-F.; Tachibana, S.; Hasegawa, A.; Sekine, T.; Takeda, Y.; Hong, J.; Kumaki, D.; Shiba, T.; Tokito, S. Printed, all-carbon-based flexible humidity sensor using a cellulose nanofiber/graphene nanoplatelet composite. Carbon Trends 2022, 7, 100166. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, Y.; Fang, Z.; Kuang, Y.; Zhang, Y.; Peng, C.; Chen, G. Flexible and Highly Sensitive Humidity Sensor Based on Cellulose Nanofibers and Carbon Nanotube Composite Film. Langmuir 2019, 35, 4834–4842. [Google Scholar] [CrossRef]
- Lin, W.-D.; Liao, C.-T.; Chang, T.-C.; Chen, S.-H.; Wu, R.-J. Humidity sensing properties of novel graphene/TiO2 composites by sol–gel process. Sens. Actuators B Chem. 2015, 209, 555–561. [Google Scholar]
- Nunes Simonetti, E.A.; Cardoso de Oliveira, T.; Enrico do Carmo Machado, Á.; Coutinho Silva, A.A.; Silva dos Santos, A.; de Simone Cividanes, L. TiO2 as a gas sensor: The novel carbon structures and noble metals as new elements for enhancing sensitivity—A review. Ceram. Int. 2021, 47, 17844–17876. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Kozbial, A.; Shenoy, G.; Zhou, F.; McGinley, R.; Ireland, P.; Morganstein, B.; Kunkel, A.; Surwade, S.P.; et al. Effect of airborne contaminants on the wettability of supported graphene and graphite. Nat. Mater. 2013, 12, 925–931. [Google Scholar] [CrossRef]
- Su, P.-G.; Chen, F.-Y.; Wei, C.-H. Simple one-pot polyol synthesis of Pd nanoparticles, TiO2 microrods and reduced graphene oxide ternary composite for sensing NH3 gas at room temperature. Sens. Actuators B Chem. 2018, 254, 1125–1132. [Google Scholar] [CrossRef]
- Sun, L.; Haidry, A.A.; Fatima, Q.; Li, Z.; Yao, Z. Improving the humidity sensing below 30% RH of TiO2 with GO modification. Mater. Res. Bull. 2018, 99, 124–131. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, S.; Wang, S.; Guo, Q.; Guo, J. The Roles of Reduced Ti Cations and Oxygen Vacancies in Water Adsorption and Dissociation on SrTiO3. J. Phys. Chem. C 2014, 118, 2469–2474. [Google Scholar]
- Lv, X.; Yang, G.; Feng, C.; Lin, T. Highly sensitive humidity sensor based on the solid solution Zr0.2Ti0.8O2 nanofibers. J. Alloys Compd. 2022, 891, 161958. [Google Scholar] [CrossRef]
- Chen, X.; Peng, X.; Jiang, L.; Yuan, X.; Fei, J.; Zhang, W. Photocatalytic removal of antibiotics by MOF-derived Ti3+− and oxygen vacancy-doped anatase/rutile TiO2 distributed in a carbon matrix. Chem. Eng. J. 2022, 427, 130945. [Google Scholar] [CrossRef]
- Jagtap, S.; Priolkar, K.R. Evaluation of ZnO nanoparticles and study of ZnO–TiO2 composites for lead free humidity sensors. Sens. Actuators B Chem. 2013, 183, 411–418. [Google Scholar]
- Marliere, C.; Poncharal, P.; Vaccarini, L.; Zahab, A. Effect of Gas Adsorption on the Electrical Properties of Single-Walled Carbon Nanotubes Mats. MRS Proc. 1999, 593, 173. [Google Scholar]
- Yusoff, M.M.; Mamat, M.H.; Ismail, A.S.; Malek, M.F.; Khusaimi, Z.; Suriani, A.B.; Mohamed, A.; Ahmad, M.K.; Rusop, M. Enhancing the performance of self-powered ultraviolet photosensor using rapid aqueous chemical-grown aluminum-doped titanium oxide nanorod arrays as electron transport layer. Thin Solid Film. 2018, 655, 1–12. [Google Scholar] [CrossRef]
- Imanishi, A.; Tsuji, E.; Nakato, Y. Dependence of the Work Function of TiO2 (Rutile) on Crystal Faces, Studied by a Scanning Auger Microprobe. J. Phys. Chem. C 2007, 111, 2128–2132. [Google Scholar] [CrossRef]
- Könenkamp, R.; Rieck, I. Electrical properties of Schottky diodes on nano-porous TiO2 films. Mater. Sci. Eng. B 2000, 69, 519–521. [Google Scholar] [CrossRef]
- Kim, J.-H.; Katoch, A.; Kim, H.W.; Kim, S.S. Realization of ppm-level CO detection with exceptionally high sensitivity using reduced graphene oxide-loaded SnO2 nanofibers with simultaneous Au functionalization. Chem. Commun. 2016, 52, 3832–3835. [Google Scholar] [CrossRef] [PubMed]


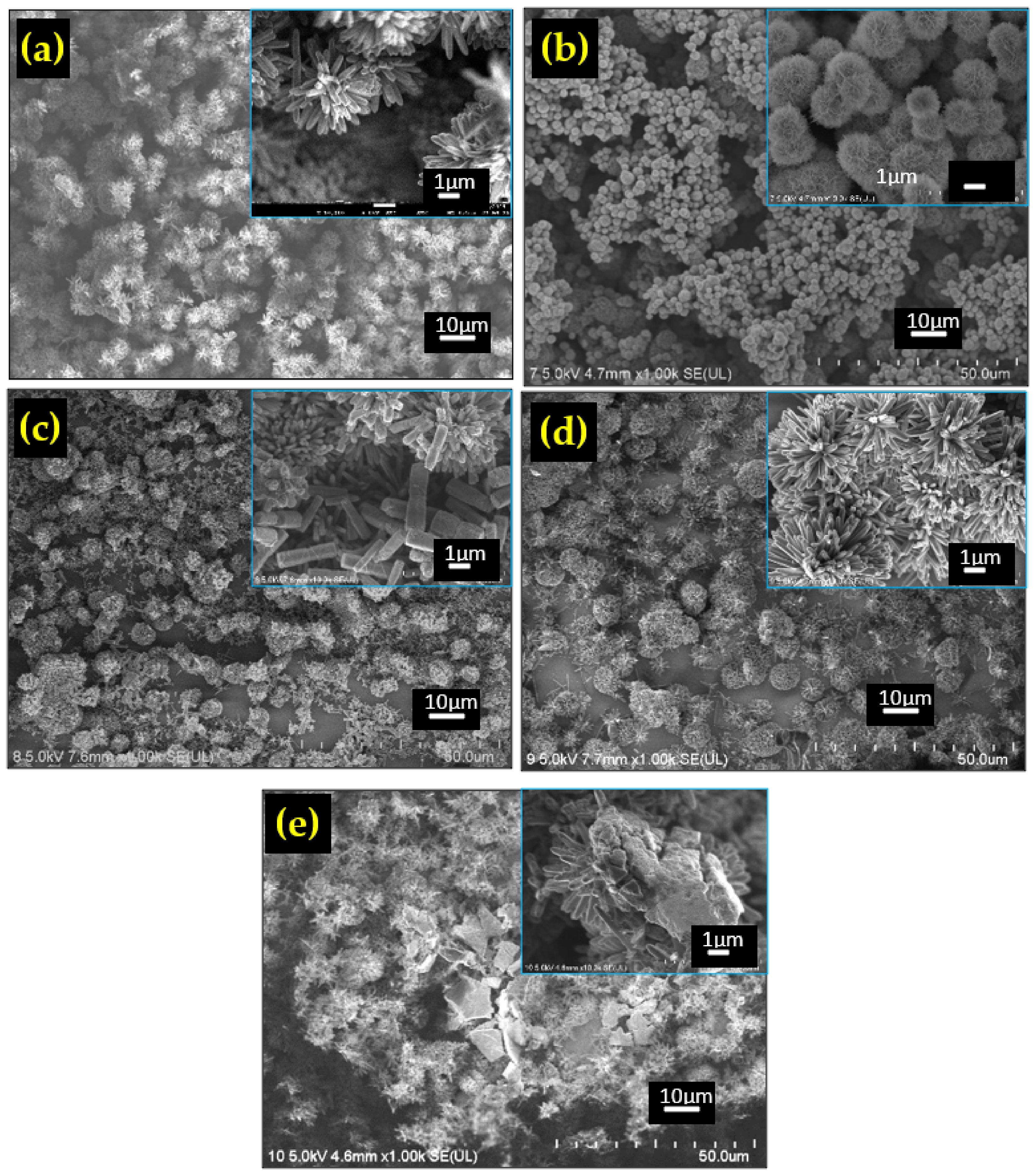
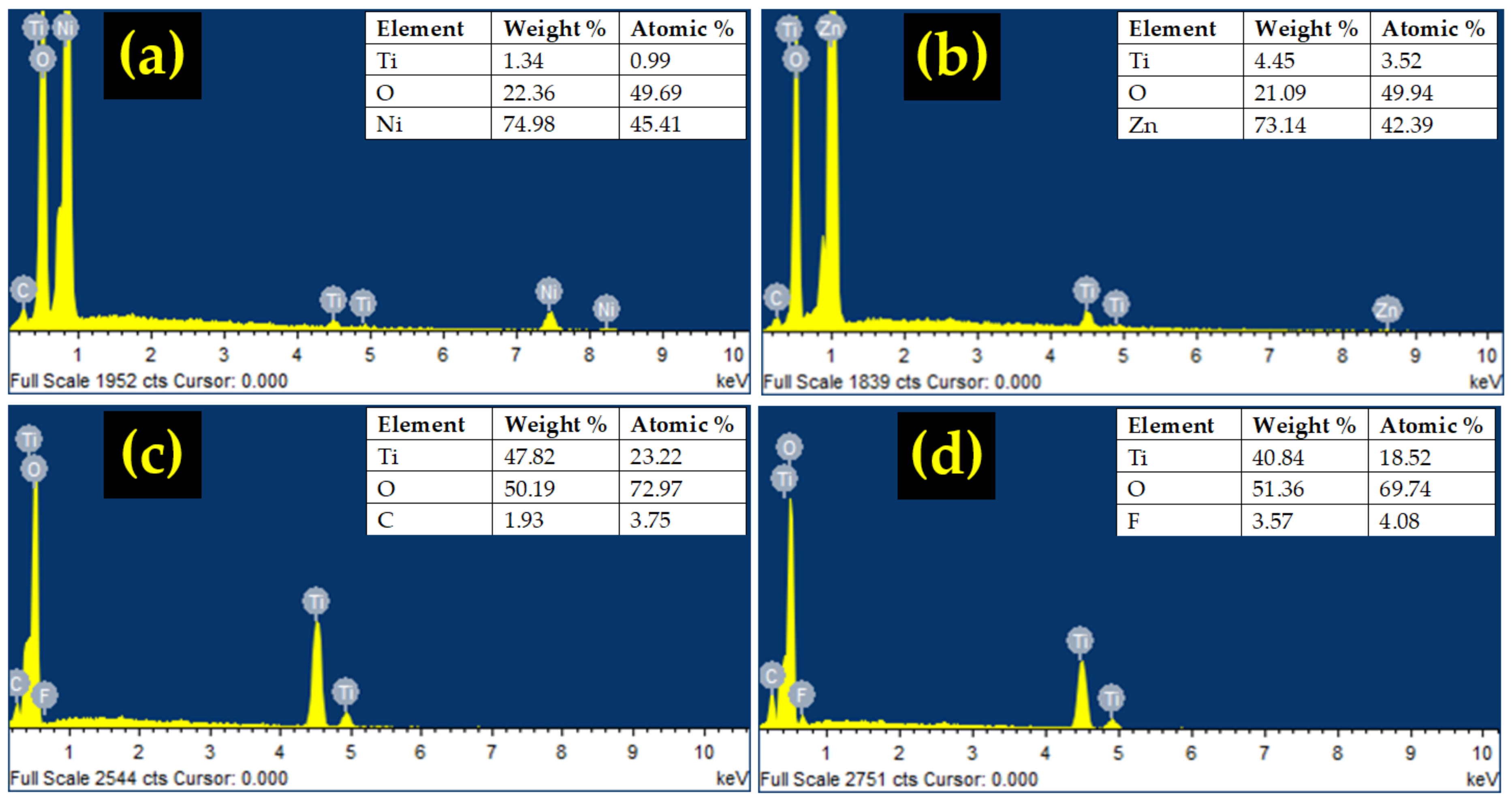

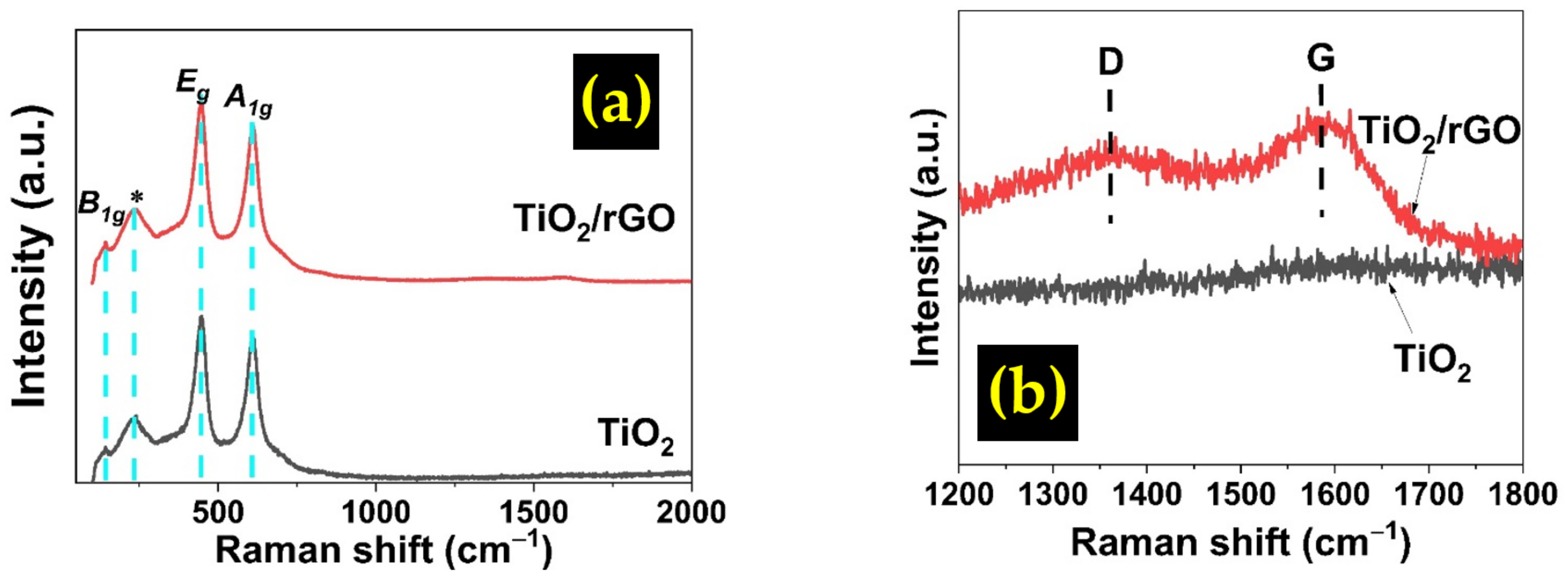


| Composite Type | Diffraction Angle, 2θ (º) | Interplanar Spacing, dhkl (Å) | Lattice Constant, a (Å) | Crystallite Size, D (nm) | Microstrain, ε (10−3) |
|---|---|---|---|---|---|
| TiO2 | 26.88 | 3.31 | 4.68 | 97.2 | 1.72 |
| TiO2/NiO | 27.16 | 3.32 | 4.64 | 75.4 | 1.86 |
| TiO2/rGO | 26.88 | 3.31 | 4.69 | 38.9 | 1.74 |
| TiO2/ZnO | 27.19 | 3.28 | 4.63 | 50.5 | 1.26 |
| TiO2/PVDF | 26.87 | 3.31 | 4.69 | 65.5 | 1.92 |
| Sample | Carrier Concentration (×1014 cm−3) | Carrier Mobility (×103 cm2/(V·s)) |
|---|---|---|
| TiO2 | 8.24 | 0.26 |
| TiO2/NiO | 0.79 | 1.55 |
| TiO2/rGO | 9.93 | 3.46 |
| TiO2/ZnO | 1.42 | 1.62 |
| TiO2/PVDF | 3.34 | 0.28 |
| Material | Sensor Type | Humidity Range | Sensor Response [Calculation Formula] |
|---|---|---|---|
| MWCNT/Polyacrylic acid [48] | Resistive | 30–90%RH | 913.8% [] |
| Graphene/Cellulose [49] | Resistive | 5–90% RH | 290% [] |
| ZnO/SnO2 [50] | Resistive | 40–90% RH | 75,440% [] |
| Mn-doped NiO/NiO [51] | Resistive | 40–90% RH | 27,000% [] |
| MWCNT/hydroxyethyl cellulose [52,53] | Resistive | 20–80% RH | 290% [] |
| Graphene/Methyl-red [54] | Resistive | 5–95% RH | 9636% [] |
| Cellulose nanofiber/graphene nanoplatelet [55] | Resistive | 30–90% RH | 14,000% [] |
| Cellulose nanofibers/CNT [56] | Resistive | 11–95% RH | 6990% [] |
| TiO2/rGO (Present work) | Resistive | 40–90% RH | 39,590% [] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Zahidi, M.; Mamat, M.H.; Malek, M.F.; Yaakob, M.K.; Ahmad, M.K.; Abu Bakar, S.; Mohamed, A.; A Subki, A.S.R.; Mahmood, M.R. Evaluating Different TiO2 Nanoflower-Based Composites for Humidity Detection. Sensors 2022, 22, 5794. https://doi.org/10.3390/s22155794
Mohamed Zahidi M, Mamat MH, Malek MF, Yaakob MK, Ahmad MK, Abu Bakar S, Mohamed A, A Subki ASR, Mahmood MR. Evaluating Different TiO2 Nanoflower-Based Composites for Humidity Detection. Sensors. 2022; 22(15):5794. https://doi.org/10.3390/s22155794
Chicago/Turabian StyleMohamed Zahidi, Musa, Mohamad Hafiz Mamat, Mohd Firdaus Malek, Muhamad Kamil Yaakob, Mohd Khairul Ahmad, Suriani Abu Bakar, Azmi Mohamed, A Shamsul Rahimi A Subki, and Mohamad Rusop Mahmood. 2022. "Evaluating Different TiO2 Nanoflower-Based Composites for Humidity Detection" Sensors 22, no. 15: 5794. https://doi.org/10.3390/s22155794
APA StyleMohamed Zahidi, M., Mamat, M. H., Malek, M. F., Yaakob, M. K., Ahmad, M. K., Abu Bakar, S., Mohamed, A., A Subki, A. S. R., & Mahmood, M. R. (2022). Evaluating Different TiO2 Nanoflower-Based Composites for Humidity Detection. Sensors, 22(15), 5794. https://doi.org/10.3390/s22155794







